Abstract
In recent studies, a crucial role for IFN-γ in immunosurveillance of tumours and in IL-12 immunotherapy has been suggested. Nevertheless, little is known about the relevance of IFN-γ and IL-12 for tumour surveillance in noncytokine immunotherapy. Adjuvant immunotherapy with viable BCG (Bacillus Calmette–Guérin) is considered to be the most powerful clinical treatment regimen of bladder cancer and is known to induce a variety of proinflammatory cytokines. Consequently, we analysed the antitumour response of IFN-γ knockout (KO), IL-12 KO and IL-10 KO mice in the absence and presence of BCG immunotherapy in a syngeneic orthotopic model of bladder cancer. IFN-γ KO and IL-12 KO mice died much earlier and by far smaller tumour inocula compared to wildtype mice, while this intrinsic antitumour response was not altered in IL-10 KO mice. BCG immunotherapy was effective in wildtype mice, but totally ineffective in IFN-γ KO and IL-12 KO mice. BCG induced a massive local immune response in the bladder of treated animals. This response was markedly increased in IL-10 KO mice, which coincides with increased therapeutic efficacy in this mouse strain compared with wildtype mice. Our data establish a crucial role for a Th1 type immune response in the intrinsic and immunotherapeutic control of local orthotopic bladder cancer.
Keywords: bladder neoplasm, IFN-gamma, IL-10, immunotherapy, orthotopic model
INTRODUCTION
Based on the profile of cytokines produced, mouse CD4 positive T helper clones are subdivided into Th1 and Th2. Th1 cells produce IFN-γ and IL-2, whereas Th2 cells secrete IL-4, IL-5, IL-6, IL-10 and IL-13 [1]. As a Th2 cytokine, IL-10 is often referred to as an immunosuppressive agent, which down-regulates cytokine production by Th1 cells, CTL generation and antigen presentation [2] and induces T cell tolerance by selective inhibition of the CD28 costimulatory pathway [3]. The induction of Th2 cytokines by tumour entities has been shown in mouse models [4,5] and in the clinical situation [6,7]. However, IL-10 does not necessarily promote tumour growth, as under certain circumstances it can also participate in immune mediated tumour rejection [8–10].
Recently, the concept of tumour immunosurveillance has regained interest and it has been shown that IFN-γ is involved in the control of tumour growth and control of metastasis formation [11,12]. In addition, IFN-γ leads to enhanced tumour immunogenicity [13], and in this way, together with lymphocytes, prevents primary tumour development [14]. By now, the important role of IFN-γ and IL-12 in cancer therapy has been established in a variety of models [15]. In particular, it has been demonstrated that IFN-γ was essential for the antitumour effect in IL-12 cytokine immunotherapy [16,17]. Notwithstanding our increasing knowledge about the protective functions of IFN-γ and IL-12 in various models of tumour control, including IL-12 immunotherapy, little is known about their role in noncytokine immunotherapy. Therefore, we investigated the relevance of these cytokines and their counterpart IL-10 in immunotherapy of bladder cancer with Mycobacterium bovis Bacillus Calmette–Guérin (BCG). BCG is clinically used for the local adjuvant treatment of bladder cancer [18,19] and leads to the local secretion of a variety of cytokines, including IFN-γ, IL-12 and IL-10 [20].
For practical reasons, thus far, most investigations in tumour immunology and immunotherapy have been carried out using heterotopic tumour models. On the other hand, it is well known that tumour growth or control depend on the outcome of multiple interactions of the tumour with its microenvironment [21]. In bladder cancer, the orthotopic animal model most closely mimics the clinical situation, which is a prerequisite for drawing meaningful conclusions [22]. Therefore, we examined in an orthotopic model of bladder cancer in parallel the role of Th1 and Th2 cytokines for the survival after tumour inoculation using the respective knockout mice.
We found that IFN-γ and IL-12 were essential for tumour surveillance in the absence and presence of immunotherapy. While intrinsic control was not altered in IL-10 KO mice, efficacy of the therapy was increased in the absence of IL-10. Improved BCG therapy in IL-10 KO mice coincided with an increased influx of T cells and NK cells into the bladder wall of these mice.
MATERIALS AND METHODS
Animals
Mice with targeted disruptions in the genes encoding IFN-γ (C57BL/6-Ifnγtm1Ts), IL-10 (C57BL/6-Il10tm1Cgn) and IL-12 (C57BL/6-Il12atm1Jm) were purchased from Jackson Laboratory (Bar Harbor, Maine). C57BL/6 wildtype controls were purchased from Charles River (Sülzfeld, Germany). Six-to-eight-week-old mice were used for experiments. All mice as well as the tumour cell line MB49 used in this study were of identical C57BL/6 background to exclude tumour rejection due to alloreactivity. The animal studies were approved by the Governmental Review Board.
Tumour cell culture
The murine 7,12-di-methylbenzanthrene-induced bladder cancer cell line MB49 (kindly provided by E.C. de Boer, University of Amsterdam) was cultured at 37°C and 5% CO2 in DMEM supplemented with 10% FCS, 1%l-glutamine, 100 U/ml penicillin and 100μg/ml streptomycin.
Orthotopic murine bladder cancer model
To compare the tumour surveillance in wildtype and cytokine knockout mice we applied an optimized murine syngeneic orthotopic bladder cancer model as published by Günther et al. 1999 [23]. In brief, MB49 tumour cells were instilled into the bladder in 50μl of DMEM without supplements. For intrinsic tumour control experiments (Fig. 1), IFN-γ KO and IL-12 KO mice (5 mice per group) received 20 000 MB49, whereas IL-10 KO mice (11 mice per group) received 100 000 MB49 cells. Survival was compared to wildtype mice, which received equal tumour inocula. Experiments were terminated when weight data and the absence of macrohematuria as a sign of bladder tumour growth suggested that the remaining mice would have no tumour outgrowth.
Fig. 1.
Impaired tumour surveillance in IFN-γ KO (a) and IL-12 KO (b), but not in IL-10 KO (c) mice. IFN-γ KO and IL-12 KO mice showed significantly reduced survival compared to immunocompetent wildtype mice (P = 0·0067, P = 0·0110 respectively), whereas survival curves of IL10 KO and wildtype mice did not differ significantly (P = 0·3811); Kaplan–Meier analysis, log-rank test. a, b and c represent three independent experiments. (a) ▪ C57BL/6; □ IFN-γ KO; (b) ▪ C57BL/6; □ IL-12 KO; (c) ▪ C57BL/6; □ IL-10 KO.
Initially, we challenged the different mouse strains with varying doses of MB49 tumour cells ranging from 800 to 100 000 cells (data not shown). These experiments revealed a different susceptibility of the different mouse strains. Therefore, in therapeutic experiments (Fig. 2), different tumour inocula were used to ensure both high tumour take rates and a high probability for the mice to receive the complete treatment course. IFN-γ and IL-12 KO mice received 20 000 MB49, whereas IL-10 KO and wildtype mice received 100 000 MB49 cells. Tumour-bearing mice were intravesically treated with 3 × 106 cfu viable BCG (strain Connaught; kindly provided by Aventis Pasteur Ltd, Toronto, Canada) in 0·9% sodium chloride on days 1, 8, 15 and 22 after tumour implantation. Control mice were treated with diluent alone. Three experiments were carried out at different occasions. Tumour growth and therapeutical effect of BCG was similar among different cohorts of wildtype mice. All of these control mice were pooled to represent one reference group consisting of 30 (BCG treated) and 35 (NaCl treated) animals, respectively, in comparison to the smaller groups with cytokine gene knockout mice (9–15 mice per treatment group). Experiments were terminated 50 days after tumour inoculation. Mice died as a result of progressive tumour growth or were euthanized after a weight loss of more than 25%. Progressive growth of bladder tumour was confirmed by autopsy.
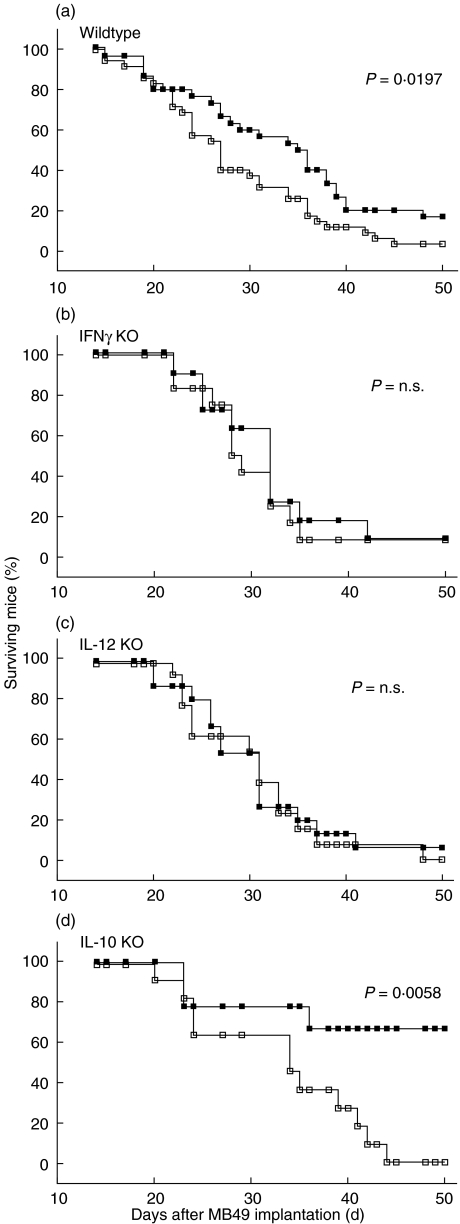
Fig. 2.
Prolonged survival of tumour bearing IL-10 KO, but not of IFN-γ KO and IL-12 KO mice after BCG immunotherapy. Tumour bearing immunocompetent wildtype mice show a significantly prolonged survival after four weekly BCG instillations (a; P = 0·0197). This immunotherapy was completely ineffective in (b) IFN-γ KO (P = 0·4235) and (c) IL-12 KO mice (P = 0·7637) and highly effective in IL-10 KO mice (d; P = 0·0058). Three experiments were carried out at different occasions. Kaplan–Meier analysis, log-rank test. ▪ MB49 + BCG; □ MB49 + NaCl.
For the immunohistochemical analysis of the local immune response mice were also treated with four weekly instillations of BCG in the absence of a tumour and killed one day after the last instillation.
Immunohistochemistry
Bladders were dissected, shock-frozen in liquid nitrogen and stored at –80°C. The immunohistochemical detection of various cell populations was performed on 5 μm frozen sections using a routine staining protocol. The binding of rat antimouse mAbs against CD4 (clone RM4-5, 500 ng/ml, BD Biosciences, Heidelberg, Germany), CD8 (clone KT15, 2 μg/ml, Biosource, Solingen, Germany), CD3 (clone CD3-12, 20 μg/ml, Biotrend, Köln, Germany), Gr-1 (clone RB6–8C5, 700 ng/ml, BD Biosciences, Heidelberg, Germany), CD11b (clone 5C6, 1 μg/ml, Biosource, Solingen, Germany) and anti-MHCII tissue culture supernatant (clone P7/7, 20%, Biosource, Solingen, Germany) was detected using peroxidase-conjugated secondary reagents (Dianova, Hamburg, Germany). The reaction was developed using substrate chromogen AEC+ (3-amino-9-ethylcarbazole; DAKO, Hamburg, Germany). The detection of NK cells using rabbit antiasialo GM1 (Wako Chemicals, Richmond, USA) was performed as described recently [24].
For the quantification of the local infiltrations, groups of two mice each were used. The number of infiltrating lymphocytes was determined by examining 7–12 randomly selected nonoverlapping microscopic fields (0·126 mm2 area) at × 250 magnification. These microscopic fields were chosen to contain equal amounts of musculature, stroma and urothelium. Immunohistochemical evaluation was blinded and performed by two independent persons. In Fig. 3, representative details are shown.
Fig. 3.
The local induction of CD4 and CD8 positive lymphocytes and NK cells is augmented in IL-10 KO mice. Representative pictures at × 250 magnification are shown. Note the increased influx of all three lymphocyte subpopulations in IL-10 KO mice.
Statistical analysis
Survival of mice was compared using the Kaplan–Meier analysis and the log-rank test. Numbers of infiltrating lymphocytes were compared using the Mann–Whitney U-test. Statistical significance was determined at P < 0·05 using SPSS statistical data analysis software for Microsoft Windows.
RESULTS
IFN-γ and IL-12, but not IL-10, are pivotal for the intrinsic antitumour response
To determine the in vivo relevance of Th1 and Th2 cytokines for the intrinsic antitumour response, we compared the survival of IFN-γ KO, IL-12 KO and IL-10 KO mice with the survival of immunocompetent wildtype mice after orthotopic inoculation of MB49 bladder tumour cells. In IFN-γ KO and IL-12 KO mice, the intrinsic antitumour response was strongly compromised, resulting in earlier death compared to immunocompetent wildtype mice (Fig. 1a,b). In contrast, intrinsic tumour surveillance of IL-10 KO was not altered (Fig. 1c).
These data suggest an important role for IFN-γ and IL-12 in the control of orthotopically implanted syngeneic bladder tumours, whereas the absence of IL-10 did not influence growth of this tumour entity.
IFN-γ and IL-12 are essential, whereas IL-10 is detrimental to immunotherapy with BCG
After we had established the in vivo relevance of IFN-γ, IL-12 and IL-10 for the intrinsic antitumour response, we next analysed their relevance for effective noncytokine immunotherapy with BCG. Female wildtype mice as well as IFN-γ KO, IL-12 KO and IL-10 KO mice were implanted with previously determined optimal tumour doses of 100 000 (wildtype, IL-10 KO) or 20 000 (IFN-γ KO, IL-12 KO) MB49 bladder tumour cells. A reduced number of tumour cells was used in IFN-γ KO and IL-12 KO mice, because these mice were more susceptible to MB49 tumour growth (see Fig. 1) compared to wildtype and IL-10 KO mice. Therefore, in the therapeutic experiments we had to prevent early death of IFN-γ KO and IL-12 KO mice, which otherwise would not receive the full treatment course. Tumour-bearing wildtype mice were successfully treated with BCG, as revealed by significantly prolonged survival (Fig. 2a; P = 0·0197). In contrast, in both IFN-γ KO and IL-12 KO mice, local BCG therapy failed, as the survival curves of treated and untreated tumour bearing mice did not differ significantly (Fig. 2b,c; P = 0·4235 and 0·7637, respectively), suggesting that both Th1 cytokines were essential for successful BCG immunotherapy. Interestingly, BCG treated IL-10 KO mice showed an even longer survival, compared to wildtype mice (Fig. 2d, P = 0·0058), suggesting that IL-10 might interfere with the establishment of a protective antitumour milieu during immunotherapy.
Control experiments were performed to show that MB49 were not able to secrete the cytokines IFN-γ and IL-10 by themselves. As expected, the secretion of both cytokines by unstimulated and BCG stimulated cell cultures turned out to be under the detection limit (less than 30 pg/ml) of sandwich ELISAs (BD Biosciences, Heidelberg, Germany) (data not shown).
BCG-induced recruitment of T cells and NK cells into the bladder is enhanced in IL-10 KO mice
In the therapeutical experiment depicted in Fig. 2 the absence of IFN-γ or IL-12 prevented successful immunotherapy whereas on the other hand the absence of IL-10 increased the efficacy of immunotherapy. To further understand the role of these three cytokines during immunotherapy we investigated the local immune response in the bladder of IFN-γ KO, IL-10 KO and IL-12 KO mice after instillation of BCG by means of immunohistology.
We could show that BCG induces a massive local immune response with the influx of CD4 and CD8 positive lymphocytes and NK cells into the bladder wall (Table 1). Recruitment of lymphocytes also occurred in IFN-γ KO and IL-12 KO, with the exception of CD8 positive cells in IL-12 KO mice. Most important, we observed an increased recruitment of CD4 (1·4-fold) and CD8 (3·1-fold) positive lymphocytes and NK cells (1·7-fold) in BCG-treated IL-10 KO compared with BCG-treated wildtype mice (Fig. 3,Table 1). This increased influx of lymphocytes into the bladder coincides with the increased therapeutic efficacy in IL-10 KO mice (Fig. 2). Initial experiments showed the murine bladder tumour cell line MB49 to be highly immunogeneic, inducing a pronounced infiltration by immunocompetent cells and granulocytes (data not shown). The induction of various cell populations by the tumour itself masked the BCG-induced cellular infiltrations and made it merely impossible to dissect tumour-induced and BCG-induced effects. Therefore, in the present manuscript, we focus on the BCG-induced effects in the absence of the tumour.
Table 1.
Local induction of T lymphocyte subsets and NK cells by BCG in wildtype, IFN-γ KO, IL-10 KO and IL-12 KO mice *
| Wildtype | IL10 KO | IL12 KO | IFN-γ KO | |
|---|---|---|---|---|
| CD4 control | 27·8 | 19·8 | 19·8 | 47·6 |
| (15·9/40·0) | (7·9/23·8) | (7·9/23·8) | (25·8/75·4) | |
| CD4 BCG | 230·2† | 317·5†‡ | 134·9†‡ | 297·6†‡ |
| (182·5/297·6) | (224·2/382·9) | (107·1/206·4) | (222·2/379·0) | |
| CD8 Control | 7·9 | 7·9 | 11·9 | 15·9 |
| (7·9/13·9) | (0/7·9) | (7·9/23·8) | (7·9/29·8) | |
| CD8 BCG | 35·7† | 111·1†‡ | 15·9‡ | 47·6† |
| (21·8/55·6) | (77·4/129·0) | (7·9/23·8) | (15·9/77·4) | |
| NK Control | 7·9 | 7·9 | 11·9 | 7·9 |
| (7·9/15·9) | (7·9/13·9) | (7·9/23·8) | (7·9/15·9) | |
| NK BCG | 59·5† | 99·2†‡ | 63·5† | 47·6† |
| (37·7/79·4) | (85·3/150·8) | (55·6/85·3) | (31·7/55·6) |
Detection of CD4 and CD8 positive lymphocytes and NK cells in bladders of BCG-treated wildtype and cytokine gene knockout (KO) mice. Staining was compared with bladders of untreated control mice. Median values of cells per mm 2 (bold script) and, in parentheses, quartiles Q1 and Q3 are shown.
Significant difference of BCG-treated bladder versus control bladder (P < 0·05; Mann–Whitney U-test).
Significant difference of BCG-induced infiltration in the respective KO mice versus wildtype mice (P < 0·05; Mann–Whitney U-test).
DISCUSSION
In literature, there are contradictory results concerning the function of Th1/Th2 cytokines in tumour immunity. This might at least partly depend upon the model used by the investigators, in most cases heterotopic models were applied. However, previous investigations made clear that the fate of a growing tumour crucially depends on the multiple and diverse interactions of the tumour with its microenvironment [21]. Insights into these complex interrelationships of the tumour and the surrounding tissue emphasize the need for an orthotopic tumour model to draw meaningful conclusions with respect to tumour immunology and immunotherapy.
Taking this into consideration, we have applied an orthotopic syngeneic murine bladder cancer model [23]. To examine the role of the Th1/Th2 cytokine system in tumour control we used mice with targeted mutations in Th1 cytokine (IFN-γ, IL-12) or Th2 cytokine (IL-10) genes in order to perform a comparative study. In addition, we investigated the role of IFN-γ, IL-12 and IL-10 in the course of BCG immunotherapy known to induce the respective cytokines besides others. We could show that the Th1 cytokines IFN-γ and IL-12 are essential for both the intrinsic antitumour response as well as effective immunotherapy. IFN-γ and IL-12 are reciprocally induced [25–28], thereby introducing a positive feedback loop to stabilize Th1 polarization. Further, neutralization of IL-12 drastically reduced IFN-γ secretion by splenocyte cultures from BCG primed mice [26] and neutralization of IFN-γ abrogated IL-12 induced tumour immunity [29]. Both IFN-γ and IL-12 were induced during in vitro stimulation of human peripheral blood mononuclear cells with BCG [30]. Further, IL-2 secreting BCG induced both IFN-γ and IL-12 in murine peritoneal exudate cells and increased cytotoxicity against a murine bladder cancer cell line [31]. Recently, the importance of IFN-γ for tumour control has been emphasized using models of spontaneous and induced tumours and in IL-12 cytokine immunotherapy [12,14,16,29]. Based on the well-known potential of BCG to induce Th1 cytokines, we obtained direct causal evidence for a role of these cytokines in BCG-mediated tumour control. In addition, to our knowledge, the data reported herein are the first demonstration of a role for IFN-γ and IL-12 in noncytokine immunotherapy of orthotopic cancer.
In our animal model, IL-10 has no influence on the intrinsic tumour surveillance. Interestingly, Halak et al. showed in a heterotopic syngeneic model that in the absence but not in the presence of IL-10, MB49 cells prime for a tumour specific CTL response leading to improved survival of IL-10 KO compared to wildtype mice [5]. A possible explanation for these contrary findings may be based on differences in the heterotopic model of Halak et al. and our orthotopic model. This view is further supported by the fact that we have found pronounced differences in subcutaneous and orthotopic tumour growth in a model of MB49 immuno-gene therapy (our own unpublished results). Notwithstanding the missing role of IL-10 in intrinsic tumour control, we observed a detrimental role of IL-10 for successful BCG immunotherapy. The improved survival of IL-10 KO mice versus wildtype mice undergoing immunotherapy might be due to an induction of IL-10 in wildtype mice by BCG. This induction could also be shown in the urine of patients [20]. Obviously, IL-10 exerts its pleiotropic effects in tumour immunity in dependence of the special cellular composition and/or cytokine milieu which predominates at the site of tumour growth.
CD4 positive cells play an important role in the in vivo generation of CTL [32] and interferon-induced tumour protection [33]. On the other hand, depletion of CD4 cells enhanced survival in a murine model of subcutaneously growing IL-12 transfected colon carcinoma [34] and lead to the regression of subcutaneously growing sarcoma [35].
Immunohistochemistry revealed that CD4 positive lymphocytes are the most prevalent lymphocyte subpopulation in BCG-treated wildtype mice. Comparing the different mouse strains, the extent of the BCG-induced influx of CD4 cells did not correlate with the treatment outcome. Our interpretation of these data is that not so much the mere density of infiltrating CD4 cells is important for tumour control, but the cytokine milieu generated by these CD4 cells. This might be a tumour protective Th1 or a tumour promoting Th2 environment.
Interestingly, there was a significant increase in CD4 and CD8 positive lymphocytes and NK cells in IL-10 KO versus wildtype mice. This might be of therapeutical relevance, as IL-10 KO mice also showed increased therapeutic efficacy. Animal experiments by Ratliff and coworkers have shown that CD4 and CD8 cells contribute to successful immunotherapy with BCG [36,37]. In addition, we have recently reported that NK cells were essential for BCG-induced cytotoxicity in vitro and for effective BCG immunotherapy in vivo [24]. In this context, it is important to note that both CD8 positive lymphocytes and NK cells are not only cytotoxic effector cells, but are also potent IFN-γ producers [38], which might shift the Th1/Th2 balance towards the tumour protective Th1 milieu at an early time point.
Taken together, depending on the experimental conditions used by different investigators, ambiguous findings are revealed with respect to the function of T cell subsets and the Th2 cytokine IL-10 in tumour immunity. We would like to emphasize that we have found different roles for IL-10 in intrinsic tumour control and in tumour control during immunotherapy. While the presence of IL-10 has no influence on the naturally occurring antitumour immune response, it seems to inhibit efficacy of BCG immunotherapy.
Recently, the construction of recombinant BCG strains has been reported [39,40]. In view of our data, the use of recombinant BCG strains, which direct the immune response towards a strong Th1 milieu and/or inhibit Th2 cytokines, might have the potential to improve current BCG therapy of bladder cancer.
Acknowledgments
We thank G. Bentien for expert technical assistance. This work was supported by DFG/Sonderforschungsbereich 367 (grant C7 to A.B.).
REFERENCES
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:3688–94. [PubMed] [Google Scholar]
- 2.Sharma S, Stolina M, Lin Y, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–8. [PubMed] [Google Scholar]
- 3.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–6. doi: 10.1046/j.1365-2567.2001.01235.x. 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAveney KM, Gomella LG, Lattime EC. Induction of TH1- and TH2-associated cytokine mRNA in mouse bladder following intravesical growth of the murine bladder tumor MB49 and BCG immunotherapy. Cancer Immunol Immunother. 1994;39:401–6. doi: 10.1007/BF01534428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halak BK, Maguire HC, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–17. [PubMed] [Google Scholar]
- 6.Maeurer MJ, Martin DM, Castelli C, et al. Host immune response in renal cell cancer: interleukin-4 (IL-4) and IL-10 mRNA are frequently detected in freshly collected tumor-infiltrating lymphocytes. Cancer Immunol Immunother. 1995;41:111–21. doi: 10.1007/BF01527407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berghella AM, Pellegrini P, Del Beato T, et al. The significance of an increase in soluble interleukin-2 receptor level in colorectal cancer and its biological regulating role in the physiological switching of the immune response cytokine network from TH1 to TH2 and back. Cancer Immunol Immunother. 1997;45:241–9. doi: 10.1007/s002620050439. 10.1007/s002620050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerard CM, Bruyns C, Delvaux A, et al. Loss of tumorigenicity and increased immunogenicity induced by interleukin-10 gene transfer in B16 melanoma cells. Hum Gene Ther. 1996;7:23–31. doi: 10.1089/hum.1996.7.1-23. [DOI] [PubMed] [Google Scholar]
- 9.Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I and enhances NK lysis. Cell Immunol. 1997;180:55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Gutierrez P, Jackson MJ, Kundu N, Fulton AM. Essential role of nitric oxide and interferon-gamma for tumor immunotherapy with interleukin-10. J Immunother. 2000;23:208–14. doi: 10.1097/00002371-200003000-00005. 10.1097/00002371-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Doherty GM, Alexander HR, Merino MJ, Venzon DJ, Norton JA. Role of endogenous interferon gamma in murine tumor growth and tumor necrosis factor alpha antitumor efficacy. Ann Surg Oncol. 1996;3:198–203. doi: 10.1007/BF02305801. [DOI] [PubMed] [Google Scholar]
- 12.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth and metastasis. Blood. 2001;97:192–7. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Nat Acad Sci. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 15.Shurin MR, Esche C, Péron J-M, Lotze MT. Antitumor activities of IL-12 and mechanisms of action. Chem Immunol. 1997;68:153–74. doi: 10.1159/000058690. [DOI] [PubMed] [Google Scholar]
- 16.Brunda MJ, Luistro L, Hendrzak JA, Fountoulakis M, Garotta G, Gately MK. Role of Interferon-γ in mediating the antitumor efficacy of Interleukin-12. J Immunol. 1995;17:71–7. doi: 10.1097/00002371-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Voest EE, Kenyon BM, O’Reilly MS, Truitt G, d'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 18.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacillus Calmette–Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–9. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 19.Herr HW, Schwalb DM, Zhang ZF, et al. Intravesical bacillus Calmette–Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13:1404–8. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 20.Schamhart DHJ, de Boer EC, de Reijke TM, Kurth K-H. Urinary cytokines reflecting the immunological response in the urinary bladder to biological response modifiers: their practical use. Eur Urol. 2000;37(Suppl.):16–23. doi: 10.1159/000052388. [DOI] [PubMed] [Google Scholar]
- 21.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Canc Metastasis Rev. 1998;17:279–84. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 22.Ratliff TL. Role of animal models in understanding intravesical therapy with bacille Calmette–Guérin. Clin Infect Dis. 2000;31(Suppl.):106–8. doi: 10.1086/314065. [DOI] [PubMed] [Google Scholar]
- 23.Günther JH, Jurczok A, Wulf T, et al. Optimizing syngeneic orthotopic murine bladder cancer (MB49) Cancer Res. 1999;59:2834–7. [PubMed] [Google Scholar]
- 24.Brandau S, Riemensberger J, Jacobsen M, et al. NK cells are essential for effective BCG-immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Chan SH, Perussia B, Gupta JW, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–79. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-gamma in response to bacillus Calmette–Guérin. J Immunol. 1999;163:4246–52. [PubMed] [Google Scholar]
- 27.Schmitt E, Rude E, Germann T. The immunostimulatory function of IL-12 in T-helper cell development and its regulation by TGF-beta, IFN-gamma and IL-4. Chem Immunol. 1997;68:70–85. doi: 10.1159/000058695. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–11. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa M, Yu WG, Umehara K, et al. Multiple roles of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998;58:2426–32. [PubMed] [Google Scholar]
- 30.Zlotta AR, van Vooren J-P, Denis O, et al. Cancer Therapy; What are the immunologically active components of Bacille Calmette–Guérin in therapy of superficial bladder cancer? Int J Cancer. 2000;87:844–52. doi: 10.1002/1097-0215(20000915)87:6<844::aid-ijc14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Murine IL-2 secreting recombinant Bacillus Calmette–Guérin augments macrophage-mediated cytotoxicity against murine bladder cancer MBT-2. J Urol. 2000;164:526–31. [PubMed] [Google Scholar]
- 32.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–82. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markovic SN, Murasko DM. Role of natural killer and T-cells in interferon induced inhibition of spontaneous metastases of the B16F10L murine melanoma. Cancer Res. 1991;51:1124–8. [PubMed] [Google Scholar]
- 34.Martinotti A, Stoppacciaro A, Vagliani M, et al. CD4 T cells inhibit in vivo the CD8-mediated immune response against murine colon carcinoma cells transduced with interleukin-12 genes. Eur J Immunol. 1995;25:137–46. doi: 10.1002/eji.1830250124. [DOI] [PubMed] [Google Scholar]
- 35.Fu T, Shen Y, Fujimoto S. Tumor specific CD4+ suppressor T-cell clone capable of inhibiting rejection of syngeneic sarcoma in A/J mice. Int J Cancer. 2000;87:680–7. [PubMed] [Google Scholar]
- 36.Ratliff TL, Gillen DP, Catalona WJ. Requirement of a thymus- dependent immune response for BCG- mediated antitumor activity. J Urol. 1987;137:155–8. doi: 10.1016/s0022-5347(17)43909-7. [DOI] [PubMed] [Google Scholar]
- 37.Ratliff TL, Ritchey JK, Yuan JJJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–23. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 38.Handa K, Suzuki R, Matsui H, Shimizu Y, Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL2) II. IL 2-induced interferon gamma production. J Immunol. 1983;130:988–92. [PubMed] [Google Scholar]
- 39.O’Donnell MA, Aldovini A, Duda RB, et al. Recombinant Mycobacterium bovis BCG secreting functional Interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Chen X, Han R, O’Donnell MA. Recombinant Bacille Calmette–Guérin (BCG) expressing human interferon-alpha 2B demonstrates enhanced immunogenicity. Clin Exp Immunol. 2001;123:264–70. doi: 10.1046/j.1365-2249.2001.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]