Abstract
Regulatory cytokines mediate the participation of oral mucosal epithelial cells (OMEC) in local immune responses. The aim of this study was to characterize the isoforms of IL-1 receptor antagonist (IL-1ra) in cultured human primary OMECs and to compare its production with that of IL-1 alpha (IL-1α) and IL-1 beta (IL-1β). Western blot analysis showed that IL-1ra was 22 kDa in size hence slightly smaller than monocyte IL-1ra (25 kDa). A minor form of 20 kDa was also found in unstimulated cell culture lysates. In culture supernatants, IL-1 bioactivity increased after IL-1ra neutralization, indicating that the baseline production of IL-1ra is biologically relevant. Immunohistochemistry showed a relation between IL-1ra and involucrin expressions, suggesting that intracytoplasmic IL-1ra may be involved in cell terminal differentiation. In unstimulated culture lysates, there was far more IL-1ra than IL-1α and IL-1β. TGF-β1 markedly increased the IL-1ra/IL-1β ratio from 93·6 : 1 to 300 : 1. IL-4, which is generally described as an anti-inflammatory cytokine, increased IL-1 but not IL-1ra production. TNF-α increased intracellular production of the three IL-1 members. IL-1ra levels were lower in supernatants than in lysates of cultured cells. Our results show that human OMECs constitutively produce significant amounts of a biologically active form of IL-1ra. TGF-β1 µp-regulation points to a positive amplification loop and IL-4 to a down-regulation loop, both including Th2 cells and OMECs. They may be important in oral tolerance and IgA production, respectively.
Keywords: epithelial cells, differentiation, IL-1, IL-1 receptor antagonist, oral
Introduction
A mucosa-associated lymphocyte population was first characterized in the oral cavity in 1981 [1]. Mucosa-associated lymphoid tissues were soon established as central to mucosal immunity and oral tolerance [2–4]. Oral mucosal epithelial cells (OMECs) act as transmucosal carriers of antigens, antibodies and immune cells [5,6]. They also produce several cytokines in functionally relevant amounts [7,8]. In vitro, this production seemed to be constitutive [9] but modulated by extraneous cytokines [10–13].
IL-1, a proinflammatory cytokine with two main forms, IL-1α and IL-1β, is produced by several cell types including OMECs [10]. Epithelial IL-1 is increased in oral inflammatory conditions such as periodontitis [14], oral lichen planus [15] and Sjogren's syndrome (SS) [16].
IL-1 receptor antagonist (IL-1ra) is a naturally occurring inhibitor which competes with IL-1 for occupancy of IL-1 cell surface receptors [17]. IL-1ra has two main variants, a secreted IL-1ra (sIL-1ra) and an intracellular IL-1ra (icIL-1ra), which characterize monocytes and epithelial cells, respectively [18,19]. We have documented previously the presence of IL-1ra in OMECs and in normal human saliva [20].
The regulation of IL-1ra production by OMECS and consequently the IL-1/IL-1ra balance have not been studied. We hypothesized that if IL-1ra production is modulated by T cell cytokines, an interplay between Th1/Th2 cytokines and epithelial cytokines may arise. Little is known about IL-1ra production in the oral mucosa. Immunohistochemistry suggested that IL-1ra is expressed in the most differentiated layers of the buccal epithelium [20]. Skin keratinocytes in culture produce IL-1 and IL-1ra [21]. IL-1ra production is increased in cells that have been differentiated in cultures with calcium [22,23].
In the present work, we studied IL-1 and IL-1ra production in cultured OMECs. We evaluated its regulation by various cytokines and for comparison by calcium or hydrocortisone. We assumed that if Th1 cytokines promote IL-1ra production and/or a decrease in IL-1 production, OMECs would down-regulate ongoing local Th1 responses. Conversely, if the Th1 cytokines act on IL-1 and IL-1ra in opposite directions, OMECs would amplify Th1 responses. OMECs could also engage in a separate dialogue with Th2 cells and their Th3 subtype [24]. The prominence of antibody-producing cells in oral mucosa makes it likely that OMECs enhance Th2 responses. This could be a new aspect of the ‘mucosal intranet’ envisioned by Yamamoto et al. [25]. In intestinal epithelium, cross-talk between intraepithelial lymphocytes and epithelial cells modulates cytokine production and participates in local immune responses or mucosal tolerance [25]. The mucosal intranet, either intestinal or oral, may have an important role in health and disease.
Materials and methods
Primary cultures
Human buccal tissue was obtained surgically during removal of wisdom teeth from healthy donors (aged 13–19, n = 10). OMEC cultures were established as described previously [26]. Briefly, mucosal samples were placed extemporaneously in sterile Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Cergy-Pontoise, France) supplemented with penicillin 100 U/ml, streptomycin 100 µg/ml and amphotericin B 1 µg/ml (all from Life Technologies). The connective tissue was discarded and mucosal samples were then washed with PBS (Life Technologies) and cut into 0·5 × 0·5 cm explants.
Each explant was plated into a well of a 12-well tissue culture plate (Falcon 3043, Elvetec, Clermont-Ferrand, France) precoated with fetal calf serum (FCS, Biosys, Compiègne, France). The explant was allowed to adhere without medium for 10 min at room temperature. A growth medium was then added consisting of a mixture in equal volumes of RPMI 1640 and DMEM supplemented with antibiotics and amphotericin B as above, FCS 0·5% and growth factors: EGF 10 ng/ml, hydrocortisone 2 µg/ml, insulin 40 µg/ml and cholera toxin 30 ng/ml (all from Sigma, St Quentin-Fallavier, France). Cell cultures were incubated at 37°C with 5% CO2. Culture supernatants were replaced every 2 days by fresh growth medium. The explants were removed after 6 days, leaving OMECs which had spread on the bottom of the well. A few contaminating fibroblasts were discarded by gentle trypsinization. The purity of the epithelial cell culture was determined by first inverted microscope and then confirmed by immunostaining (monoclonal anticytokeratin protein 13, clone KS-1A3, Sigma) on cytospins. Most cell cultures reached confluency at 15 days or later. They were then used for further experiments or for subcultivations.
Subcultivations
OMEC monolayers were rinsed in PBS-EDTA (phosphate-buffered saline containing 5 mm ethylene diamine tetracetate), incubated in PBS-EDTA for 5 min at 37°C and detached with 0·5% trypsin (1/250) and 5 mm EDTA (Life Technologies). After trypsin neutralization by FCS and centrifugation at 400 g, cell pellets were resuspended in culture medium and seeded into 24-well culture plates (Falcon 3047, Elvetec) at 100 000 cells/well (1 ml). For all stimulation experiments, cells were used in first or second passages.
Reagents and cell stimulation
Epithelial cells were washed with calcium-free PBS and cultured for 24 h in RPMI 1640 with or without the following test reagents: 10 ng/ml recombinant human TNF-α, IL-1α, IL-6, IFN-γ, IL-4, IL-10 and TGF-β1 (R&D Systems, Abingdon, UK), 50 µg/ml hydrocortisone succinate (Sigma) or 1 mm calcium (Life Technologies). According to R&D Systems, all the recombinant cytokines contained less than 0·01% of LPS. Cell culture supernatants (1 ml/well) were harvested and non-adherent cells were discarded after centrifugation at 9000 g for 5 min at 4°C. Adherent OMECs were lysed by three cycles of freezing/thawing. Supernatants and cell lysates were stored at −20°C until assayed.
Cytokine assays
IL-1ra, IL-1α and IL-1β concentrations were measured by sandwich ELISA according to the kit manufacturer's recommendations (Quantikine R&D Systems, Abingdon, UK). Limits of detection were 14 pg/ml for IL-1ra, 0·5 pg/ml for IL-1α and 1 pg/ml for IL-1β (reading at 450 nm). The tests did not discriminate between secreted or intracellular forms in any of the members of the IL-1/IL-1ra family.
Western blotting
In order to determine the size distribution of IL-1ra protein in OMECs, a pool of 15 ml of supernatant from unstimulated cultures was further enriched in IL-1ra (400 ng/ml according to ELISA assay) by ultrafiltration on a Centricon-10 concentrator (YM membrane, Amicon, Epernon, France). Cell lysates from unstimulated primary cultures (106 cells) were used in similar IL-1ra concentrations to those in cell supernatants (450 ng/ml). Proteins were separated on 15% SDS-PAGE under reducing conditions, then transferred electrophoretically to PVDF membrane (Biorad, Ivry-sur-Seine, France). Blots were developed using specific polyclonal antihuman IL-1ra goat antibody (R&D Systems, Abingdon, UK) at 1/100 dilution in PBS with 1% skimmed milk, biotinylated mouse antigoat secondary antibody (Sigma) at 1/100 dilution and extravidin–phosphatase complex (Sigma) at 1/100 dilution in phosphate-free buffer. Staining was revealed by bromo-chloro-indolyl-phosphate/nitro blue tetrazolium (BCIP/NBT) chromogen (Sigma). Protein size was determined from low-range biotinylated SDS-PAGE standard (Biorad) and compared with that of secreted IL-1ra from monocyte cultures (MW: 25 kD). The specificity of the reaction was checked by using a preimmune serum (goat serum, Dako, Trappes, France) instead of the specific anti-IL-1ra serum.
Biological activity of OMECs IL-1ra
IL-1 is known to induce IL-6 production by OMECs [10]. This and other evidence suggest that IL-1 can compete successfully for IL-1 receptor occupancy with any IL-1ra normally present in the same culture. If so, anti-IL-1ra antibodies should enhance IL-6 production. OMEC cultures were incubated for 24 h with neutralizing goat antihuman IL-1ra IgG (10 µg/ml, R&D Systems) or goat serum (Dako). Direct IL-1 stimulation (recombinant human IL-1α, 10 ng/ml, R&D Systems) was used as positive control and RPMI alone as negative control. IL-6 concentrations were measured in cell culture supernatants by sandwich ELISA kit (R&D Systems, Quantikine D6050, Abingdon, UK) with a sensitivity of 0·7 pg/ml.
Immunohistochemistry
Cytospins of OMEC suspensions were fixed in methanol/acetone (1 : 1) at −20°C for 10 min. They were subsequently air-dried, washed in PBS and incubated for 10 min in PBS containing 1% bovine serum albumin (BSA, Sigma). Cells were then incubated for 1 h at 25°C with either a polyclonal goat antihuman IL1ra antibody (R&D Systems) or a monoclonal mouse antihuman involucrin antibody (Novocastra, Le Perray-en-Yvelines, France) at 1/20 dilution. In negative controls, the primary antibody was replaced by preimmune serum or the diluted anti-IL-1ra serum (50 µg/ml) was preincubated with 10 ng/ml of human recombinant IL-1ra (R&D Systems). The second antibody, biotinylated mouse antigoat IgG (Sigma, Extra 1-A) or biotinylated goat antimouse IgG (Sigma, Extra 2-A) and the extravidin–phosphatase complex were applied sequentially for 30 min and revealed by Fast Red TR/Naphtol substrate containing levamisol (Sigma). The immunostaining was counterstained with Mayer's haematoxylin.
Statistics
Dunnett's test was used to compare the set of stimulating agents and a single control. Analysis was performed using SAS (SAS Institute, Cary, NC, USA). Results were considered significant when P < 0·05. Error bars are s.d.
Results
Western blot analysis
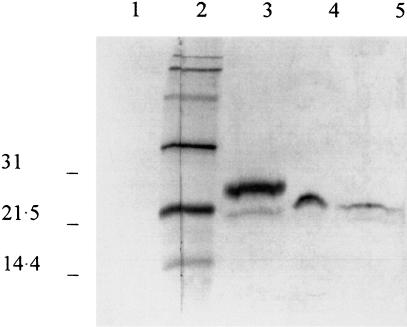
When analysed by SDS-PAGE, pooled concentrated supernatants from unstimulated OMECs showed only one IL-1ra band (Fig. 1, lane 4). Its apparent molecular weight was lower than that of monocyte IL-1ra (25 kDa, Fig. 1, lane 3) and estimated at 22 kDa. Lysates from unstimulated OMECs showed the 22 kDa band and an additional faint band of 20 kDa (Fig. 1, lane 5).
Fig. 1.
Western-blot analysis of IL-1ra protein in lysate and supernatant of oral mucosal cells. Lane 4 is a fraction of a concentrated pool of supernatants from three unstimulated cultures. Cell-associated IL-1ra is shown from unstimulated cells (lane 5). For comparison, lane 3 is the 25 kD monocyte IL-1ra. Lane 2 is the biotinylated standard (mol. wt. × 103 daltons). Lane 1 is the control with preimmune serum.
Biological activity of OMECs IL-1ra
The addition of a blocking antihuman IL-1ra antibody from goat increased IL-6 production in unstimulated OMECs by 261% (Table 1). Goat serum did not modify IL-6 production (data not shown). Positive controls stimulated by extraneous recombinant IL-1α showed a 552% increase in IL-6 production.
Table 1.
IL-1-induced IL-6 secretion in OMECs, which were incubated with either RPMI (control), 20 µg/ml of neutralizing human IL-1ra (anti-IL-1ra) or recombinant IL-1α (10 ng/ml) for 24 h. IL-6 immunoreactivity in cell culture supernatants was assayed by ELISA. Exogenous IL-1 enhanced IL-6 secretion by 552%, whereas endogenous IL-1 enhanced IL-6 secretion by 261% after IL-1ra neutralization. The ‘% increase’ represents the mean of (test/control) × 100 calculated for the three experiments, each performed with different cell cultures
| IL-6 production (pg/ml) | ||||
|---|---|---|---|---|
| expt 1 | expt 2 | expt 3 | % increase (mean ±s.d.) | |
| Control | 796 | 186 | 223 | |
| anti-IL-1ra (20 µg/ml) | 2732 | 558 | 316 | 261 ± 106 |
| rec IL-1α (10 ng/ml) | 4409 | 1359 | 833 | 552 ± 178 |
Immunohistochemical study
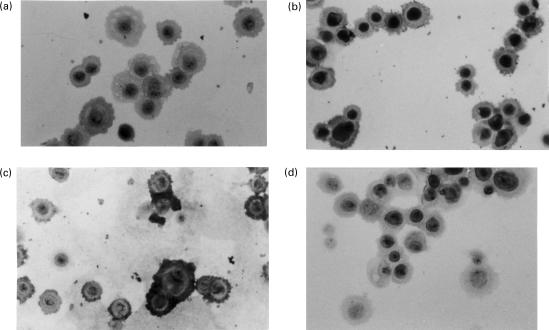
IL-1ra was expressed in resting OMEC cytoplasm (Fig. 2a). Cells stimulated by TGF-β1 became strongly positive for IL-1ra (Fig. 2b). Staining for involucrin, a marker of cell terminal differentiation, was increased by TGF-β1 (Fig. 2c). Unstimulated OMECs incubated in medium containing recombinant IL-1ra (10 ng/ml) did not have a stronger IL-1ra expression than unstimulated OMECs in medium (data not shown), which suggests that exogenous IL-1ra was not taken up by the cells.
Fig. 2.
Immunohistological staining in scattered oral mucosal cells (×20). These results are representative of two similar experiments. (a) IL-1ra staining is observed in a few unstimulated cells. (b) It is more intense in the cytoplasm of cells stimulated with TGF-β1. (c) These cells stain markedly positive for involucrin. (d) Staining was nil in control with preimmune serum. Control was also negative with anti-IL-1ra serum preincubated with recombinant IL-1ra.
Cell-associated cytokines
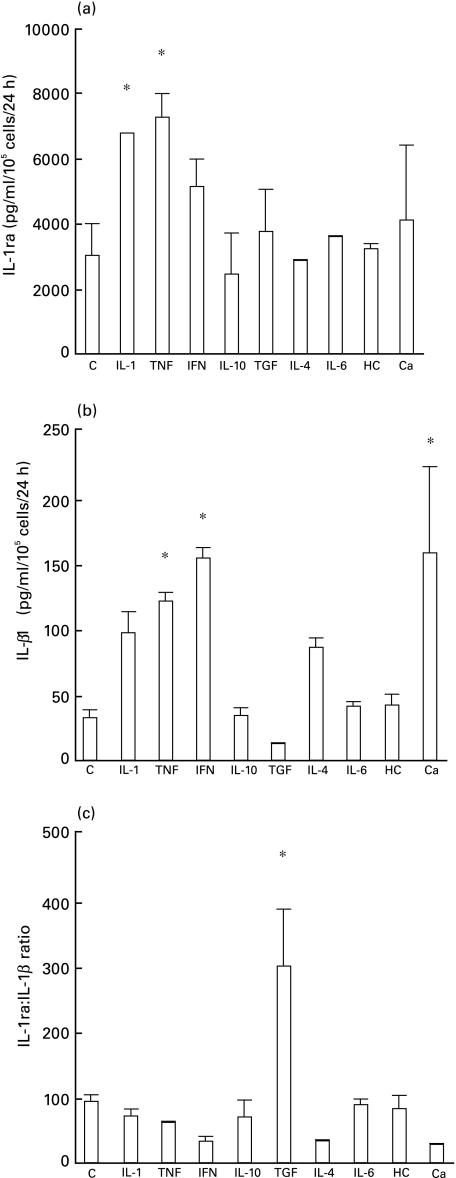
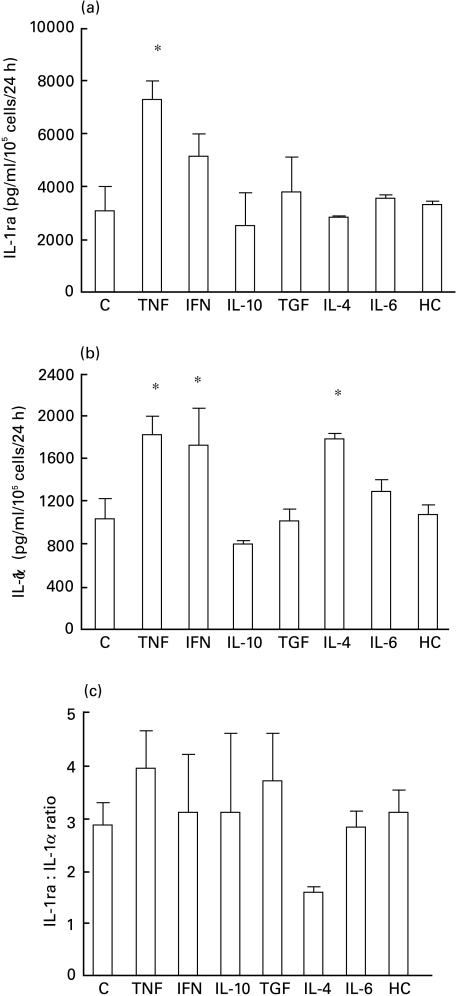
Cell lysates from unstimulated cultures contained large amounts of IL-1ra (3031 + 979 pg/ml/105 cells/24 h). IL-1-α and TNF-α increased intracellular IL-1ra production to 6783 + 2 pg/ml/105 cells/24 h and 7291 + 719 pg/ml/105 cells/24 h, respectively (P < 0·05, Fig. 3a) while IFN-γ, TGF-β1 and calcium induced only a small increase. Conversely, intracellular IL-1β was increased significantly by TNF-α, IFN-γ and calcium (Fig. 3b). TGF-β1 significantly raised the IL-1ra/IL-1β ratio, from 93·6 to 300 (Fig. 3c). IFN-γ and IL-4 selectively stimulated IL-1α production (Fig. 4b). TNF-α did not change the IL-1ra/IL-1α ratio as it increased equally IL-1ra and IL-1α(Fig. 4). For clarity, results are summarized in Table 2 and, for comparison, are represented along with those in epidermal keratinocytes described by Phillips et al. [21].
Fig. 3.
Modulation of intracellular IL-1ra (a), IL-1β (b) and IL-1ra : IL-1β ratio (c) by cytokines in oral mucosal cells. Cells were incubated for 24 h with RPMI 1640 alone as control (C), IL-1α, TNFα, IFNγ, IL-10, TGFβ1, IL-4, IL-6, hydrocortisone (HC) or calcium. The concentration of cytokines in culture lysates was determined by ELISA. The results represent the mean of two experiments, each performed from different cultures. P < 0·05.
Fig. 4.
Modulation of intracellular IL-1ra (a), IL-1α (b) and IL-1ra : IL-1α ratio (c) by cytokines in oral mucosal cells. Cells were incubated for 24 h with RPMI 1640 alone as control (C), IFN-γ, IL-10, TGFβ1, IL-4, TNFα, IL-6 or hydrocortisone (HC). The concentration of cytokines in culture lysates was determined by ELISA. The results represent the mean of two experiments, each performed from different cultures. P < 0·05.
Table 2.
Summary of intracellular IL-1 family regulation in OMECs, compared with that in skin keratinocytes
| IL-1ra production | IL-1 production | IL-1ra/IL-1 | ||||
|---|---|---|---|---|---|---|
| Cytokines (derived cells) | OMEC | SK* | OMEC | SK* | OMEC | SK* |
| IFN-γ (Th1) | 0 | 0 | ↑ | ↑ | 0 | ↓ |
| TNF-α (Th1/Th2) | ↑ | 0 | ↑ | ↑ | 0 | ↓ |
| IL-4 (Th2) | 0 | 0 | ↑ | 0 | 0 | 0 |
| IL-6 (Th2) | 0 | 0 | 0 | ↑ | 0 | ↓ |
| IL-10 (Th1/Th2) | 0 | 0 | 0 | 0 | 0 | 0 |
| TGF-β1 (Th3) | 0 | 0 | 0 | ↑ | ↑ | ↓ |
Results in SK (skin keratinocytes) from Phillips et al. [21].
In all OMEC lysates, the relative amounts of the three IL-1 receptor ligands were consistently in the order IL-1ra > IL-1α > IL-1β. However, we observed variations in IL-1ra production (2000–10000 pg/ml) from one culture to another, according to donors and to the number of passages.
Cytokines in supernatants
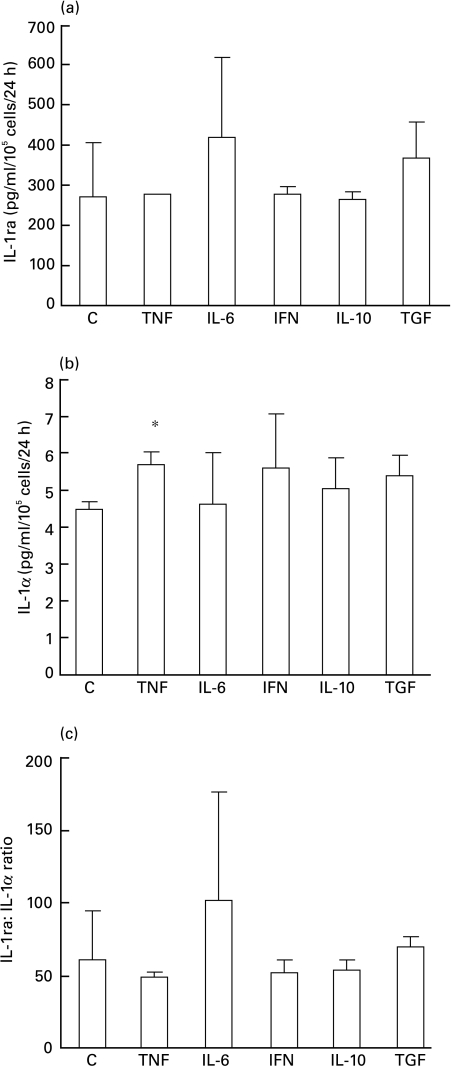
OMECs constitutively secreted both IL-1ra and IL-1α (268 pg/ml/105 cells/24 h and 4·45 + 0·2 pg/ml/105 cells/24 h, respectively, Fig. 5). TNF-α significantly increased IL-1 α secretion (5·65 + 0·35 pg/ml/105 cells/24 h) but not IL-1ra secretion (276 pg/ml/105 cells/24 h). Other cytokines had no significant effect on either IL-1α or IL-1ra secretion. In supernatants, IL-1β levels were about 10 times lower than those of IL-1α and often undetectable (data not shown).
Fig. 5.
Modulation of secreted IL-1ra (a), IL-1α (b) and IL-1ra : IL-1α ratio (c) by cytokines in oral mucosal cells. Cells were incubated with RPMI 1640 alone as control (C), TNFα, IL-6, IFN-γ, IL-10 or TGFβ1. The concentration of cytokines in culture supernatants was determined by ELISA. The results represent two experiments, each performed from different cultures. P < 0·05.
Discussion
The present study demonstrates that epithelial cells from human oral mucosa produce IL-1ra in culture. Western blot analysis showed a major band of 22 kD corresponding to the secreted form of IL-1ra [18] and a minor band of 20 kD corresponding to the intracellular form [19], a finding that concurs with our previous observations on salivary IL-1ra [20], and confirms its epithelial origin. This is the first report of sIL-1ra production by such cells. mRNAs of sIL-1ra were detected recently in resting oral keratinocytes [27]. sIL-1ra, whose role is not yet understood, could be of physiological importance since, unlike icIL-1ra, it is available in extracellular compartments in the absence of cell lesions. Incubation of unstimulated OMECs with an IL-1ra-blocking antibody increased IL-6 production, proving that OMECs secrete biologically relevant amounts of IL-1ra which compete with IL-1 in resting cultures. Our finding also establishes that OMECs constitutively express IL-1 receptors.
Larger amounts of IL-1ra were found in cell lysates, in spite of interindividual and interculture variations (see Results). IL-1ra concentrations were higher in primary cultures than in subcultures, probably because of a declining viability of subcultivated cells [28]. OMECs also constitutively produced intracellular and secreted IL-1, mainly IL-1α as in epidermal keratinocytes [29]. However, modulation of the IL-1 ligand family seems to be quite different in OMECs (Table 2). The more striking discrepancy is the IL-1ra/IL-1 modulation by TGF-β1.
IL-1 and IL-1ra expressions in OMECs were not merely constitutive but modulated by several cytokines (Table 2). TNF-α increased equally OMEC IL-1ra and OMEC IL-1, thereby producing a stable IL-1/IL-1ra ratio. In contrast, IL-4, which inhibits IL-1 production by monocytes and induces that of sIL-1ra [30], increased IL-1 α without affecting IL-1ra expression. IL-4 stimulation of IL-1α production was in the same range as that of TNFα or IFN-γ. Our findings raise the possibility that IL-4, a Th2 cell-derived cytokine, plays a proinflammatory role amplified by OMECs in oral mucosa while decreasing immune and inflammatory responses in monocytes. In periodontitis, IL-4 is secreted by CD4+ T cells [31] and possibly by other resident cells of the mucosal environment. OMECs may thus enhance Th2, IL-4 dependent inflammatory responses, rather than Th1 responses.
TGF-β1, a cytokine produced by mucosally derived CD4+ cells termed Th3 [24], may be more relevant to protection than to inflammation. TGF-β1 is a multi-functional cytokine that regulates several events in many cell types [32]. It is a strong inhibitor of cell growth and a differentiation factor in various epithelial cells [33–36], including OMECs [37]. TGF-β1 could be involved in inflammatory conditions of epithelium by increasing IL-1α production over that of IL-1ra, as described in skin keratinocytes [21]. In OMECs, however, TGF-β1 increased the IL-1ra/IL-1β ratio more than 100-fold (Fig. 3c), reaching the excess of IL-1ra needed to inhibit IL-1 bioactivity. In the present work, TGF-β1 was the only cytokine that induced an imbalance in the IL-1ra/IL-1 ratio in favour of IL-1ra. Immunohistochemistry showed that IL-1ra was expressed concurrently with involucrin, a differentiation marker restricted to the granular cell layers. The increased IL-1ra immunostaining in the most differentiated layers of oral mucosa noted by Dubost et al. [20] probably reflected the IL-1/IL-1ra imbalance. Thus, TGF-β1 may promote both IL-1ra inhibitory function and mucosa cell differentiation.
A pathological decrease in TGF-β1 along with that of IL-1ra may contribute to mucosal damages such as oral erosions associated with infections. It is noteworthy that in severe SS, IL-1ra and TGF-β1 salivary concentrations are both reduced [20,38].
TGF-β1, which induces cell differentiation and apoptosis in normal OMECs [37], could exert its effects via the modulation of the IL-1 cytokine network. The role of IL-1ra in cell apoptosis has been described in leukaemia cells only [39]. It may also be effective in OMECs. An interplay between TGF-β1-producing T cells and IL-1ra producing OMECs could also be envisioned. Th3 clones producing TGF-β1 can transfer oral tolerance and suppress autoimmune encephalomyelitis [24]. One may speculate that TGF-β1-producing Th3 cells arising in the gut colonize oral mucosa and down-regulate local immune responses through IL-1ra. Oral IL-1ra could play a part in subliminal antigen stimulation, from which tolerance arises in other models.
In conclusion, our results demonstrate the production of biologically active, secreted and intracellular forms of IL-1ra in human oral mucosal cells. Intraepithelial IL-1ra increased with mucosal cell differentiation. Its expression and that of IL-1 were modulated by TGF-β1 which promoted the former, and by IL-4 which promoted the latter. In diseases such as SS, a decreased production of IL-1ra with normal or increased levels of the proinflammatory cytokine IL-1 may induce and/or maintain chronic mucosal inflammation. IL-1ra may also contribute to the integrity and protective capacity of the superficial layers of the oral mucosa by enhancing cell differentiation. In normal conditions, IL-4 may shift the balance in epithelial cell production of IL-1 and IL-1ra in favour of IL-1, thereby allowing for a positive interaction of OMECs in local, classical Th2 response. The converse may be true for the Th2 subtype, Th3 response and for Th1 response.
Acknowledgments
The authors thank Dr Jean-Michel Dagrosa and Dr François Malpuech who kindly provided surgery samples, Annie Dosgilbert for her skilful technical assistance and Jeffrey Watts for improving the English rendition of the manuscript. This work was supported in part by a grant from ‘Direction Générale des Hopitaux de Clermont-Ferrand’ and two grants for clinical research.
REFERENCES
- 1.Lebendiger M, Lehner T. Characterization of mononuclear cells in the human oral mucosa. Arch Oral Biol. 1981;26:1041–9. doi: 10.1016/0003-9969(81)90115-1. [DOI] [PubMed] [Google Scholar]
- 2.Reibel J, Dabelsteen E, Kenrad B, Buschard K. Pattern of distribution of T lymphocytes, Langerhans cells and HLA-DR bearing cells in normal human oral mucosa. Scand J Dent Res. 1985;93:513–21. doi: 10.1111/j.1600-0722.1985.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Loon LA, Krieg SR, Davidson CL, Bos JD. Quantification and distribution of lymphocyte subsets and Langerhans cells in normal human oral mucosa and skin. J Oral Pathol Med. 1989;18:197–201. doi: 10.1111/j.1600-0714.1989.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Wilsem EJ, Van Hoogstraten IM, Breve J, Scheper RJ, Kraal G. Dendritic cells of the oral mucosa and the induction of oral tolerance. A local affair. Immunology. 1994;83:128–32. [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin CJ. The fine structure of epithelial cells in normal and pathological buccal mucosa. IV. Interactions between epithelial cells, lymphocytes, and macrophages. Aust Dent J. 1980;25:284–94. doi: 10.1111/j.1834-7819.1980.tb05202.x. [DOI] [PubMed] [Google Scholar]
- 6.Squier CA. The permeability of oral mucosa. Crit Rev Oral Biol Med. 1991;2:13–32. doi: 10.1177/10454411910020010301. [DOI] [PubMed] [Google Scholar]
- 7.Younes F, Quartey EL, Kiguwa S, Partridge M. Expression of TNF and the 55-kDa TNF receptor in epidermis, oral mucosa, lichen planus and squamous cell carcinoma. Oral Dis. 1996;2:25–31. doi: 10.1111/j.1601-0825.1996.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 8.Yumoto H, Nakae H, Fujinaka K, Ebisu S, Matsuo T. Interleukin-6 and interleukin-8 are induced in human oral epithelial cells in response to exposure to periodontopathic Eikenella corrodens. Infect Immun. 1999;67:384–94. doi: 10.1128/iai.67.1.384-394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Formanek M, Knerer B, Kornfehl J. Cytokine expression of human oral keratinocytes. ORL J Otorhinolaryngol Relat Spec. 1999;61:103–7. doi: 10.1159/000027650. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Farthing PM, Ireland GW, Thornhill MH. IL-1 alpha and IL-6 production by oral and skin keratinocytes: similarities and differencies in response to cytokine treatment in vitro. J Oral Pathol Med. 1996;25:157–62. doi: 10.1111/j.1600-0714.1996.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 11.Bickel M, Nothen SM, Freiburghaus K, Shire D. Chemokine expression in human oral keratinocyte cell lines and keratinized mucosa. J Dent Res. 1996;75:1827–34. doi: 10.1177/00220345960750110301. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Ireland GW, Farthing PM, Thornhill MH. Epidermal and oral keratinocytes are induced to produce RANTES and IL-8 by cytokine stimulation. J Inv Dermatol. 1996;106:661–6. doi: 10.1111/1523-1747.ep12345482. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Osaki T, Yoneda K, Ueta E. Cytokine production by keratinocytes and mononuclear infiltrates in oral lichen planus. J Oral Pathol Med. 1994;23:309–15. doi: 10.1111/j.1600-0714.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 14.Hillmann G, Hillmann B, Geurtsen W. Immunohistological determination of interleukin-1 beta in inflamed human gingival epithelium. Arch Oral Biol. 1995;40:353–9. doi: 10.1016/0003-9969(94)00136-y. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Osaki T. Characteristic cytokines generated by keratinocytes and mononuclear infiltrates in oral lichen planus. J Invest Dermatol. 1995;104:784–8. doi: 10.1111/1523-1747.ep12606990. [DOI] [PubMed] [Google Scholar]
- 16.Fox RI, Kang H, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- 17.Hannum CH, Wilcox CJ, Arend WP, et al. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–40. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 18.Arend WP, Joslin FG, Thompson RC, Hannum CH. An IL-1 inhibitor from human monocytes; production and characterization of biological properties. J Immunol. 1989;143:1851–8. [PubMed] [Google Scholar]
- 19.Haskill S, Martin G, Van Le L, et al. cDNA cloning of an intracellular form of the human interleukin-1 receptor antagonist associated with epithelium. Proc Natl Acad Sci. 1991;88:3681–5. doi: 10.1073/pnas.88.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubost JJ, Perrier S, Afane M, et al. IL-1 receptor antagonist in saliva; characterization in normal saliva and reduced concentration in Sjogren's syndrome. Clin Exp Immunol. 1996;106:237–42. doi: 10.1046/j.1365-2249.1996.d01-824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips WG, Feldmann M, Breathnach SM, Brennan FM. Modulation of the IL-1 cytokine network in keratinocytes by intracellular IL-1 alpha and IL-1 receptor antagonist. Clin Exp Immunol. 1995;101:177–82. doi: 10.1111/j.1365-2249.1995.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigler CF, Norris DA, Weston WL, Arend WP. Interleukin-1 receptor antagonist production by human keratinocytes. J Invest Dermatol. 1992;98:38–44. doi: 10.1111/1523-1747.ep12494196. [DOI] [PubMed] [Google Scholar]
- 23.Gruaz-Chatellard D, Baumberger C, Saurat JH, Dayer JM. Interleukin-1 receptor antagonist in human epidermis and cultured keratinocytes. FEBS Lett. 1991;294:137–40. doi: 10.1016/0014-5793(91)81360-k. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Kuchroo VK, Inobe J-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Fujihashi K, Kawabata K, McGhee JR, Kiyono H. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J Immunol. 1998;160:2188–96. [PubMed] [Google Scholar]
- 26.Southgate J, Williams HK, Trejdosiewicz LK, Hodges GM. Primary culture of human oral epithelial cells. Growth requirements and expression of differentiated characteristics. Lab Invest. 1987;56:211–23. [PubMed] [Google Scholar]
- 27.Formanek M, Knerer B, Temmel A, Thurnher D, Millesi W, Kornfehl J. Oral keratinocytes derived from the peritonsillar mucosa express the proinflammatory cytokine IL-6 without prior stimulation. J Oral Pathol Med. 1998;27:202–6. doi: 10.1111/j.1600-0714.1998.tb01942.x. [DOI] [PubMed] [Google Scholar]
- 28.Arenholt-Bindslev D, Jepsen A, MacCallum DK, Lillie JH. The growth and structure of human oral keratinocytes in culture. J Invest Dermatol. 1987;88:314–9. doi: 10.1111/1523-1747.ep12466191. [DOI] [PubMed] [Google Scholar]
- 29.Kupper TS, Ballard DW, Chua AO, et al. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1α and β mRNA. J Exp Med. 1986;164:2095–100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin-4: interleukin 4 suppresses interleukin-1 production but up-regulates gene expression and synthesis of interleukin-1 receptor antagonist. Proc Natl Acad Sci. 1992;89:4076–80. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabashima H, Nagata K, Hashiguchi I, et al. Interleukin-1 receptor antagonist and interleukin-4 in gingival crevicular fluid of patients with inflammatory periodontal disease. J Oral Pathol Med. 1996;25:449–55. doi: 10.1111/j.1600-0714.1996.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 32.Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-beta: biological function and chemical structure. Science. 1986;233:532–4. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- 33.Masui T, Wakefield LM, Lechner JF, LaVeck MA, Sporn MB, Harris CC. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc Natl Acad Sci. 1986;83:2438–42. doi: 10.1073/pnas.83.8.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto K, Hashimoto K, Hashiro M, Yoshimasa H, Yoshikawa K. Modulation of growth and differentiation in normal human keratinocytes by transforming growth factor-beta. J Cell Physiol. 1990;145:95–101. doi: 10.1002/jcp.1041450114. [DOI] [PubMed] [Google Scholar]
- 35.Jetten AM, Shirley JE, Stoner G. Regulation of proliferation and differentiation of respiratory tract epithelial cells by TGF beta. Exp Cell Res. 1986;167:539–49. doi: 10.1016/0014-4827(86)90193-x. [DOI] [PubMed] [Google Scholar]
- 36.Kurokowa M, Lynch K, Podolsky DK. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987;142:775–82. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- 37.Min BM, Woo KM, Lee G, Park NH. Terminal differentiation of normal human oral keratinocytes is associated with enhanced cellular TGF-beta and phospholipase C-gamma 1 levels and apoptotic cell death. Exp Cell Res. 1999;249:377–85. doi: 10.1006/excr.1999.4489. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa N, Dang H, Lazaridis K, McGuff HS, Aufdemorte TB, Talal N. Analysis of transforming growth factor beta and other cytokines in autoimmune exocrinopathy (Sjogren's syndrome) J Interferon Cytokine Res. 1995;15:759–67. doi: 10.1089/jir.1995.15.759. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa Y, Kikuchi J, Terui Y, et al. Preferential production of interleukin-1 beta over interleukin-1 receptor antagonist contributes to proliferation and suppression of apoptosis in leukemic cells. Jpn J Cancer Res. 1995;86:208–16. doi: 10.1111/j.1349-7006.1995.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]