Abstract
Psoriasis is a chronic, inflammatory, hyperproliferative skin disease, in which autoimmunity plays a great role. Natural killer T cells (NK T cells), are suggested to be involved in the pathogenesis of different autoimmune diseases. To examine the involvement of CD3+CD56+ NK T cells in the pathogenesis of psoriasis, we investigated the lymphocyte subpopulations obtained from blood samples of psoriatic patients before and after treatment, and of healthy controls, using two-colour flow cytometry. We found no significant differences between total T cells, total B cells, T helper cells, T cytotoxic cells and NK cells in patients with psoriasis before and after treatment and in controls. Increased percentage of memory T cells and decreased percentage of naive T cells was detected in psoriatic patients compared to controls, but these changes were not statistically significant. The CD3+CD56+ cells of psoriatic patients were significantly decreased relative to controls. The percentage of CD3+CD56+ cells increased after different antipsoriatic therapies, but remained significantly lower than those found in controls. CD3+CD56+ cells of healthy controls were capable of rapid activation, while in psoriatic patients activated NK T cells were almost absent. The decrease in the number of CD3+CD56+ cells may represent an intrinsic characteristic feature of patients with psoriasis, which is supported by the fact that after treatment NK T cells do not reach the values found in controls. In conclusion our results suggest that CD3+CD56+ NK T cells could be actively involved in the development of Th1 mediated autoimmune diseases.
Keywords: CD3+CD56+ NK T cells, autoimmunity, psoriasis
INTRODUCTION
Although the pathogenesis of psoriasis is not yet clear, there are characteristic features of the disease which suggest an immunological mediated process. Several direct and indirect evidences suggest that T cells play a crucial role in the pathogenesis of psoriasis [1–7]. The presence of T helper cells, that secrete type 1 cytokines (IFN-γ, IL-2, TNF-α), was demonstrated in psoriatic skin lesions [8–13]. A type 1 differentiation bias was also observed in circulating blood T cells of psoriatic patients [14]. The existence of an imbalance between Th1 and Th2 cells in psoriasis was supported further by findings which demonstrated that IL-10 was decreased in psoriatic lesions [15]. Moreover, during antipsoriatic therapy an increase in IL-10 mRNA expression was observed in peripheral blood mononuclear cells [16]. IL-10 therapy given either intralesionally or subcutaneously resulted in marked reduction of psoriatic lesions [16,17]. These data suggest that psoriasis is an inflammatory Th1 mediated autoimmune disorder, but the triggering autoantigens are still not identified.
Although the factors that induce the imbalance between Th1 and Th2 cells in psoriasis are unknown, a possible role could be attributed to natural killer T cells (NK T cells) [18]. NK T cells are a heterogeneous T cell population characterized by the co-expression of αβ or γδ TCRs and various NK receptors, including CD16, CD56, CD161, CD94, CD158a and CD158b [19–21]. NK T cells have the ability to rapidly secrete large amounts of cytokines following activation [22–24]. NK T cell clones secrete type 1, type 2 or both types of cytokines, which could influence the differentiation of Th0 cells towards Th1 or Th2 cells [25,26]. CD3+CD56+ cells represent one of the NK T cell populations.
The number of CD3+CD56+ NK T cells has been shown to be significantly decreased in the peripheral blood of patients with rheumatoid arthritis, another Th1 mediated autoimmune disease [27].
In the present study we determined the number of CD3+CD56+ NK T cells in the peripheral blood of patients with psoriasis (before and after treatment) and in healthy controls. Other lymphocyte subpopulations (total T cells, T helper cells, T cytotoxic cells, T memory cells, T naive cells, B lymphocytes and NK cells) were also analysed and compared in psoriatic patients and healthy volunteers.
Our results show that CD3+CD56+ NK T cells are significantly decreased in the peripheral blood of patients with psoriasis relative to the healthy controls. This finding is discussed in relation with the development of a type 1 immune response in psoriasis.
PATIENTS AND METHODS
Patients’ profile
Peripheral blood samples of 15 patients with psoriasis (one erythrodermic, two guttate type and 12 chronic plaque psoriasis) were obtained with informed consent for use in this study. The Ethical Committee of the University of Szeged, Hungary approved this investigation. Blood samples were collected before and after treatment from all patients. The patients’ characteri-stics are presented in Table 1. Therapeutic modalities included monotherapy and combined therapeutic regimens (Table 2). The second blood sample was taken at the time when the applied treatment regimen had been completed. At this time the majority of patients was symptom-free or had minimal skin changes (PASI < 4).
Table 1.
Patient profile
| Sex (male:female) | 12:3* | |
| Age (years) | 42·35 ± 14·32 | (20–67)‡ |
| Age of onset (years) | 25·7 ± 10·38† | (9–45) |
| Disease duration (years) | 16·7 ± 9·21 | (3–37) |
| Psoriasis type (chronic: eruptive) | 13:2 | |
| PASI score | 18·77 ± 12·25 | (6–54) |
| Family history (positive: negative) | 6:9 |
Number of patients;
mean ± s.d.;
range.
Table 2.
Treatment regimens used in the study
| Treatment | Number of patients |
|---|---|
| Dithranol † | 6 |
| PUVA * | 6 |
| Dithranol + PUVA ‡ | 1 |
| Dithranol + narrowband ultraviolet B (311 nm)§ | 1 |
| Re-PUVA ¶ | 1 |
Dithranol treatment was used in slowly increasing concentrations starting with 0·5% up to 8% depending on the induced erythema.
Oral 8-methoxypsoralen + UVA (four times a week) was administered from 1 J/cm2 up 5 J/cm2 depending on the induced erythema.
Dithranol treatment with slowly increasing concentrations starting with 0·5% up to 8% was combined with oral 8-methoxypsoralen + UVA (four times a week) from 1 J/cm2 up 5 J/cm2 depending on the induced erythema.
Dithranol treatment with slowly increasing concentrations starting with 0·5% up to 8% was combined with narrowband ultraviolet B from 0·2 J/cm2 up to 2·2 J/cm2 (five times a week) depending on the induced erythema.
Oral acitretin (0·5 mg/kg body wt) + oral 8-methoxypsoralen + UVA (four times a week) from 1 J/cm2 up 5 J/cm2 was administered depending on the induced erythema.
The control group consisted of 12 healthy hospital employees (seven females and five males), aged 37·08 ± 7·21 years with informed consent for use in the study. In the control group nobody took any medication and nobody suffered from any known acute or chronic disease.
Reagents
Anti-CD3 FITC, anti-CD3 PE (clone UCHT1), anti-CD19 PE (clone HD37), anti-CD4 FITC (clone MT310), anti-CD8 PE, anti-CD8 FITC (clone DK 25), anti-CD25 FITC (clone ACT-1) and isotype-matched labelled mouse immunoglobulins were obtained from DAKO (Copenhagen, Denmark), anti-CD56 FITC (clone NKH-1), anti-CD45RA PE (clone F8-11–13) were obtained from Serotec (Oxford, UK), anti-CD4 FITC (clone SK3), anti-CD56 PE (MY31) from Becton-Dickinson (San Jose, CA, USA) and anti-CD45RO FITC (clone UCHL1) was obtained from Immunotech (Beckman Coulter, Fullerton, CA, USA).
Immunostaining and flow cytometry
Peripheral blood, anticoagulated with EDTA, was collected. Each blood sample (50 μl) was stained with two monoclonal antibodies, one conjugated with FITC and the other with PE (10 μl from each) at room temperature, in the dark for 20 min Erythrocytes were lysed with FACS Lysing Solution (Becton Dickinson San Jose, CA, USA). After two washes with PBS the cells were resuspended in PBS for immediate analyses or were fixed with 2% paraformaldehyde for overnight storage before analyses. Two-colour flow cytometry was performed by using a FACSCalibur cytometer and the data were analysed using Cell Quest software (Becton Dickinson, San Jose, CA, USA). In each stain 30 000 events were acquired.
Isolation and stimulation of T cells from peripheral blood
Mononuclear cells (PBMC) were isolated from peripheral venous blood samples of psoriatic patients and healthy controls by Ficoll-Hypaque density gradient centrifugation (Biotech Inc, Piscataway, NJ, USA). PBMC were recovered at the interface and washed in PBS supplemented with 2% fetal calf serum (FCS). T cells were isolated by positive selection using uniform magnetizable polystyrene beads coated with monoclonal antibodies specific for CD3 (Dynabeads M-450 CD3 from Dynal, Oslo, Norway) and a magnetic particle separator (Dynal MPC, from Dynal, Oslo, Norway) following the protocol provided by the manufacturer. Briefly, PBMC were incubated with CD3 monoclonal antibody coated magnetic beads (bead: cell ratio 5: 1, Dynabeads concentration 1 × 107/ml) for 6 h in RPMI 1640 culture medium supplemented with 10% FCS, at 37°C in a humid 5% CO2 incubator. After the incubation time Dynabeads were detached from the cells by pipetting the cell suspension 10 times through an automated pipette. The beads were than removed from the cell suspension using a Dynal MPC. The beads attached to the tube wall while the cells remained in the suspension. This isolation procedure through the CD3 binding results in stimulation of the CD3+ cells [28]. The isolated and stimulated cells were then analysed by two-colour flow cytometry, using monoclonal antibodies for detection of CD56, CD25, CD4 and CD8. After 6h of anti-CD3 antibody stimulation, the CD3 antigen was transiently down-regulated. By growing the cells for further 24 h in culture medium the CD3 antigen was reexpressed on the cell surface, and could be detected using FITC conjugated CD3 antibodies. Vitality of isolated cells was tested by trypan blue exclusion.
Statistical analysis
Statistical analysis of the data was made by anova, Pearson correlation test and Spearman’s rank order correlation test. For significant ANOVA values, groups were compared by Tukey’s post hoc test for multiple comparisons with unequal cell size. A probability level of 0·05 was accepted as indicating significant differences.
RESULTS
Analyses of lymphocyte subsets
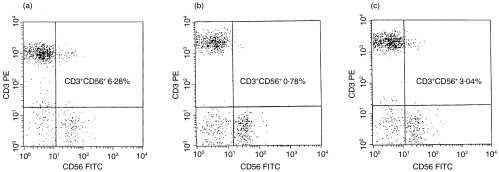
The lymphocyte subsets in patients with psoriasis before treatment and in healthy controls were analysed. The percentage of CD3+CD56+ NK T cells was significantly decreased in the peripheral blood of patients with psoriasis before treatment compared with healthy controls (1·79 ± 1·07% in patients versus 5·22 ± 1·74% in controls, P < 0·0001) (Table 3). Representative flow cytometric analyses show CD3+CD56+ NK T cells in a patient before treatment (0,78%) (Fig. 1b) versus in a healthy control (6·28%) (Fig. 1a). The absolute number of circulating CD3+CD56+ NK T cells was also significantly lower in psoriatic patients before treatment than the values found in healthy controls (29·43 ± 17·12 μl in psoriasis before treatment versus 120·15 ± 45·35μl in controls, P < 0·0001). Memory T cells (CD3+CD45RO+) represented a larger (35·36 ± 9·38% in patients versus 27·21 ± 7·34% in controls) and naive T cells (CD3+CD45RA+) a smaller population (37·71 ± 8·34% in patients versus 45·00 ± 7·19%) in the peripheral blood of patients relative to controls; however, these differences did not reach statistical significance. Similarly, a slight but statistically not significant increase in the proportion of helper CD4+ T helper cells was observed in patients with psoriasis (44·52 ± 9·05% in patients versus 38·97 ± 5·66% in controls P > 0·05). There was no difference in the percentages of B lymphocytes, conventional NK cells (CD3−CD56+), total T cells and T cytotoxic CD8+ cells between the two groups (Table 3).
Table 3.
Percentage of lymphocyte subsets (mean ± s.d.) in peripheral blood of patients with psoriasis before treatment, after treatment and of healthy controls
| Psoriasis before treatment (%) | Psoriasis after treatment (%) | Healthy controls (%) | |
|---|---|---|---|
| CD3+ | 72·46 ± 8·59 | 70·04 ± 8·59 | 70·78 ± 4·71 |
| CD19+ | 11·24 ± 4·87 | 11·5 ± 5·41 | 13·97 ± 4·63 |
| CD3+CD4+ | 44·52 ± 9·05 | 42·54 ± 8·22 | 38·97 ± 5·66 |
| CD3+CD8+ | 27·12 ± 8·21 | 26·39 ± 7·18 | 28·95 ± 7·43 |
| CD3+CD56+ | 1·79 ± 1·07 | 2·68 ± 1·04 | 5·22 ± 1·74 |
| CD3–CD56+ | 10·20 ± 5·69 | 12·47 ± 6·98 | 10·30 ± 4·7 |
| CD3+CD45RA+ | 37·71 ± 8·34 | 37·90 ± 8·60 | 45·00 ± 7·19 |
| CD3+CD45RO+ | 35·36 ± 9·38 | 35·13 ± 9·87 | 27·21 ± 7·34 |
Fig. 1.
Representative dot-plot diagrams of peripheral blood cells stained with PE conjugated anti-CD3 monoclonal antibodies and PE conjugated anti-CD56 monoclonal antibodies. CD3+CD56+ NK T cells in a healthy control (a), in a patient with psoriasis before treatment (b) and in the same patient after treatment (c) are in the upper-right quadrants of each dot-plot diagrams. CD3+CD56+ NK T cells are significantly decreased in the peripheral blood of patients with psoriasis (b, 0·78%), the percentage of these cells increases after therapy (c, 3·04%), but remains significantly lower than in healthy controls (a, 6·28%).
Comparing the lymphocyte subsets of patients with psoriasis before and after treatment, the only lymphocyte population in which changes were statistically significant was the NK T (CD3+CD56+) subset. Both the percentage and the absolute cell number of NK T cells were significantly increased in the peripheral blood of patients with psoriasis after treatment (2·68 ± 1·04%, 29·43 ± 17·12μl versus 1·79 ± 1·07%, 58·95 ± 31·56μl, P < 0·001), but did not reach the values found in healthy controls (Table 3). A representative flow cytometric analysis shows the comparison of CD3+CD56+ NK T cells in a patient before treatment (0·78%) (Fig. 1b) versus in a patient after treatment (3·04%) (Fig. 1c). We found no statistically significant changes in the other lymphocyte subsets (Table 3). The antipsoriatic treatments used in this study had no effect on the number of memory and naive T cells (Table 3).
After treatment the absolute cell number of CD3+CD56+ NK T cells remained significantly decreased in patients with psoriasis compared to healthy controls (58·95 ± 31·56μl in patients after treatment versus 120·15 ± 45·35μl in controls, P < 0·001). The same was observed when the percentage of NK T cells among lymphocytes was analysed in peripheral blood samples (2·68 ± 1·04% in patients versus 5·22 ± 1·74% in controls, P < 0·001) (Table 3). Representative flow cytometric analyses show CD3+CD56+ NK T cells in a patient after treatment (3·04%) (Fig. 1c) versus in a healthy control (6·28%) (Fig. 1a). Memory T cells remained elevated (35·13 ± 9·87% in patients versus 27·21 ± 7·34% in controls) and naive T cells were decreased in the psoriasis group compared to healthy controls (37·90 ± 8·60% in patients versus 45·00 ± 7·19% in controls) (Table 3), without reaching statistical significance. Similarly, the number of T helper cells showed a slight, statistically not significant elevation in treated patients compared to controls (42·54 ± 8·22% in patients versus 38·97 ± 5·66% in controls P > 0·05) (Table 3). No difference between the two groups was found regarding B lymphocytes, NK cells, total T cells and cytotoxic T cells (Table 3).
CD3+CD56+ NK T cells and patients’ profile
To determine whether the number or the percentage of CD3+CD56+ NK T cells in patients with psoriasis shows any correlation with age, PASI score and disease duration the appropiate correlation test has been applied. The analysis of the possible correlation of NKT cells with age was also performed in healthy controls. Statistical analyses of our data showed a slight, but significant direct correlation between NK T cells and the age of controls (r1 =+0·39). In contrast in psoriatic patients a slight, but statistically significant inverse correlation (r2 =– 0·31) was detected (data not shown). We observed that patients with a long-term history of frequent relapses, who did not respond well to treatment, had the lowest NKT cell counts. In these patients the recovery of NK T cells following therapy was slower and generally poor. However, CD3+CD56+ cells showed no correlation with PASI score and disease duration (data not shown).
Stimulation of peripheral blood T cells with anti-CD3 monoclonal antibodies
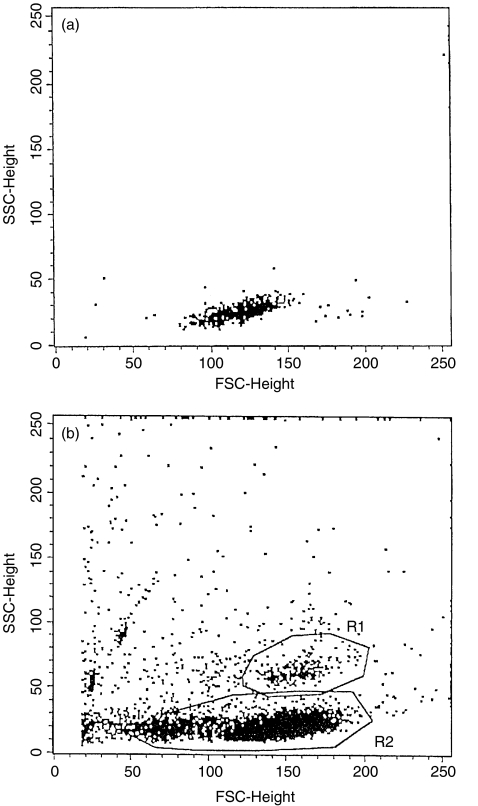
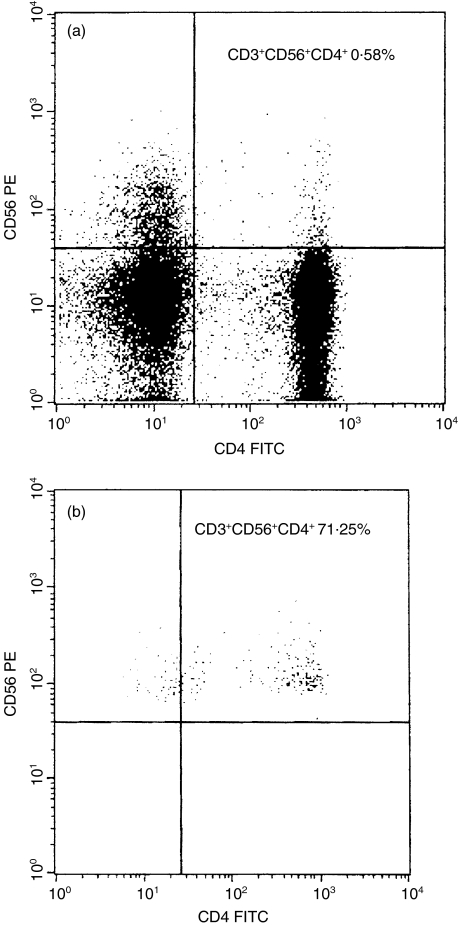
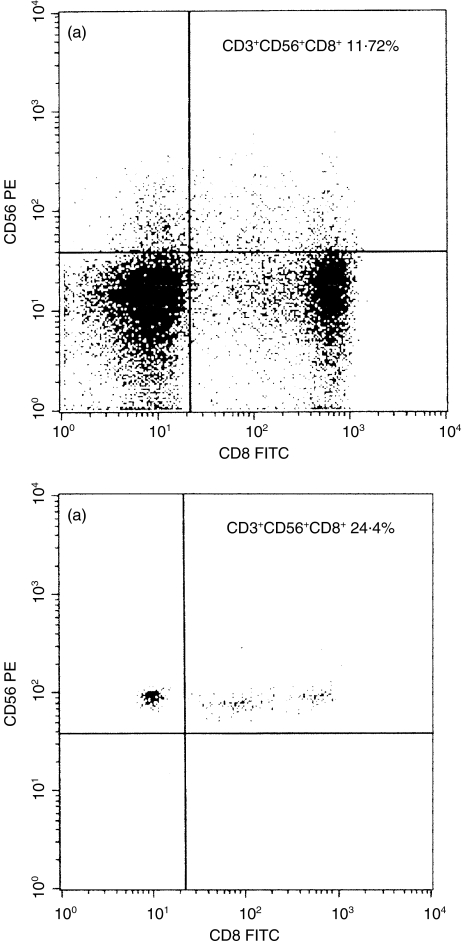
To examine the activation status of CD3+CD56+ NK T cells, we separated T cells from the peripheral blood of healthy controls and of patients with psoriasis. These T cells were stimulated for 6h using anti-CD3 monoclonal antibodies, and then flow cytometric analysis was performed. On the FSC/SSC dot-plot of separated and stimulated T cells obtained from healthy controls a distinct cell population was recognized, which was not present as a distinct population on the dot-plot of unstimulated CD3+ T cells (Fig. 2). These cells showed a marked granulated pattern. Analysis of stimulated T cells was performed by using two gates: R1 for these granulated cells and R2 for the other cells. All the granulated cells (gate R1) expressed the surface molecule CD56, but only a minority of the other cells (gate R2) expressed this molecule (Fig. 3). Thus the granulated cells were NK T (CD3+CD56+) cells. More than half of NK T cells (gate R1) expressed the CD4 marker (71·25%), while very few of the less granulated CD3+CD56+ NK T cells (gate R2) were CD4+ (0·58%) (Fig. 3). The CD8 molecule was expressed by more than half of the less granulated CD3+CD56+ NK T cells (gate R2) and by about one-third of the granulated NK T cells (gate R1) (Fig. 4). The low affinity receptor for IL-2, an early activation marker for T cells, was detected with an anti-CD25 FITC labelled monoclonal antibody. Almost all the granulated NK T cells (gate R1) expressed CD25 molecules (93·2%), indicating that they were activated cells. Among the less granulated cell population (gate R2) only 59·65% of the cells expressed the low affinity IL-2 receptor (data not shown). After analysing the separated and stimulated T cells collected from patients with psoriasis we found that CD56+ T cells with marked granulated pattern were almost absent. Scattered CD56+ T cells were present between the cells with normal granulation pattern, characteristic for lymphocytes (data not shown). These findings are in concordance with the low levels of CD3+CD56+ NK T cells that were detected in the peripheral blood samples of patients with psoriasis by flow cytometric analyses of unseparated cells.
Fig. 2.
Light scatter analysis (FSC versus SSC) of unstimulated peripherial blood CD3+ T cells (a) and of separated and stimulated CD3+ T cells (b). Peripheral venous blood sample was obtained from a healthy control. Cell separation and stimulation was performed using magnetic beads coated with anti-CD3 monoclonal antibodies. After stimulation of the CD3+ T cells a distinct population of granulated cells appeared (R1).
Fig. 3.
Phenotypic analyses of separated and stimulated CD3+ T cells. T cells were stained with PE conjugated anti-CD56 and FITC conjugated anti-CD4. The expression of CD56 and CD4 surface molecules on granulated CD3+ T cells, gate R1 in Fig. 2b and on the less granulated CD3+ T cells, gate R2 in Fig. 2a are shown. All the granulated CD3+ T cells express the surface molecule CD56 (b, upper left and right quadrants), but of the less granulated CD3+ T cells only a minor population express this molecule (a, upper left and right quadrants). 71·25% of granulated CD3+CD56+ cells express the CD4 marker (b, upper right quadrant), at the same time very few (0·58%) of the less granulated T cells express both CD56 and CD4 on the surface (a, upper right quadrant).
Fig. 4.
Phenotypic analyses of separated and stimulated CD3+ T cells. T cells were stained with PE conjugated anti-CD56 and FITC conjugated anti-CD8. The expression of CD56 and CD4 surface molecules on granulated CD3+ T cells, gate R1 in Fig. 2b and on the less granulated CD3+ T cells, gate R2 in Fig. 2a are shown. 24·4% of granulated CD3+CD56+ cells express the CD8 marker (b, upper right quadrant), at the same time 11·72% of the less granulated T cells express both CD56 and CD8 on the surface (a, upper right quadrant).
In each experiment controls staining of separated and stimulated T cells was performed after 24h of culture in medium alone, using FITC conjugated anti-CD3 antibodies. The percentage of separated cells that expressed the surface molecule CD3 was 95–98% (data not shown). The vitality of separated and stimulated cells was tested using trypan blue, and was always above 95%.
DISCUSSION
NK T cells are phenotypically and functionally diverse [28]. Initially, NK T cells were described as cells that express an invariant TCR Valpha14 in mouse and Valpha24 in humans [29]. Recently NK T cells expressing diverse TCRs have been also recognized [22,23,30]. The CD3+ CD56+ cells represent one of these NK T cell subpopulations.
Our results show that the number of CD3+CD56+ NK T cells is significantly decreased in the peripheral blood of patients with psoriasis and that the percentage of these cells increases after different therapies used in psoriasis, but remains significantly lower than those found in healthy controls. The full relevance of this finding is still speculative. The decrease in the number of CD3+CD56+ cells may represent an intrinsic characteristic feature of patients with psoriasis. This hypothesis is supported by the fact that NK T cells do not reach the values found in healthy controls, so it is possible that the percentage of this cell population is permanently decreased in the peripheral blood of patients with psoriasis. Another possible cause that leads to decreased number of NK T cells may be represented by the early activation of these cells by antigens involved in the relapse of the disease, followed by apoptosis [31]. Since we did not study NK T cells in the skin we could not exclude that the decrease in NK T cells in the peripheral blood might come from the differential homing of NK T cells to the skin lesions. Further studies are needed to elucidate the direct role of NK T cells in the skin of patients with psoriasis.
In our study we found that CD3+CD56+ NK T cells were capable of rapid activation. After stimulation of CD3+ T cells one population of activated NK T cells appeared. NK T cells are able to recognize non-peptide antigens [32]. In vivo administration of synthetic ceramide induces the secretion of both type 1 and type 2 cytokines [24], but repeated doses polarize NK T cells towards Th2 cytokine synthesis [33,34]. Thus IL-4 produced by the NK T cells plays a major role in promoting the differentiation of Th0 cells into Th2 cells [34,35]. Although the precise role of NK T cells is not yet elucidated, they can be a regulatory cell type playing a pivotal role in the development of peripheral tolerance and in the modulation of immune responses by inducing a shift in early activation and consequently in the cytokine secretion of classical T cells [36,37]. Psoriasis is an autoimmune disease in which type 1 cytokine secretion pattern can be demonstrated in T cells derived from lesional skin and from peripheral blood [8,14]. It is possible that this feature is a consequence of an inefficient type 2 response, because of lack of NK T cells. This issue might be clarified by comparing the IL-4 producing capacity of CD3+CD56+ NK T cells from psoriatic patients with that from healty controls. However, the study of the cytokine production in psoriasis on a per cell basis was beyond the scope of our present work. Our results are the first clear-cut evidence showing that low NK T cell counts are a characteristic of psoriasis patients.These findings raised the question of whether NK T cell deficiency might result in the missing contraregulatory signals needed for the development of a normal immune response upon antigen stimulation, and favours excessive activation of Th1 cells. Type 2 cytokines are also important in the development of tolerance [38]; thus deficit in these cytokines can favour the development of autoimmunity.
The dysfunction of NK T cells correlates with the pathogenesis of other T cell-mediated autoimmune diseases [39,40]. In lpr/lpr mice, in which a spontaneous autoimmune syndrome resembling human systemic lupus erythematosus occurs, NK T cells disappear from the periphery by the time the autoimmune disease develops. Selective experimental depletion of NK T cells from the peripheral blood results in early onset and exacerbation of the autoimmune phenomena [39]. The selective reduction of NK T cells has been also detected in non-obese diabetic mice [40,41]. Studies in humans have showed that decreased number of NK T cells are present in the peripheral blood of patients with rheumatoid arthritis, systemic sclerosis and insulin-dependent diabetes mellitus [27,42,43].
Other lymphocyte populations were also investigated. We found no significant differences between total T cells, total B cells, T helper cells, T cytotoxic cells and NK cells in patients with psoriasis and healthy controls. These results are in concordance with observations of other authors and highlight further the significance of CD3+CD56+ NK T cells in the pathogenesis of psoriasis [44–46].
We found increased percentage of memory T cells and decreased percentage of naive T cells. However, these changes were not statistically significant, they might be related to the chronic activation of the immune system in patients with psoriasis. Increased numbers of memory T cells have been found in lesional skin and in the synovial tissue of patients with psoriatic arthritis [38,47]. It is interesting that the treatments used in this study had no effect on memory and naive T cells. It is possible that the persistence of the increased number of memory T cells is one of the factors that contribute to the relapses observed in psoriasis, but these assumptions are still speculative and need further investigations.
In conclusion, our results suggest that CD3+CD56+ NK T cells have a role in the pathogenesis of psoriasis and that reduced NK T cells can be of importance in the development of psoriasis, a Th1 mediated autoimmune disease.
Acknowledgments
This work was supported by the following grants: OTKA T030749, MKM 1271, ETT 073 99, OTKA T032494, OTKA T032498, ETT 412 05, János Bolyai Foundation of the Hungarian Academy of Sciences.
REFERENCES
- 1.Bos JD, de Rie MA. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today. 1999;20:40–5. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 2.Schlaak JF, Buslau M, Jochum W, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–9. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- 3.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–87. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilhar A, David M, Ullmann Y, et al. T-lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to SCID mice. J Invest Dermatol. 1997;109:283–8. doi: 10.1111/1523-1747.ep12335758. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Matsuchi M, Katagama I, et al. Repeated subcutaneous injection of staphylococcal enterotoxin B-stimulated lymphocytes retain epidermal thickness of psoriatic skin-graft onto severe combined immunodeficient mice. J Dermatol Sci. 1998;17:8–14. doi: 10.1016/s0923-1811(97)00064-9. [DOI] [PubMed] [Google Scholar]
- 6.Boehncke WH, Dressel D, Zollner TM, et al. Pulling the trigger on psoriasis. Nature. 1996;379:777. doi: 10.1038/379777a0. [DOI] [PubMed] [Google Scholar]
- 7.Bos JD, Hulseboxch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. Arch Dermatol Res. 1983;275:181–9. doi: 10.1007/BF00510050. [DOI] [PubMed] [Google Scholar]
- 8.Nickoloff BJ. Cytokine network in psoriasis. Arch Dermatol. 1991;127:871–84. [PubMed] [Google Scholar]
- 9.Nickoloff BJ. The immunologic and genetic basis of psoriasis. Arch Dermatol. 1999;135:1104–10. doi: 10.1001/archderm.135.9.1104. [DOI] [PubMed] [Google Scholar]
- 10.Bata-Csorgo Z, Hammerberg C, Voorhees JJ, et al. Kinetics and regulation of human keratinocyte stem cell growth in short term primary ex-vivo culture: cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J Clin Invest. 1995;95:317–27. doi: 10.1172/JCI117659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bata-Csorgo Zs, Hammenberg C, Voorhees JJ, et al. Intralesional T-lymphocyte activation as a mediator of psoriatic epidermal hyperplasia. J Invest Dermatol. 1995;105:89S–93S. doi: 10.1111/1523-1747.ep12316121. [DOI] [PubMed] [Google Scholar]
- 12.Uyemura K, Yamamura M, Fiverson DF, et al. The cytokine network in lesional and lesional-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701–5. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- 13.Olaniran AK, Baker BS, Paige DG, et al. Cytokine expression in psoriatic skin during PUVA therapy. Arch Dermatol Res. 1996;288:421–5. doi: 10.1007/BF02505228. 10.1007/s004030050076. [DOI] [PubMed] [Google Scholar]
- 14.Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–9. doi: 10.1046/j.1523-1747.1999.00749.x. 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 15.Mussi A, Bonifati C, Carducci M, et al. IL-10 levels are decreased in psoriatic lesional skin as compared to the psoriatic lesion-free and normal skin suction blister fluids. J Biol Regul Homeost Agents. 1994;8:117–20. [PubMed] [Google Scholar]
- 16.Asadullah K, Sterry W, Stephanek K, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–94. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asadullah K, Sabat R, Wiese A, et al. Interleukine-10 in cutaneous disorders. implication for its pathophysiological importance and therapeutic use. Arch Dermatol Res. 1999;291:628–36. doi: 10.1007/s004030050467. [DOI] [PubMed] [Google Scholar]
- 18.Seder RA, Moemann TM. Differentation of effector phenotypes of CD4+ and CD8+ T cells. 4. Bethesda, Maryland: Lippincott-Raven: Fundamental immunology; 1998. pp. 879–909. [Google Scholar]
- 19.Ortaldo JR, Winkler-Pickett RT, Yagita H, et al. Comparative studies of CD3- and CD3+CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol. 1991;136:486–95. doi: 10.1016/0008-8749(91)90369-m. [DOI] [PubMed] [Google Scholar]
- 20.Ferrini SA, Cambiaggi A, Meazza R, et al. T cell clones expressing the natural killer cell-related p58 receptor molecule display heterogeneity in phenotypic properties and p58 function. Eur J Immunol. 1994;24:2294–8. doi: 10.1002/eji.1830241005. [DOI] [PubMed] [Google Scholar]
- 21.Mingari MC, Vitale C, Cambiaggi A, et al. Cytolitic T lymphocytes dispalying natural killer (NK)-like activity. Expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 22.Skold M, Faizunnessa NN, Wang CR, et al. CD1d-specific NK1.1+ T cells with transgenic variant TCR. J Immunol. 2000;165:168–74. doi: 10.4049/jimmunol.165.1.168. [DOI] [PubMed] [Google Scholar]
- 23.Chiu YH, Jayawardena J, Weiss A, et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189:103–10. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Paul WE. Cultured NK1.1+CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–9. [PubMed] [Google Scholar]
- 25.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- 26.Mendes R, Bromelow KV, Westby M, et al. Flow cytometric visualisation of cytokine production by CD3-CD56+ NK cells and CD3+CD56+ NK-T cells in whole blood. Cytometry. 2000;39:72–8. doi: 10.1002/(sici)1097-0320(20000101)39:1<72::aid-cyto10>3.0.co;2-r. 10.1002/(sici)1097-0320(200001)39:1<72::aid-cyto10>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Yanagihara Y, Shiozawa K, Takai M, et al. Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;118:131–6. doi: 10.1046/j.1365-2249.1999.01018.x. 10.1046/j.1365-2249.1999.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond KJ, Pelikan SB, Crowe NY, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–81. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. 10.1002/(sici)1521-4141(199911)29:11<3768::aid-immu3768>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-CD8- T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberl G, Lees R, Smiley ST, et al. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–19. [PubMed] [Google Scholar]
- 31.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–53. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 32.Kronenberg M, Brossay L, Kurepa Z, et al. Conserved lipid and peptide presentation functions of nonclassical class I molecules. Immunol Today. 1999;20:515–21. doi: 10.1016/s0167-5699(99)01521-2. [DOI] [PubMed] [Google Scholar]
- 33.Burdin N, Brossay L, Kronenberg M. Immunization with α- galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–25. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. 10.1002/(sici)1521-4141(199906)29:06<2014::aid-immu2014>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Kitamura H, Ohta A, Sekimoto M, et al. α-Galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 35.Yashimoto T, Paul WE. CD4pos, NK1.1pos T cells promtly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaman WE. Natural killer cells and natural killer T cells. Arthritis Rheum. 2000;43:1204–17. doi: 10.1002/1529-0131(200006)43:6<1204::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Stein-Streilein J, Sonoda KH, Fraunce D, et al. Regulation of adaptive immune responses by innate cells expressing NK markers and antigen-transporting macrophages. J Leukoc Biol. 2000;67:488–94. doi: 10.1002/jlb.67.4.488. [DOI] [PubMed] [Google Scholar]
- 38.Servitje O, Bordas X, Seron D, et al. Changes in T-cell phenotype and adhesion molecules expression in psoriatic lesions after low-dose cyclosporin therapy. J Cutan Pathol. 1996;23:431–6. doi: 10.1111/j.1600-0560.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 39.Mieza MA, Itoh JQ, Cui Y, et al. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–40. [PubMed] [Google Scholar]
- 40.Falcone M, Yeung B, Tucker L, et al. A defect in interleukin 12-induced activation and interferon γ secretion of peripheral natural killer T cells in nonobese diabetic mice suggest new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999;190:963–72. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gombert JM, Herbelin E, Tancrede-Bohin M, et al. Early quantitative and fuctional deficiency of NK1+-like thymocytes in the NOD mouse. Eur J Immunol. 1996;26:2989–98. doi: 10.1002/eji.1830261226. [DOI] [PubMed] [Google Scholar]
- 42.Sumida T, Sakamoto A, Murata H, et al. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with sistemic sclerosis. J Exp Med. 1995;182:1163–8. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–81. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 44.Ventura M, Colizzi M, Ottolenghi A, et al. Cell-mediated immune response in psoriasis and psoriatic arthritis. Recent Prog Med. 1989;80:449–54. [PubMed] [Google Scholar]
- 45.Hodl S, Beaufort F, Soyer HP, et al. A fluorescence-activated cell sorter – analysis of peripheral T lymphocyte subpopulations in psoriasis. Z Hautkr. 1988;63:415–16. [PubMed] [Google Scholar]
- 46.Chen Z, Foca A, Norton-Koger B, et al. Mononuclear cell phenotypes in patients with psoriasis. Dermatologica. 1986;172:291–7. doi: 10.1159/000249364. [DOI] [PubMed] [Google Scholar]
- 47.Veale DJ, Barnes L, Rogers S, FitzGerald O. Immunohistochemical markers for arthritis in psoriasis. Ann Rheum Dis. 1994;53:450–4. doi: 10.1136/ard.53.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]