Abstract
B7-1 (CD80) and B7-2 (CD86) molecules on antigen presenting cells play important roles in providing co-stimulatory signals required for activation and expansion of autoreactive T cells. Moreover, some reports have suggested that these molecules may have distinct functions in the differentiation of Th1 and Th2 cells. Mercury-induced autoimmunity in H-2s mice is characterized by lymphoproliferation of T and B cells, serum increases in IgG1 and IgE and production of antinucleolar antibodies (ANoA). The mechanisms responsible for the various manifestations of this syndrome have yet to be elucidated. To examine the contributions of B7 co-stimulatory molecules to this model, susceptible mice were treated with antibodies to B7-1, B7-2, or both during the development of mercury-induced autoimmunity. The combination of anti-B7-1 and anti-B7-2 antibodies prevented Hg-induced disease in H-2s mice. Additionally, single anti-B7-1 antibody treatment was sufficient to prevent Hg-induced ANoA production, but not IgG1 and IgE hypergammaglobulinaemia. Further, single antibody treatment with anti-B7-2 resulted in a partial reduction of ANoA titres but had no significant effect on total serum IgG1 and IgE levels. Taken together, these results indicate that B7-1 and B7-2 molecules are critical for the development of Hg-induced autoimmunity and suggest that the different manifestations of the syndrome are regulated by independent mechanisms.
Keywords: autoimmunity, co-stimulatory molecules, mercury, mouse, rodent, Th1/Th2
Introduction
In addition to antigen-specific signals mediated through the T cell receptor, T cells require additional, antigen non-specific co-stimuli for activation. The B7 family of molecules, which includes B7-1 (CD80) and B7-2 (CD86), is a major provider of co-stimulatory signals required for the development of immune responses to foreign and self antigens. B7 molecules, which are expressed on antigen-presenting cells, are specific for both CD28 and CTLA-4 coreceptors found on T cells. CD28/B7 interactions are important for augmenting and sustaining T cell responses, and can have multiple effects upon the production of various cytokines, chemokines and anti-apoptotic proteins [1,2]. In contrast, CTLA-4/B7 interactions usually result in an inhibition of T cell activation (reviewed in [1]). Another recently described B7 family member, PD-L1, inhibits proliferation and cytokine secretion after engaging the PD−1 receptor on T lymphocytes [3].
Recently, much attention has focused on the role of B7 co-stimulatory interactions in the pathogenesis of various autoimmune phenomena, including EAE [4,5], diabetes [6], GVHD [7] and SLE [8,9]. Investigators have utilized soluble CTLA-4 Ig, individual MoAbs directed against B7-1 or B7-2 or mice lacking expression of these molecules to explore the roles of these interactions. While these studies have shed light on our understanding of co-stimulatory events and T cell activation requirements in the context of autoimmunity, they have also raised important questions concerning the individual contributions of B7-1 vs. B7-2 signals to these processes. In particular, it remains highly controversial whether B7-1 and B7-2 possess distinct roles in Th subset differentiation and regulation. For instance, administration of anti-B7-1 MoAb inhibits development of EAE in SJL mice and blocks the pathogenic Th1 response while single MoAb to B7-2 exacerbates disease [4]. In contrast, in NOD mice which develop a Th1-mediated diabetes, treatment with anti-B7-2 MoAb prevents development of diabetes whereas anti-B7-1 MoAb accelerates disease [6]. Similarly, treatment of mice with anti-B7-2 antibodies prevents Th1-mediated autoimmune lesions in a murine model of Sjögren's syndrome through up-regulation of Th2 responses [10]. Thus, it is unclear whether B7 molecules have distinct or overlapping functions in the development of an autoimmune response.
Mercury (Hg)-induced autoimmunity in genetically susceptible H-2s mice is characterized by a loss of tolerance to nucleolar antigens such as fibrillarin as well as a profound lymphoproliferation and hypergammaglobulinaemia. Mice receiving injections of mercuric chloride (HgCl2) develop IgG antinucleolar antibodies (ANoA) and serum increases in IgG1 and IgE (reviewed in [11–13]). Although both Th1 and Th2 cells participate in Hg-induced immune responses, the mechanisms for induction and regulation of this autoimmune syndrome are unclear. The aim of our study was to investigate the individual roles for B7 molecules in the development of Hg-induced disease and to determine whether the blockade of signalling through either of these molecules selectively affects the manifestations of this syndrome.
Materials and methods
Mice
Female A.SW/SnJ (H-2s) mice were obtained from the Jackson Laboratories (Bar Harbor, ME, USA) and maintained in our animal facilities. All mice used in experiments were at least 2 months old.
Antibodies
Anti-B7-1 (IG10, γ2a) and anti-B7-2 (2D10, γ2b) antibody-producing hybridomas were a kind gift from Dr V. Kuchroo (Harvard University) [4]. Rat antiras p21(Y13-238) and rat anti-DR5 (SFR-DR5) antibody-producing hybridomas were obtained from ATCC (Manassas, VA, USA) and used as IgG2a and IgG2b isotype controls, respectively. Monoclonal antibodies were purified from culture supernatants by affinity chromatography over a protein G column.
HgCl2 and antibody treatment
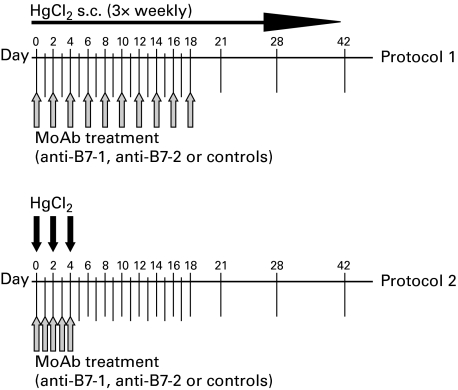
Mercury-induced autoimmunity was induced in groups of A.SW mice (H-2s) according to a standard protocol by subcutaneous injection (30 µg HgCl2 in 0·1 ml sterile PBS) three times weekly [14]. In addition to HgCl2, some groups of mice received anti-B7-1, anti-B7-2 or control antibodies. In the first series of experiments (protocol 1), control groups received either rat γ2a isotype control antibody, rat γ2b control antibody or a combination of both antibodies. No statistically significant differences were observed among these groups in their responses to HgCl2 injections, thus these values were pooled into one isotype control group. In the second series of experiments (protocol 2), control mice received a combination of γ2a and γ2b isotype control MoAbs. Antibody injection in protocols 1 and 2 were adapted from those used by other investigators [4] and are described in detail in Fig. 1. In all cases, antibodies were administered intraperitoneally and mice received 100 µg of each antibody in 0·2 ml sterile PBS per injection.
Fig. 1.
Time-course of HgCl2 and anti-B7 antibody injection protocols. In protocol 1, mice received subcutaneous injections of 30 µg HgCl2 in 0·1 ml sterile PBS three times weekly throughout the duration of the experiment. In protocol 2, only three injections of 30 µg HgCl2 were administered at days 0, 2 and 4. Arrows indicate dates of antibody injections (anti-B7-1, anti-B7-2, both, or isotype controls). In all cases, mice received 100 µg of each antibody intraperitoneally in 0·2 ml sterile PBS for each injection. Serum was collected at weeks 0, 1, 2, 3, 4 and 6 in all animals.
Antinucleolar antibodies (ANoA) immunofluorescence
ANoA levels in serially diluted mouse serum were determined by indirect immunofluorescence, as described previously [14]. Sera diluted in PBS containing 1% BSA and 0·02% sodium azide were incubated with HEp-2 slides (Antibodies, Inc., CA, USA) for 30 min, and ANoA were detected with FITC-conjugated goat antimouse IgG1 or IgG2a antibodies (Southern Biotechnology Associates). The initial serum dilution was 1/100. The inverse of the highest serum dilution at which nucleolar fluorescence could be detected was defined as the ANoA titre.
ELISA for mouse serum IgG1
Total serum IgG1 was determined using a sandwich ELISA adapted from a previously described method [14]. Briefly, plates were coated overnight at 4°C with goat antimouse Ig (Southern Biotechnology Associates, Birmingham, AL, USA) diluted 2 µg/ml in carbonate buffer. Following three washes with PT buffer (PBS + 0·05% Tween), wells were blocked with PBTN (PBS containing 1% BSA, 0·05% Tween 20 and 0·02% sodium azide) for 30 min. Sera diluted 1/60 000 in PBTN containing 20% goat serum (PBTN-G) were then added to wells and incubated at room temperature for 2 h. Samples were washed out six times with PT, and alkaline phosphatase (AP)-conjugated goat antimouse IgG1 secondary antibody (Southern Biotechnology Associates) diluted 1/4000 in PBTN-G was added for 1·5 h. Secondary antibody was washed out with two washes each of PT and AP substrate buffer (10 mm diethanolamine and 0·5 mm MgCl2 in dH2O). p-nitrophenylphosphate substrate (1 mg/ml in AP buffer) was then added and allowed to develop 20 min. Absorbances were read at 405 nm. A standard curve was generated using varying concentrations of ASWU1 (IgG1) MoAb [15].
ELISA for mouse serum IgE
Total serum IgE levels were determined using a sandwich ELISA which has been described previously [14]. Briefly, plates were coated overnight at 4°C with a rat antimouse IgE capture MoAb (clone R35-72, Pharmingen, San Diego, CA, USA) diluted 2 µg/ml in carbonate buffer. Following several washes and a blocking step with PBTN, sera diluted 1/100 in PBTN with 20% rat serum were then added to wells and incubated at room temperature for 2 h. After several washes, the secondary antibody, biotinylated rat antimouse IgE (clone R35-92, Pharmingen) diluted 2 µg/ml in PBTN containing 20% rat serum was added to wells and incubated at room temperature for 45 min Secondary antibody was then washed out and streptavidin-AP (Southern Biotechnology Associates) diluted 1/2000 in PBTN was added to each well and allowed to stand at room temperature for 45 min. Plates were then washed several times with PT, and p-nitrophenylphosphate substrate (1 mg/ml in AP buffer) was added to each well. Absorbance values were measured at 405 nm after 2 h. A standard curve was generated using varying concentrations (3–800 ng/ml) of purified mouse IgE (clone IgE-3, Pharmingen).
Measurement of mouse serum antibodies to administered rat Ig
Mouse serum antibodies were tested by ELISA for reactivity to the administered rat IG10 (antimouse B7-1) and 2D10 (antimouse B7-2) MoAb. Plates were coated overnight with IG10, 2D10, or both (each at 1 µg/ml in carbonate buffer). Plates were blocked with PBTN for 30 min. Serum samples diluted 1 : 1000 in PBTN were added and incubated for 2 h at room temperature. Plates were washed and incubated with biotinylated rat antimouse IgG (H + l) (Jackson Immunoresearch, West Grove, USA), diluted 1 : 50 000 in PBTN, for 1 h. Plates were then incubated with streptavidin-AP for 30 min Following washing, plates were developed with PNPP (1 mg/ml) and absorbances were measured at 405 nm.
Statistical analyses
Prior to analysis, all data were tested for normality using the Shapiro–Wilk test [16] and for homogeneity of variance using the Levene test [17]. The dependent variable data were significantly non-normal. In order to apply anova methods, a ‘normalized-rank’ transformation was applied to the data [18,19]. The rank-transformed data were analysed using an anova for repeated measures followed by multiple comparisons to detect significant individual pairwise mean differences. Multiple pairwise comparisons used the Dunn–Bonferroni adjustment [20] to maintain an overall experiment-wise type I error of 0·05 or less. Significant individual differences were represented as P ≤ 0·05 to indicate the adjusted P-values for the multiple comparisons.
Results
Combination of anti-B7-1 + anti-B7-2 MoAbs prevent Hg-induced autoimmunity
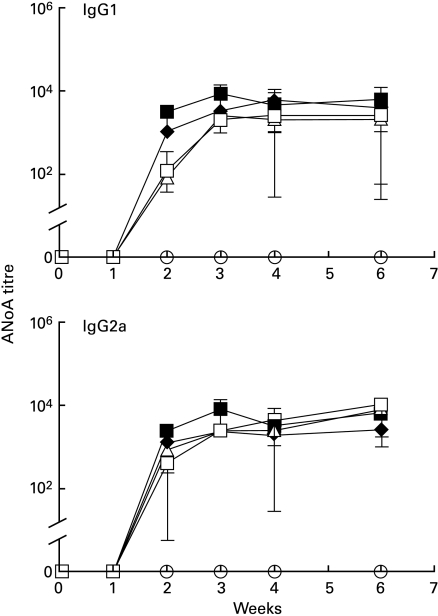
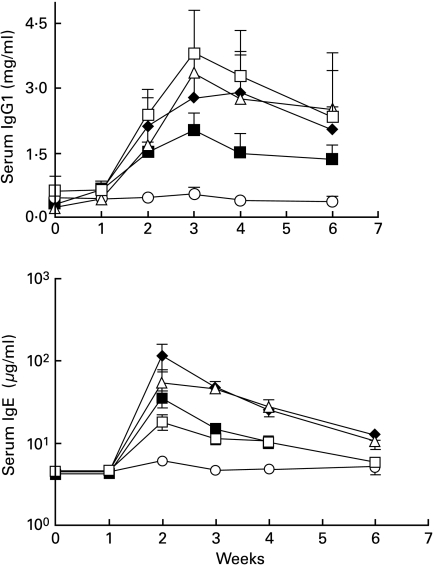
To assess the roles of B7-1 and B7-2 in Hg-induced autoimmunity, we initially conducted a series of experiments (protocol 1) in which groups of five ASW mice received HgCl2 injections three times a week throughout the experiment. Antibody treatments were administered every other day from day 0 until day 18 (see details in Fig. 1). Mice receiving either HgCl2 or HgCl2 plus isotype control antibodies developed IgG1 and IgG2a ANoA responses which peaked at week 3 and remained elevated thereafter (Fig. 2). In contrast, mice treated with both anti-B7-1 and B7-2 MoAbs had no ANoA detectable in the serum by week 6. Treatment with either anti-B7-1 or anti-B7-2 alone did not prevent the production of Hg-induced ANoA, although IgG1 and IgG2a ANoA titres in these groups were slightly, but significantly (P < 0·05) reduced at week 2 in comparison with control groups. In addition to autoantibody production, increases in serum IgG1 and IgE are an important feature of Hg-induced autoimmunity. Mice receiving either HgCl2 or HgCl2 plus rat isotype controls developed dramatic serum IgG1 and IgE increases (Fig. 3) peaking at 3 and 2 weeks, respectively, and gradually diminishing thereafter. As was the case with the ANoA response, mice receiving a combination of anti-B7-1 and anti-B7-2 antibodies maintained low levels of IgG1 and IgE, and did not show the striking increases of the control groups. Single treatment with either anti-B7-1 or anti-B7-2 MoAbs did not prevent the Hg-induced increases in IgG1 and IgE, although serum IgE levels were moderately, but significantly (P < 0·05) lower at weeks 2 and 3 in mice treated with anti-B7-1 MoAb.
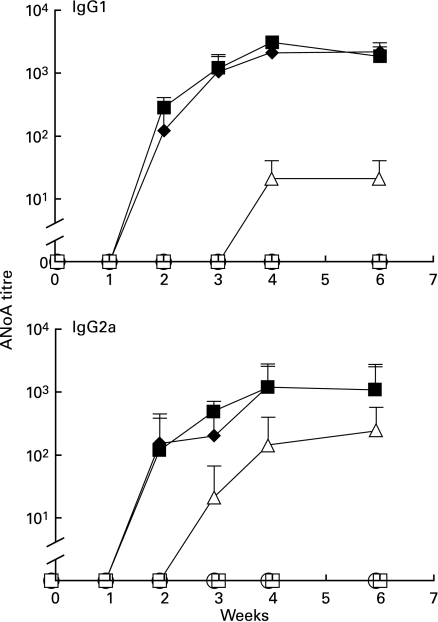
Fig. 2.
B7-1 and B7-2 blockade prevents autoantibody formation. Groups of five mice received HgCl2 and antibody injections as detailed in protocol 1 (Fig. 1). ANoA were detected by immunofluorescence on HEp-2 cells using isotype-specific FITC conjugates [14]. Results are expressed as serum titres (inverse of the highest serum dilution that yielded nucleolar fluorescence) ± s.d. None of the mice receiving both anti-B7-1 and anti-B7-2 MoAbs developed ANoA. Mice receiving single anti-B7-1 or anti-B7-2 MoAb treatment did not differ significantly from the control groups except for slightly lower ANoA titres at weeks 2 and 3 than in the control groups. □, Anti-B7-1; ▵, anti-B7-2; ○, anti-B7-1 + anti-B7-2; ♦, isotype control; ▪, mercury alone.
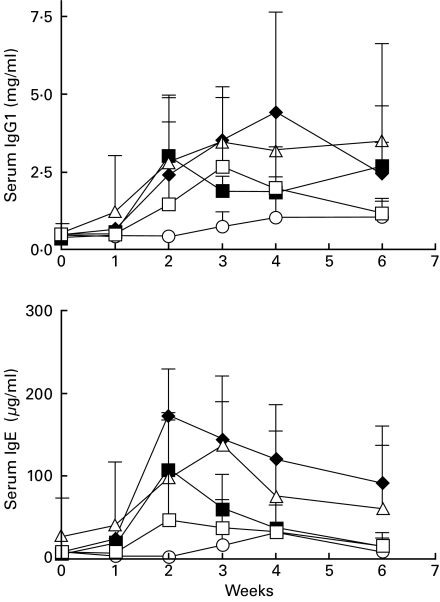
Fig. 3.
B7-1 and B7-2 blockade prevents the increase in serum IgG1 and IgE during Hg-induced autoimmunity.Groups of five mice were treated as described in protocol 1 (Fig. 1). Serum immunoglobulin levels were measured by ELISA as described in the Materials and methods section and are expressed in mg/ml ± s.d. (IgG1) or µg/ml ± s.d. (IgE). From week 2 and thereafter, serum IgG1 and IgE levels were significantly (P < 0·05) lower in mice receiving both anti-B7-1 and anti-B7-2 MoAbs than in mice treated with control MoAbs. Levels of serum IgG1 and IgE were not significantly different in mice receiving single anti-B7-1 or anti-B7-2 MoAb treatment than those in mice treated with control isotypes (except for lower levels of serum IgE at weeks 2 and 3 in mice treated with anti-B7-1, p <0·05). □, Anti-B7-1; ▵, anti-B7-2; ○, anti-B7-1 + anti-B7-2; ♦, isotype control; ▪, mercury alone.
Co-stimulation through both B7-1 and B7-2 is critical for Hg-induced ANoA production
In the experiments conducted under protocol 1, interruption of either B7-1 or B7-2 co-stimulatory interactions alone by means of single antibody administration was not sufficient to prevent the production of Hg-induced autoantibodies. A concern, however, was that mice receiving single antibody treatment may have mounted a host antirat Ig immune response that would have neutralized the anti-B7-1 or anti-B7-2 antibody treatment. Indeed, several investigators have reported significant mouse antirat responses elicited in mice treated with rat MoAbs to CD80 (B7-1) or CD86 (B7-2) [8,21]. The host immune response is not a concern when animals receive both anti-B7-1 and anti-B7-2 antibodies since blockade of both B7-1 and B7-2 molecules prevents the formation of mouse antirat Ig antibodies [8,21].
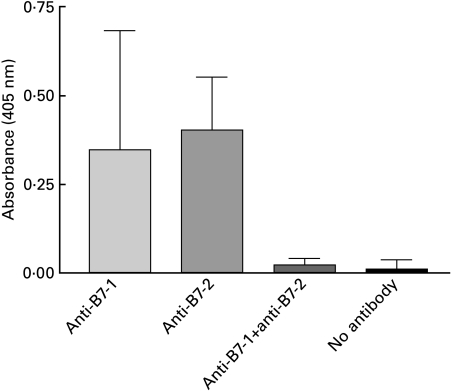
To verify whether our mice indeed developed a host immune responses to the administered xenogenic rat MoAb, their sera (at week 2 of protocol 1) were tested by ELISA for reactivity to IG10 (anti-B7-1) or 2D10 (anti-B7-2). The results in Fig. 4 show that mouse antirat antibodies were readily detectable in mice treated singly with either anti-B7-1 or anti-B7-2, but were not present in mice receiving both anti-B7-1 and anti-B7-2 rat antibodies. These data suggest that mouse antirat immune responses may have neutralized single antibody treatments to either B7-1 or B7-2.
Fig. 4.
Anti-rat Ig responses in anti-B7-1 or anti-B7-2 treated mice. Groups of five mice were treated as described in protocol 1. Week 2 sera were evaluated for the presence of antibodies to rat Ig by ELISA as described in the Materials and methods section and results are expressed in absorbances ± s.d. Only animals receiving single anti-B7-1 or anti-B7-2 MoAbs developed mouse antirat Ig responses. Absorbances in mice treated with both anti-B7-1 and anti-B7-2 MoAbs or in mice receiving HgCl2 without rat MoAb treatment were similar to those of animals receiving no treatment at all.
To offset the role of the host immune response, we therefore designed a new treatment regimen in which we limited the duration of mercury and antibody administration. In protocol 2 (Fig. 1), mice received three HgCl2 injections on days 0, 2 and 4 and a daily antibody injection from day 0 to day 4 (for a total of five MoAb injections). The purpose of this protocol was not to prevent the mouse antirat Ig response; rather, it was to ensure that the effects of Hg would take place before the production of antirat antibodies. Indeed, a series of five daily injections of rat antibodies is certainly immunogenic in mice, but this antiglobulin response takes several days to develop and does not interfere with the induction phase of autoimmunity since the last Hg injection was given on day 4 (Fig. 1). Following this protocol, mice receiving either HgCl2 alone or HgCl2 plus a combination of rat γ2a and γ2b isotype controls developed elevated titres of IgG1 and IgG2a ANoA by week 4 (Fig. 5). In contrast to protocol 1, Hg-injected mice receiving only anti-B7-1 MoAb within the first week of treatment had no detectable levels of either IgG1or IgG2a ANoA. Similarly, mice receiving treatment with anti-B7-2 MoAb had very low levels of serum IgG1 ANoA (only 20% of mice tested positive by IF) and significantly (P < 0·05) reduced levels of IgG2a ANoA compared to control groups. Moreover, mice receiving a combination of both antibodies had no detectable levels of serum ANoA, confirming our initial findings of experiment 1.
Fig. 5.
Single anti-B7-1 or anti-B7-2 MoAb treatment can inhibit ANoA induction In Hg-induced autoimmunity. Groups of five mice received HgCl2 and antibody injections as detailed in protocol 2 (Fig. 1). ANoA were detected by immunofluorescence on HEp-2 cells using isotype-specific FITC conjugates [14]. Results are expressed as serum titres (inverse of the highest serum dilution that yielded nucleolar fluorescence) ± s.d. None of the mice receiving either anti-B7-1 MoAb alone or both anti-B7-1 and anti-B7-2 MoAbs developed ANoA. Although some anti-B7-2-treated mice developed ANoA after week 3, their titres were significantly (P < 0·05) lower than those in the control groups. □, Anti-B7-1; ▵, anti-B7-2; ○, anti-B7-1 + anti-B7-2; ♦, isotype control; ▪, HgCl2 alone.
Blockade of both B7-1 and B7-2 interactions is required to prevent Hg-induced increase in total serum IgG1 and IgE
Serum samples from mice treated according to protocol 2 were also used to measure total serum levels of IgG1 and IgE by ELISA. Overall, the increases in IgG1 and IgE serum levels were of a lesser magnitude (with more interanimal variation) than those seen with protocol 1, probably as a consequence of the shorter duration of HgCl2 treatment. Mice receiving HgCl2 alone developed increased levels of serum IgG1 which peaked at week 2 (Fig. 6). These levels remained elevated for some time and gradually tapered off. Similarly, mice receiving a combination of rat isotype controls in addition to HgCl2 had increased levels of total serum IgG1. While this response was slightly delayed in comparison with mice receiving HgCl2 alone, there were no statistically significant differences in the magnitude of the response between these two groups. In contrast, at week 2 mean serum IgG1 levels in mice receiving both anti-B7-1 and anti-B7-2 antibodies were almost sixfold lower than those in mice receiving isotype controls (0·4 mg/ml versus 2·4 mg/ml). Therefore, administration of both antibodies resulted in a significant inhibition of the Hg-induced IgG1 increase (P < 0·05). In contrast, administration of either anti-B7-1 or anti-B7-2 alone had no statistically significant effect on serum IgG1 levels in comparison with mice receiving isotype controls.
Fig. 6.
Serum immunoglobulin levels in A.SW mice receiving HgCl2 and anti-B7 antibody treatment. Groups of five mice were treated as described in protocol 2 (Fig. 1). Serum immunoglobulin levels were measured by ELISA as described in the Materials and methods section and are expressed in mg/ml ± s.d. (IgG1) or µg/ml ± s.d. (IgE). From week 2 and thereafter, serum IgG1 and IgE levels remained significantly (P < 0·05) lower in mice receiving both anti-B7-1 and anti-B7-2 MoAbs than in mice treated with control MoAbs. Levels of serum IgG1 and IgE were not significantly different in mice receiving single anti-B7-1 or anti-B7-2 MoAb treatment than those in mice treated with control isotypes (except for lower levels of serum IgE at weeks 2, 4 and 6 in mice treated with anti-B7-1, p <0·05). □, Anti-B7-1; ▵, anti-B7-2; ○, anti-B7-1 + anti-B7-2; ♦, isotype control; ▪, HgCl2.
As expected, mice receiving either HgCl2 alone or in combination with rat isotype controls increased their serum IgE levels, which peaked 2 weeks after HgCl2 was administered (Fig. 6). Additionally, mice treated with anti-B7-2 antibody alone developed an increase in the IgE response which was similar to control mice levels. Serum IgE levels also increased in mice treated with anti-B7-1 antibody alone, but remained significantly (P < 0·05) lower than those detected in the control groups. Serum IgE levels remained low in mice receiving both anti-B7-1 plus anti-B7-2 antibodies. Levels of serum IgE for mice in this group showed virtually no increase in response to Hg treatment.
Discussion
Recent reports have demonstrated that B7-1 and B7-2 molecules on APCs play critical roles in the co-stimulation and activation of autoreactive T cells. The present study indicates that B7-1 and B7-2 co-stimulatory interactions are necessary for the loss of tolerance and subsequent production of autoantibodies seen in mercury-induced autoimmunity, as mice receiving antibodies to both B7-1 and B7-2 had no detectable ANoA in their serum. Our data support the view that the production of ANoA in Hg-induced autoimmunity is self-Ag driven [15]. These findings are also consistent with a growing body of evidence implicating critical roles for B7 molecules in the activation of autoreactive T and B cells required for autoantibody production. For instance, complete interruption of all B7 interactions by means of anti-B7-1 and B7-2 MoAbs or CTLA-4 Ig is effective at preventing autoantibody production in lupus-prone MRL-lpr/lpr [9] and NZB/W F1 mice [8], in a model of collagen-induced arthritis [22], and in GVHD [23]. Our results confirm an earlier study showing treatment with CTLA4-Ig prevents the development of Hg-induced autoimmunity in SJL mice [24]. Our data also indicate that, in our model, the recently described ICOS/B7 h pathway cannot substitute for CD28/B7 interactions. This is not unexpected since B7 co-stimulation is needed to optimally induce ICOS expression [25].
Since cognate MHC-restricted T cell help is required for ANoA production [26], we anticipated that interruption of B7/CD28 interactions might prevent the development of an autoantibody response directed towards specific self antigens. It is also significant that B7 blockade suppressed the Hg-induced increase in serum IgG1 and IgE levels. The B7-CD28 pathway provides co-stimulatory signals that are essential for the T–B interactions involved in immunoglobulin class switching and germinal centre formation during a conventional immune response (reviewed in [2]). Anti-B7-1 and B7-2 antibodies can inhibit class switching by at least two different mechanisms: (i) in an indirect manner, by interruption of CD28/B7 interactions between APCs and interleukin-producing helper T cells which regulate class switching; (ii) in a direct fashion by preventing the necessary T–B interactions required for B cell activation and immunoglobulin synthesis. Further, B7-1 and B7-2 molecules appear to have overlapping or compensatory functions in Hg-induced isotype switching, as single blockade of either molecule in our study did not affect overall Ig increases in Hg-treated mice. Our data are in agreement with findings reported by Borriello et al. in which antigen-specific IgG1 and IgG2a responses and germinal centre formation were defective in mice deficient in both B7 molecules, but functional in B7-1 or B7-2 singly deficient mice [27]. Nevertheless, conventional isotype switching mechanisms involve cognate, antigen-specific interactions between T and B cells. In contrast, most of the increase in serum IgG1 and IgE immunoglobulins during Hg-induced autoimmunity is polyclonal in nature and not targeted against a specific antigen. This phenomenon presumably reflect a Hg-driven, non-antigen specific isotype switching process in B lymphocytes.
Although several lines of evidence have suggested that B7-1 and B7-2 have differential effects on Th1 and Th2 differentiation and activation, the issue remains controversial. Some of this evidence derives from murine models of autoimmunity in which susceptibility to disease can be modulated by selectively blocking either B7-1 or B7-2 co-stimulatory events. As mentioned above, blocking the pathogenic Th1 response in EAE with anti-B7-1 MoAbs inhibits development of disease, while antibodies to B7-2 exacerbate disease, presumably by suppressing the protective Th2 response [4]. Additionally, anti-B7-1 MoAbs can increase IL-4 production in mice, and anti-B7-2 antibodies can increase IFN-γ production in vitro [4]. Taken together, these studies support a model whereby B7-1 co-stimulation promotes Th1 responses and B7-2 co-stimulation promotes Th2 responses (for review see [28,29]). Hg-induced autoimmunity has been traditionally viewed as a Th2-driven disease, but recent studies using IL-4 deficient mice have demonstrated that IL-4 is not required for the Hg-induced loss of tolerance [30,31]. Similarly, treatment with IL-12, a Th1-promoting cytokine, does not fully prevent Hg-induced autoimmunity [14,32]. Moreover, Kono and colleagues have suggested that IFN-γ, and therefore the Th1 subset of T cells is required for development of Hg-induced disease [30]. The results from protocol 2 indicate that treatment with anti-B7-1 or anti-B7-2 MoAb equally affects IgG1 and IgG2a ANoA production and does not promote either a Th1 (IgG2a) or Th2 (IgG1) isotype profile. Further, treatment with anti-B7-1 MoAb partially inhibits the Hg-induced increase in serum IgE in both protocols 1 and 2, arguing against a role for B7-1 co-stimulation in promoting the Th1 subset. Therefore, our data do not support the view that blockade of only B7-1 or B7-2 can bias Hg-induced autoimmunity towards a Th1 or Th2 phenotype, respectively. This lack of a specific association between B7-1/B7-2 and Th1/Th2 phenotype has been observed by others [33–36] and a distinct role for B7 molecules in T helper subset differentiation may thus be limited to certain experimental settings.
As mentioned earlier, mercury induces a dramatic polyclonal activation of the immune system, marked by lymphoproliferation of T and B cells as well as a resulting hypergammaglobulinaemia in genetically susceptible mice. Mercury and other heavy metals can also prevent apoptosis, have mitogen-like effects on cells and are strong activators of the immune system [37–41]. Significantly, in H-2s mice mercury up-regulates expression of IL-2 receptor proteins CD25 and CD122 and the proliferation marker CD71 on T cells [42], and MHC class II molecules on B cells [43]. These molecules, along with co-stimulatory molecules such as B7-1 and B7-2, can facilitate interactions between T and B cells and may contribute to the T cell-dependent activation of autoreactive B cells seen in this syndrome. A role for both B7-1 and B7-2 in mercury-induced autoimmunity is further supported by our observation that the percentages of both B7-1- and B7-2-positive cells increase after 7 days of HgCl2 exposure (18·3% B7-1 + splenocytes in mice receiving PBS versus 60·7% positive cells after HgCl2 treatment; 10·5% B7-2 + splenocytes in mice receiving PBS versus 45·8% positive cells after HgCl2 treatment) (unpublished data). We are currently evaluating which cell populations increase their expression of B7 markers and other co-stimulatory molecules during mercury-induced autoimmunity.
Our data suggest that B7-1 may play a more critical role in the events leading to autoimmunity in this model, as a single antibody blockade by anti-B7-1 MoAb is sufficient to block induction of both IgG1 and IgG2a ANoA in protocol 2. In comparison, mice in which B7–2 interactions alone were blocked showed only a partial reduction of ANoA levels. Our observation is similar to that recently made in a model of Goodpasture's disease, where the treatment of rats immunized with glomerular basement membrane with a mutant CTLA-4 Ig which selectively binds B7-1 resulted in reduced glomerulonephritis [44]. Similarly, our results suggest that, in the absence of B7–2 interactions, signalling through B7-1 is able to partially compensate for the co-stimulation required for the breaking of tolerance to self antigens. These results are in agreement with a recent report that quantitative differences exist between B7-1 and B7-2 in their ability to signal through CD28 [45]. This study indicated that co-stimulation via B7-1 resulted in more CD28 tyrosyl phosphorylation than via B7-2 [45], supporting our observation that B7-1 signalling is more critical than B7-2 in Hg-induced autoimmunity. We cannot, however, formally exclude that our anti-B7-2 treatment was less efficient than the anti-B7-1 MoAb at blocking interactions with CD28. This could be due to intrinsic properties of the two MoAbs such as a stronger affinity of the anti-B7-1 than that of the anti-B7-2 antibody for their respective targets resulting in a more efficient blockade with the anti-B7-1 MoAb. It is also possible that the anti-B7-2 elicited a stronger antirat response resulting in a decreased efficiency for this particular MoAb. This was observed by Nakajima et al. in a previous study of the effects of anti-B7-1 and anti-B7-2 MoAbs upon the development of lupus in NZB/W F1 mice [8]. They indeed detected a stronger mouse antirat response to the anti-B7-2 MoAb than to the anti-B7-1 MoAb [8]. These authors, however, used a different set of anti-B7 antibodies than the pair that we used in our studies and it is unknown whether anti-B7-2 antibodies would always elicit stronger xenogenic responses than anti-B7-1 antibodies. Irrespective of this issue, the respective roles of B7-1 and B7-2 will be definitively addressed when mouse lines that are genetically deficient for either of these two molecules will be crossed onto a susceptible H-2s background and will be evaluated for their autoimmune response to mercury.
A caveat of studies employing xenogenic antibodies to examine their effects on disease is the development of host immune responses to the administrated MoAb. While host responses to single administration of rat anti-B7-1 or B7-2 alone can reduce or eliminate their efficacy, administration of both MoAbs may prevent this host response to the xenogenic Ig [21]. Initially (protocol 1), we administered HgCl2 throughout the duration of the experiment while antibody treatment was administered every other day for 18 days, identical to the protocol used to successfully block EAE in susceptible mouse strains [4]. Since mice receiving individual MoAbs to B7-1 or B7-2 developed antibodies to rat IgG, the host immune response probably neutralized single antibody treatments. In protocol 2, we limited the number of HgCl2 injections to three over a span of 96 h (simultaneous with anti-B7-1 or anti-B7-2 treatment) and therefore prevented the antirat immune response in protocol 2 to interfere at the initiation stage of this autoimmune syndrome. This approach allowed us to observe an alteration of the autoimmune response by blocking only B7-1 or B7-2, a finding which was not apparent in the first protocol. Our results suggest that some earlier studies performed with anti-B7-1 and anti-B7-2 MoAbs as biological response modifiers should be re-evaluated since the host immune response has not always been taken into consideration.
Loss of tolerance to nucleolar antigens arises within 10 days after the beginning of mercury injections and is dependent on cognate interactions between T cells and B cells, as depletion of T cells by anti-CD4 MoAb [46] or treatment of mice with CTLA-4 Ig or anti-CD40L MoAb [24] prevent disease. Nevertheless, it is still unknown how heavy metals such as mercury exert their effects and induce autoantibody production and activation of the immune system. Mercury may modify self antigens or their processing, thus rendering them immunogenic [47,48]. Additionally, mercury and other heavy metals can induce proliferation and cytokine production by cells of the immune system [39, 40, 49–53, ]. However, several features of this model suggest that the polyclonal activation and the production of specific autoantibodies may be regulated by separate mechanisms [14,31]. Indeed, our observation that single blockade of either B7-1 or B7-2 co-stimulation can inhibit ANoA production without preventing total serum IgG1 and IgE increase lends support to the existence of independent regulatory mechanisms.
Acknowledgments
We are grateful to Dr Kuchroo for his generous gift of the anti-B7-1 and anti-B7-2 hybridomas and to Dr Gaughan for his assistance with the statistical analyses. This work was supported by NIH grants AI-26665 and ES-09409 to MM.
REFERENCES
- 1.Chambers CA, Allison JP. Co-stimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–10. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 2.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–47. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuchroo VK, Das MP, Brown JA, et al. B7–1 and B7–2 co-stimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 5.Perrin PJ, Scott D, June CH, Racke MK. B7-mediated co-stimulation can either provoke or prevent clinical manifestations of experimental allergic encephalomyelitis. Immunol Res. 1995;14:189–99. doi: 10.1007/BF02918216. [DOI] [PubMed] [Google Scholar]
- 6.Lenschow DJ, Ho SC, Sattar H, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetes mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito K, Yagita H, Hashimoto H, Okumura K, Azuma M. Effect of CD80 and CD86 blockade and anti-interleukin-12 treatment on mouse acute graft-versus-host disease. Eur J Immunol. 1996;26:3098–106. doi: 10.1002/eji.1830261241. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima A, Azuma M, Kodera S, et al. Preferential dependence of autoantibody production in murine lupus on CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:3060–9. doi: 10.1002/eji.1830251112. [DOI] [PubMed] [Google Scholar]
- 9.Liang B, Gee RJ, Kashgarian MJ, Sharpe AH, Mamula MJ. B7 co-stimulation in the development of lupus: autoimmunity arises either in the absence of B7.1/B7.2 or in the presence of anti-b7.1/B7.2 blocking antibodies. J Immunol. 1999;163:2322–9. [PubMed] [Google Scholar]
- 10.Saegusa K, Ishimaru N, Yanagi K, et al. Treatment with anti-CD86 co-stimulatory molecule prevents the autoimmune lesions in murine Sjögren's syndrome (SS) through up-regulated Th2 response. Clin Exp Immunol. 2000;119:354–60. doi: 10.1046/j.1365-2249.2000.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieson PW. Mercuric chloride-induced autoimmunity. Autoimmunity. 1992;13:243–7. doi: 10.3109/08916939209004830. [DOI] [PubMed] [Google Scholar]
- 12.Bagenstose LM, Salgame P, Monestier M. Murine mercury-induced autoimmunity. a model of chemically related autoimmunity in humans. Immunologic Res. 1999;20:67–78. doi: 10.1007/BF02786508. [DOI] [PubMed] [Google Scholar]
- 13.Pollard KM, Hultman P. Effects of mercury on the immune system. Met Ions Biol Syst. 1997;34:421–40. [PubMed] [Google Scholar]
- 14.Bagenstose LM, Salgame P, Monestier M. IL-12 down-regulates autoantibody production in mercury-induced autoimmunity. J Immunol. 1998;160:1612–7. [PubMed] [Google Scholar]
- 15.Monestier M, Losman MJ, Novick KE, Aris JP. Molecular analysis of mercury-induced antinucleolar antibodies in H-, 2s mice. J Immunol. 1994;152:667–75. [PubMed] [Google Scholar]
- 16.Armitage P, Berry G. Statistical methods in medical research. 3. Cambridge, MA: Blackwell Science; 1994. pp. 395–8. [Google Scholar]
- 17.Snedecor GW, Cochran WG. Statistical methods. 8. Ames, Iowa: Iowa State University Press; 1989. pp. 252–3. [Google Scholar]
- 18.Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–9. [Google Scholar]
- 19.Harter HL. Expected values of normal order statistics. Biometrika. 1961;48:151–65. [Google Scholar]
- 20.Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design. 3. New York: McGraw-Hill; 1991. pp. 158–66. [Google Scholar]
- 21.Daikh DI, Wofsy D. Effects of anti-B7 monoclonal antibodies on humoral immune responses. J Autoimmun. 1999;12:101–8. doi: 10.1006/jaut.1998.0258. [DOI] [PubMed] [Google Scholar]
- 22.Webb LM, Walmsley MJ, Feldmann M. Prevention and amelioration of collagen-induced arthritis by blockade of the CD28 co-stimulatory pathway: requirement for both B7-1 and B7-2. Eur J Immunol. 1996;26:2320–8. doi: 10.1002/eji.1830261008. [DOI] [PubMed] [Google Scholar]
- 23.Via CS, Rus V, Nguyen P, Linsley P, Gause WC. Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD. J Immunol. 1996;157:4258–67. [PubMed] [Google Scholar]
- 24.Biancone L, Andres G, Ahn H, et al. Distinct regulatory roles of lymphocyte co-stimulatory pathways on T helper type-2 mediated autoimmune disease. J Exp Med. 1996;183:1473–81. doi: 10.1084/jem.183.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdam AJ, Chang TT, Lumelsky AE, et al. Mouse inducible co-stimulatory molecule (ICOS) expression is enhanced by CD28 co-stimulation and regulates differentiation of CD4 (+) T cells. J Immunol. 2000;165:5035–40. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 26.Hanley GA, Schiffenbauer J, Sobel ES. Resistance to HgCl2-induced autoimmunity in haplotype-heterozygous mice is an intrinsic property of B cells. J Immunol. 1998;161:1778–85. [PubMed] [Google Scholar]
- 27.Borriello F, Sethna MP, Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 co-stimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CB. Distinct roles for the co-stimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 30.Kono DH, Balomenos D, Pearson DL, et al. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-γ and not Th1/Th2 imbalance. J Immunol. 1998;161:234–40. [PubMed] [Google Scholar]
- 31.Bagenstose LM, Salgame P, Monestier M. Mercury-induced autoimmunity in the absence of interleukin-4. Clin Exp Immunol. 1998;114:9–12. doi: 10.1046/j.1365-2249.1998.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorrie MJ, Qasim FJ, Whittle CJ, et al. Exogenous type-1 cytokines modulate mercury-induced hyper-IgE in the rat. Clin Exp Immunol. 2000;121:17–22. doi: 10.1046/j.1365-2249.2000.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine BL, Ueda Y, Craighead N, Huang ML, June CH. CD28 ligands CD80 (B7–1) and CD86 (B7–2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int Immunol. 1995;7:891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- 34.Natesan M, Razi-Wolf Z, Reiser H. Co-stimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783–91. [PubMed] [Google Scholar]
- 35.Schweitzer AN, Borriello F, Wong RC, Abbas AK, Sharpe AH. Role of co-stimulators in T cell differentiation: studies using antigen- presenting cells lacking expression of CD80 or CD86. J Immunol. 1997;158:2713–22. [PubMed] [Google Scholar]
- 36.Elloso MM, Scott P. Expression and contribution of B7-1 (CD80) and B7-2 (CD86) in the early immune response to Leishmania major infection. J Immunol. 1999;162:6708–15. [PubMed] [Google Scholar]
- 37.Whitekus MJ, Santini RP, Rosenspire AJ, McCabe MJJ. Protection against CD95-mediated apoptosis by inorganic mercury in Jurkat T cells. J Immunol. 1999;162:7162–70. [PubMed] [Google Scholar]
- 38.Enestrom S, Hultman P. Does amalgam affect the immune system? A controversial issue. Int Arch Allergy Immunol. 1995;106:180–203. doi: 10.1159/000236843. [DOI] [PubMed] [Google Scholar]
- 39.Reardon CL, Lucas DO. Heavy-metal mitogenesis: Zn++ and mercury++ induce cellular cytotoxicity and interferon production in murine T lymphocytes. Immunobiology. 1987;175:455–69. doi: 10.1016/S0171-2985(87)80073-6. [DOI] [PubMed] [Google Scholar]
- 40.Prigent P, Saoudi A, Pannetier C, et al. Mercuric chloride, a chemical responsible for T helper cell (Th) 2-mediated autoimmunity in Brown Norway rats, directly triggers T cells to produce interleukin-4. J Clin Invest. 1995;96:1484–9. doi: 10.1172/JCI118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waalkes MP, Fox DA, States JC, Patierno SR, McCabe MJJ. Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol Sci. 2000;56:255–61. doi: 10.1093/toxsci/56.2.255. [DOI] [PubMed] [Google Scholar]
- 42.Johansson U, Sander B, Hultman P. Effects of the murine genotype on T cell activation and cytokine production in murine mercury-induced autoimmunity. J Autoimmunity. 1997;10:347–55. doi: 10.1006/jaut.1997.0149. [DOI] [PubMed] [Google Scholar]
- 43.van Vliet E, Uhrberg M, Stein C, Gleichmann E. MHC control of IL-4-dependent enhancement of B cell Ia expression and Ig class switching in mice treated with mercuric chloride. Int Arch Allergy Immunol. 1993;101:392–401. doi: 10.1159/000236482. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds J, Tam FW, Chandraker A, et al. CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest. 2000;105:643–51. doi: 10.1172/JCI6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slavik JM, Hutchcroft JE, Bierer BE. CD80 and CD86 are not equivalent in their ability to induce the tyrosine phosphorylation of CD28. J Biol Chem. 1999;274:3116–24. doi: 10.1074/jbc.274.5.3116. [DOI] [PubMed] [Google Scholar]
- 46.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity. The role of T-helper cells. J Autoimmunity. 1995;8:809–23. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 47.Griem P, Gleichmann E. Metal ion induced autoimmunity. Curr Opin Immunol. 1995;7:831–8. doi: 10.1016/0952-7915(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 48.Kubicka-Muranyi M, Griem P, Lubben B, Rottmann N, Luhrmann R, Gleichmann E. Mercuric-chloride-induced autoimmunity in mice involves up-regulated presentation by spleen cells of altered and unaltered nucleolar self antigen. Int Arch Allergy Immunol. 1995;108:1–10. doi: 10.1159/000237110. [DOI] [PubMed] [Google Scholar]
- 49.Gillespie KM, Qasim FJ, Tibbatts LM, Thiru S, Oliveira DB, Mathieson PW. Interleukin-4 gene expression in mercury-induced autoimmunity. Scand J Immunol. 1995;41:268–72. doi: 10.1111/j.1365-3083.1995.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 50.Gillespie KM, Saoudi A, Kuhn J, et al. Th1/Th2 cytokine gene expression after mercuric chloride in susceptible and resistant rat strains. Eur J Immunol. 1996;26:2388–92. doi: 10.1002/eji.1830261018. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira DB, Gillespie K, Wolfreys K, Mathieson PW, Qasim F, Coleman JW. Compounds that induce autoimmunity in the Brown Norway rat sensitize mast cells for mediator release and interleukin-4 expression. Eur J Immunol. 1995;25:2259–64. doi: 10.1002/eji.1830250822. [DOI] [PubMed] [Google Scholar]
- 52.Badou A, Savignac M, Moreau M, et al. HgCl2-induced interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through l-type calcium channels. J Biol Chem. 1997;272:32411–8. doi: 10.1074/jbc.272.51.32411. [DOI] [PubMed] [Google Scholar]
- 53.Wu Z, Turner DR, Oliveira DB. IL-4 gene expression up-regulated by mercury in rat mast cells. a role of oxidant stress in IL-4 transcription. Int Immunol. 2001;13:297–304. doi: 10.1093/intimm/13.3.297. [DOI] [PubMed] [Google Scholar]