Abstract
Heat shock protein 60 (hsp60) has been increasingly recognized as an important molecule in infectious and autoimmune diseases. We have demonstrated previously that serum antibodies to both human hsp60 and Porphyromonas gingivalis GroEL were elevated in periodontitis patients compared with healthy subjects. In order to clarify the relative importance of hsp60 in the inflammatory response in periodontal disease, the stimulatory effect of human and bacterial hsp60 on the production of tumour necrosis factor-α (TNF-α) was examined in phorbol myristate acetate (PMA)-stimulated THP-1 cells. As bacterial hsp60s, recombinant P. gingivalis and Actinobacillus actinomycetemcomitans GroEL was used. Human hsp60 but not P. gingivalis or A. actinomycetemcomitans GroEL demonstrated stimulatory activity similar to lipopolysaccharide (LPS) derived from the bacteria. The activity of hsp60 was inhibited by anti-CD14 and anti-Toll-like receptor 4 (TLR4) antibodies, suggesting that both CD14 and TLR4 mediate hsp60 signalling. Immunohistochemical analysis demonstrated that hsp60 is abundantly expressed in periodontitis lesions. Therefore, it is postulated that periodontopathic bacteria stimulate the cells in the periodontium to up-regulate the expression of hsp60, which in turn may stimulate macrophage and possibly other cells to produce proinflammatory cytokines. These mechanisms may be involved in the chronicity and tissue destruction of periodontal disease.
Keywords: Hsp60, macrophage, THP-1, TNF-α
INTRODUCTION
As with other infectious diseases or autoimmune disorders, activation of immune cells by bacterial components is an important feature of chronic inflammatory periodontal diseases. Subsequent production of the proinflammatory cytokines such as interleukin (IL)-1, tumour necrosis factor (TNF)-α or IL-6 are thought to induce connective tissue destruction and alveolar bone resorption [1]. Among the various bacterial components, microbial heat shock protein 60 (hsp60) is known to be a major target of the immune response in infection, particularly in mycobacterial infection [2]. The mycobacterial 65-kDa protein has a high amino acid sequence identity with the GroEL protein of Escherichia coli. Mammalian hsp60 is also known to act as an autoantigen during chronic inflammation.
Hsp60 belongs to a family of related proteins, which have been conserved during evolution. It has been reported that GroEL-like protein belonging to the hsp60 family can be expressed by periodontopathic bacteria such as Porphyromonas gingivalis [3] and Actinobacillus actinomycetemcomitans (the GroEL-like proteins of P. gingivalis and A. actinomycetemcomitans are designated hereafter as P. gingivalis GroEL and A. actinomycetemcomitans GroEL, respectively) [4]. The sequence homologuey between human hsp60 and P. gingivalis GroEL or A. actinomycetemcomitans GroEL at an amino acid level is 49% and 52%, respectively. Despite being highly homologous between prokaryotic and eukaryotic cells, hsp60s are strongly immunogenic and immune responses to microbial hsp60s are thought to initiate chronic inflammatory diseases in which autoimmune responses to human hsp 60 may be central to pathogenesis [5]. In fact we have previously demonstrated that the frequency of seropositivity and the antibody titre to human hsp60 and P. gingivalis GroEL were significantly higher in periodontitis patients than in periodontally healthy control subjects [6]. Furthermore, affinity purified serum antibodies to human hsp60 and P. gingivalis GroEL cross-reacted with P. gingivalis GroEL and human hsp60, respectively. In addition, Maeda et al. demonstrated that highly conserved peptide between P. gingivalis GroEL and human hsp60 were recognized by the serum antibodies [7]. These results suggest that an immune response based on the molecular mimicry between P. gingivalis GroEL and human hsp60 may play a role in periodontitis.
Bacterial heat shock proteins have been reported to stimulate human monocytes to produce proinflammatory cytokines [8–12] or to up-regulate the expression of adhesion molecules [13,14]. Recently it has been demonstrated that human hsp60 can also activate the innate immune system [15–17].
Therefore, the aim of the present study was to examine the effects of human hsp60, P. gingivalis GroEL and A. actinomycetemcomitans GroEL and on the production of TNF-α from human macrophages. Our results demonstrated that in spite of the putative pathogenicity of P. gingivalis and A. actinomycetemcomitans, human hsp60 but not P. gingivalis nor A. actinomycetemcomitans GroEL had potent stimulatory properties on macrophages.
MATERIALS AND METHODS
Reagents
Recombinant human hsp60 and monoclonal antihuman hsp60 antibody (LK-1) were obtained from StressGen Biotechnologies Corp., Victoria, Canada. P. gingivalis GroEL [6] and A. actinomycetemcomitans GroEL [18] was prepared as described previously. Anti-CD14 monoclonal antibody (MY4) was purchased from Coulter (Hialearh, FL, USA). Anti-human TLR4 (HTA125) was kindly provided by S. Akashi and K. Miyake (Department of Immunology, Saga Medical School, Saga, Japan) [19]. Lipopolysaccharide (LPS) from E. coli O111:B4 was purchased from List Biological Laboratories (Campbell, CA, USA). LPS from P. gingivalis 381 was kindly provided by H. Kumada and T. Umemoto (Department of Microbiology, Kanagawa Dental University, Yokosuka, Japan). LPS from A. actinomycetemcomitans Y4 was a generous gift from LION Co. (Odawara, Japan). Phorbol myristate acetate (PMA), Polymyxin B, trypsin and soybean trypsin inhibitor were all purchased from Sigma Chemical Co. (St Louis, MO, USA). Polymyxin B binds to the endotoxins and suppress their biological activity. In order to examine whether the contaminated endotoxins in the recombinant protein could affect the results, polymyxin B was added to some cultures.
Cell preparation and culture
The monocytic cell line THP-1 was maintained in 25 mm Hepes-buffered RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, hereafter referred as medium. All incubations were carried out at 37°C in the atmosphere of 5% CO2 in air.
For the experiments, the cells were incubated in a 24-well culture plate (Costar, Cambridge, MA, USA) at a concentration of 2 × 106 cells/ml in the medium supplemented with 200 nm of PMA to induce differentiation into macrophage-like cells, hereafter referred as macrophages. After 48 h of incubation, the cells were extensively washed with RPMI 1640 and cultured further in the medium for 12 h and the medium was changed to remove the cytokines induced by cell adherence. Various stimulants were then added to the culture in the fresh medium and incubated for another 12 h. Bacterial and human hsp60 were used at 10 μg/ml, whereas LPS was used at 1 μg/ml.
In order to examine the role of CD14 and TLR4 in signalling by hsp60 stimulation, anti-CD14 antibody (MY4) or anti-TLR4 (HTA125) was added to the culture at a concentration of 10 μg/ml 1 h prior to the addition of the stimulants. To ensure the stimulatory activity is not attributable to contaminants, LPS and hsp60 were subjected to heating at 100°C for 20 min, incubation with 0·25% trypsin at 37°C for 30 min and then added to the culture, and incubated for 12 h. Residual trypsin activity was neutralized with an excess amount of soybean trypsin inhibitor. Polymyxin B was added simultaneously at a concentration of 10 μg/ml with stimulants. At the end of incubation period, culture supernatants were collected and stored at –70°C until assayed.
TNF-α assay
TNF-α levels in the supernatants of macrophage culture were determined by using commercially available ELISA kits (Endogen Inc., Woburn, MA, USA) according to the manufacturer’s instruction. The effects of various inhibitors on the TNF-α production were determined.
Immunohistochemistry
Expression of hsp60 in periodontitis lesion was determined by immunohistochemistry. Cryostat sections were prepared from a gingival specimen of a patient with severe periodontitis obtained at periodontal surgery under informed consent. Monoclonal antihuman hsp60 (LK-1; StressGen) was as primary antibody.
After rehydration in 0·05% Tris-buffered saline (pH 7·6) and blocking with normal rabbit serum (Dako, Glostrup, Denmark), the sections were incubated with LK-1 at 1/100 dilution followed by rabbit antimouse immunoglobulins (Dako) and finally with monoclonal mouse APAAP (Dako). Colour was developed with an alkaline phosphatase substrate III kit (Vector, Burlingame, CA, USA).
Endotoxin measurement
Endotoxin was determined by the limulus amebocyte lysate assay (Endospecy, Seikagaku Corporation, Tokyo, Japan).
Statistical analysis
The differences in TNF-α production by the different stimulants and the effects of antibodies were analysed using paired t-test. The statistical significance risk rate was set at P < 0·05.
RESULTS
TNF-α production by monocyte-derived macrophage
Each individual experiment was repeated three times. Although the results demonstrated similar trends, there were slight variations between experiments.
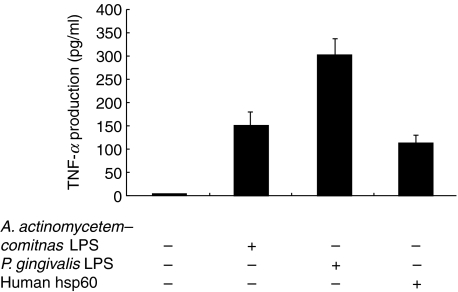
TNF-α production by macrophages stimulated with LPS and hsp60 from different source was compared. The concentrations of stimulants were 1 μg/ml for LPS and 10 μg/ml for hsp60, respectively, which were determined in a preliminary experiment. As shown in Fig. 1, P. gingivalis LPS induced a maximal response two times higher than A. actinomycetemcomitans LPS and three times higher than human hsp60. Both P. gingivalis GroEL and A. actinomycetemcomitans GroEL did not induce significant TNF-α production although P. gingivalis GroES (hsp10) and A. actinomycetemcomitans GroES demonstrated weak stimulatory effect (data not shown). Therefore, we examined only human hsp60 in the subsequent experiments. Contaminating endotoxin in the human hsp60 preparation was undetectable whereas the recombinant P. gingivalis GroEL and A. actinomycetemcomitans GroEL prepared in our laboratory had 12·5 pg/ml and 49·7 pg/ml, respectively, as measured by limulus assay. The results of the limulus assay clearly refute arguments that the elevated TNF-α production by macrophage is induced by trace amount of contaminating endotoxin in the preparation as the stimulatory effect of the contaminated P. gingivalis GroEL was even lower than that of the uncontaminated human hsp60.
Fig. 1.
Production of TNF-α by THP-1-derived macrophages. Differentiated THP-1 cells were stimulated with either LPS derived from P. gingivalis 381 or A. actinomycetemcomitans, or human hsp60 at concentrations of 1 μg/ml and 10 mg/ml, respectively, for 12 h. Culture supernatants were harvested and TNF-α levels were determined by ELISA. Data are expressed as means ± standard deviations of three replicates. Representative result of three independent experiments was shown. *Significantly higher in LPS- and hsp60-stimulated cultures than in the control.
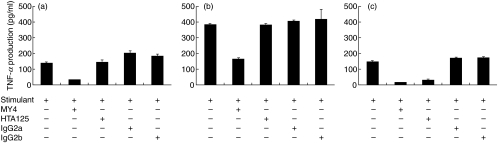
Effects of anti-CD14 and anti-TLR4 antibodies on the production of TNF-α
As shown in Fig. 2a,b, the inhibitory effect of anti-CD14 MAb was obvious for both A. actinomycetemcomitans LPS and P. gingivalis LPS; however, the effect was more prominent for A. actinomycetemcomitans LPS. The antibody also effectively inhibited human hsp60-induced TNF-α production. In contrast to the effect of MY4, HTA125 did not demonstrate any inhibitory activity for TNF-α production by macrophages stimulated with LPS of both bacteria (Fig. 2a,b). However, HTA125 clearly down-regulated the stimulatory effect of human hsp60 by 75%. Isotype-matched control antibodies had no inhibitory effect (Fig. 2c).
Fig. 2.
Effects of anti-CD14 monoclonal antibody and anti-TLR4 monoclonal antibody on TNF-α production by differentiated THP-1 cells. The cells were stimulated with 1 μg/ml of P. gingivalis LPS (a), 1 μg/ml of A. actinomycetemcomitans LPS (b) and 10 μg/ml of human hsp60 (c) in the presence or absence of antibodies at concentrations of 20 μg/ml for 12 h. TNF-α levels in the culture supernatants were determined by ELISA. Data are expressed as means ± standard deviations of three replicates. Representative result of three independent experiments was shown.
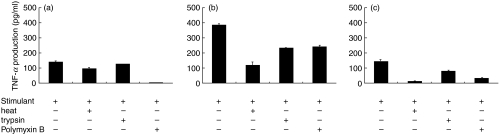
Effects of treatments with heat, trypsin and polymyxin B on the production of TNF-α
In order to confirm that the stimulatory effects were not due to the contaminants in the preparation, stimulants were subjected to various treatments prior to the addition to the culture. As shown in Fig. 3, heat treatment did not abolish the stimulatory effect of A. actinomycetemcomitans LPS although some effect was observed with P. gingivalis LPS. Trypsin treatment did not have any inhibitory effect on the TNF-α production stimulated by LPS from either A. actinomycetemcomitans or P. gingivalis. In contrast the stimulatory effect of hsp60 was sensitive both to heating and to trypsin treatment. Whereas heating of hsp60 inhibited TNF-α production by 96%, trypsin treatment inhibited the activity by 47%. Polymyxin B inhibited the TNF-α inducing activity of the LPS from both A. actinomycetemcomitans and P. gingivalis but the effect was lower for P. gingivalis LPS. Although the contaminating endotoxin was not detected in the recombinant human hsp60, inhibitory effect of polymyxin B was also observed for hsp60 stimulation in some extent.
Fig. 3.
Effects of various treatments of stimulants on TNF-α production by differentiated THP-1 cells. The cells were stimulated with 1 μg/ml of P. gingivalis LPS (a), 1 μg/ml of A. actinomycetemcomitans LPS (b) and 10 μg/ml of human hsp60 (c) treated with heating at 90°C for 30 min, incubation with trypsin at 37°C for 30 min or 10 μg/ml of polymyxin B for 12 h. TNF-α levels in the culture supernatants were determined by ELISA. Data are expressed as means ± standard deviations of three replicates. Representative result of three independent experiments was shown.
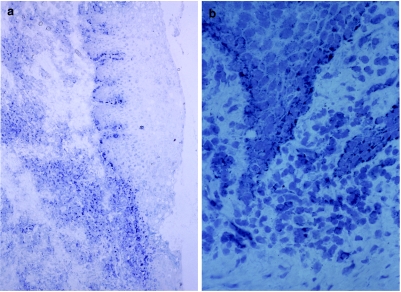
Hsp60 expression in periodontitis lesion
Immunohistochemical analysis of hsp60 expression demonstrated that basal cells of both oral and pocket epithelium were positive for LK-1 antibody. Many mononuclear cells in the inflammatory cell infiltrate beneath the pocket epithelium were also positively stained. Although hsp60 can be expressed on the cell surface, our finding demonstrated mainly cytoplasmic staining, which cannot be distinguished from cell surface staining and a few cell surface staining (Fig. 4). We have also confirmed that THP-1 cells express both hsp60 and TLR4 on their cell surface (data not shown).
Fig. 4.
Expression of hsp60 in periodontitis tissues. Staining of hsp60 was carried out by using an alkaline-phosphatase anti-alkaline-phosphatase (APAAP) method on cryostat sections. Hsp60-expressing cells appeared as blue. (a) Anti-hsp60 antibody reacted with either cells in the inflammatory infiltrate or basal cells of the oral epithelium (×50). (b) Higher magnification (×100).
DISCUSSION
The main conclusion to be drawn from the present study is that human but not periodontopathic bacterial hsp60 can induce TNF-α production in macrophages and this activity is mediated at least in part by CD14 and TLR4, both of which are known to be LPS receptors. We initially postulated that hsp60 derived from periodontopathic bacteria would have a stimulatory effect on macrophages to produce TNF-α. This concept was based on the finding that mycobacterial hsp65 [8–10] and E. coli GroEL [12,14] could induce the production of proinflammatory cytokines in human monocytes. Furthermore, hsp60 or GroEL-like protein from P. gingivalis and A. actinomycetemcomitans has been implicated in the pathogenesis of periodontal diseases in terms of the induction of a humoral immune response [3,6,20]. However, hsp60 from P. gingivalis and A. actinomycetemcomitans are both different from those of mycobacteria or E. coli inasmuch as GroEL from P. gingivalis and A. actinomycetemcomitans had little effect on the TNF-α production by macrophages. In this respect, Retzlaff et al. demonstrated that hsp60 family proteins from different species of bacteria have different inducing activity of proinflammatory cytokines in mouse macrophages [11]. Therefore, hsp60 of P. gingivalis and A. actinomycetemcomitans, both representative periodontopathic bacteria, may have less potency in the induction of proinflammatory cytokines than that of other bacteria.
The striking finding was that human hsp60 was a potent inducer of TNF-α in human macrophages. As the protein was produced in E. coli as a recombinant protein, contaminating small amount of endotoxin may have had a profound effect on the macrophage cell line. In order to confirm that the cytokine inducing activity came from hsp60 itself, we measured the endotoxin level in hsp60 preparation using a commercially available kit and showed it to be undetectable. Although the kit we used is highly sensitive, the possibility that the contamination of very small amount of endotoxin which is below detection limit cannot be excluded since the stimulatory activity of human hsp60 was partially abolished by polymyxin B treatment. Contrary, P. gingivalis GroEL and A. actinomycetemcomitans GroEL, both of which were prepared as recombinant proteins in our laboratory and demonstrated higher endotoxin levels than human hsp60, had only weak stimulatory activity. To ensure further the stimulatory effect of hsp60 is not mediated by the possible contaminating endotoxin, hsp60 was heat denatured or degradated with trypsin before addition to the culture. Heat treatment almost completely abolished the stimulatory activity. Since LPS is heat stable, the activity is therefore considered to be mediated by protein, not by LPS. However, the reason for the partial inhibition by trypsin treatment is not known.
We also confirmed recent reports that CD14 signalling [17] and TLR4 signalling [21] are important pathways in mediating the activation of macrophages in response to human hsp60. Although the TLR4 signalling of hsp60 was examined previously in murine macrophages [21], this study is the first to show that human TLR4 can confer responsiveness to human hsp60. Human hsp60 has been proposed as a danger signal of stressed or damaged cells [15]. The role of hsp60 in the pathogenesis of periodontal diseases is not fully elucidated, however, considering the abundant expression of hsp60 and also TLR4 [22] by various cell types in the periodontitis lesion and the stimulatory effect on the innate immune system, a pathological role of hsp60 should be expected.
In addition to hsp60, hsp70 [23] and hsp90 [24] have also been implicated in the macrophage activation which in turn results in TNF gene induction using similar signalling pathway. Therefore, apart from the role of chaperonin, these families of protein can also induce tissue pathology suggests that they may play important roles in many aspects of inflammation seen in both autoimmune and infectious diseases such as rheumatoid arthritis [25] and atherosclerosis [26].
This study demonstrated the possibility that proinflammatory cytokine production induced by autologous hsp60 could be another pathway leading to periodontal tissue destruction. The bacterial homologueue of human hsp60, P. gingivalis GroEL and A. actinomycetemcomitans GroEL did not show TNF-α inducing activity. A. actinomycetemcomitans GroEL has, however, been reported to act as the potent bone-resorbing factor in a murine calvarial resorption assay [27]. In spite of the strong bone-resorbing activity of bacterial components such as LPS, several lines of evidence suggest that active bone resorption is induced by mediators elaborated from host cells during immune response [1]. Although this activity has not been reported for either P. gingivalis GroEL or human hsp60, further study is clearly needed to clarify this issue.
Our findings suggested a possible new mechanism for human hsp60 in inflammatory periodontal disease, in which proinflammatory cytokines are directly induced. This is in addition to a possible cross-reactive immune response mediated by molecular mimicry of epitopes of microbial and human hsp60. Further studies are clearly needed to clarify the role of hsp60 in periodontal disease. This will, in turn, lead to develop new therapeutic modalities in controlling inflammation in periodontal diseases.
Acknowledgments
The authors would like to thank S. Akashi and K. Miyake (Department of Immunology, Saga Medical School) for kindly providing HTA125 and H. Kumada and T. Umemoto (Department of Microbiology, Kanagawa Dental University). The authors are also grateful to G. J. Seymour (Oral Biology and Pathology, Department of Dentistry, The University of Queensland) for critical reading of this manuscript. This work was supported by grants from the Ministry of Education, Science Sports and Culture of Japan (13470462, 10470458, 10307054).
REFERENCES
- 1.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontology. 2000;1997:112–43. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 2.Munk ME, Schöel B, Modrow S, Karr RW, Young RA, Kaufmann SHE. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989;143:2844–9. [PubMed] [Google Scholar]
- 3.Maeda H, Miyamoto M, Hongyo H, Nagai A, Kurihara H, Murayama Y. Heat shock protein 60 (GroEL) from Porphyromonas gingivalis. Molecular cloning and sequence analysis of its gene and purification of the recombinant protein. FEMS Microbiol Lett. 1994;119:129–36. doi: 10.1111/j.1574-6968.1994.tb06879.x. [DOI] [PubMed] [Google Scholar]
- 4.Nakano Y, Inai Y, Yamashita Y, et al. Molecular and immunological characterization of a 64-kDa protein of Actinobacillus actinomyctemcomitans. Oral Microbiol Immunol. 1995;10:151–9. doi: 10.1111/j.1399-302x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 5.Kiessling R, Gronberg A, Ivanyi J, et al. Role of hsp60 during autoimmune and bacterial inflammation. Immunol Rev. 1991;121:91–112. doi: 10.1111/j.1600-065x.1991.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–93. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda H, Miyamoto M, Kokeguchi S, et al. Epitope mapping of heat shock protein 60 (GroEL) from Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2000;28:219–24. doi: 10.1111/j.1574-695X.2000.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 8.Friedland JS, Shattock R, Remick DG, Griffin GE. Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells. Clin Exp Immunol. 1993;91:59–62. doi: 10.1111/j.1365-2249.1993.tb03354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Doerfler M, Lee TC, Guillemin B, Rom WN. Mechanisms of stimulation of interleukin-1β and tumor necrosis factor-α by Mycobacterium tuberculosis components. J Clin Invest. 1993;91:2076–83. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peetermans WE, Raats CJI, Langermans JAM, van Furth R. Mycobacterial heat-shock protein 65 induces proinflammatory cytokines but does not activate human mononuclear phagocytes. Scand J Immunol. 1994;39:613–7. doi: 10.1111/j.1365-3083.1994.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 11.Retzlaff C, Yamamoto Y, Hoffman PS, Friedman H, Klein TW. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage culture. Infect Immun. 1994;62:5689–93. doi: 10.1128/iai.62.12.5689-5693.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabona P, Reddi K, Khan S, et al. Homogeneous Escherichia coli chaperonin 60 induces IL-1β and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J Immunol. 1998;161:1414–21. [PubMed] [Google Scholar]
- 13.Verdegaal EME, Zegveld ST, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–76. [PubMed] [Google Scholar]
- 14.Galdiero M, DeLero GC, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect Immun. 1997;65:699–707. doi: 10.1128/iai.65.2.699-707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–9. [PubMed] [Google Scholar]
- 16.Kol A, Bourcier T, Lichtman AH, Libby P. Clamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–7. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Heat shock protein (HSP) 60 activates the innate immune response. CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–7. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Tabeta K, Yoshie H, Yamazaki K. Characterization of serum antibody to Actinobacillus actinomycetemcomitans GroEL-like protein in periodontitis patients and healthy subjects. Oral Microbiol Immunol. 2001;16:290–295. doi: 10.1034/j.1399-302x.2001.016005290.x. [DOI] [PubMed] [Google Scholar]
- 19.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga T, Kusuzaki T, Asakawa H, Senpuku H, Nishihara T, Noguchi T. The 64-kilodalton GroEL-like protein of Actinobacillus actinomycetemcomitans. J Periodont Res. 1993;28:475–7. doi: 10.1111/j.1600-0765.1993.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi K, Burkart V, Flohe S, Kolb H. Heat shock protein 60 is a putative endogeneous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 22.Tabeta K, Yamazaki K, Akashi S, et al. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect Immun. 2000;68:3731–5. doi: 10.1128/iai.68.6.3731-3735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asea A, Kraeft S-K, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 24.Byrd C, Bornmann W, Erdjument-Bromage H, et al. Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1999;96:5645–50. doi: 10.1073/pnas.96.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Graeff-Meeder ER, Rijkers GT, Voorhorst-Ogink MM, et al. Antibodies to human hsp60 in patients with juvenile chronic arthritis, diabetes mellitus, and cystic fibrosis. Pediatric Res. 1993;34:424–8. doi: 10.1203/00006450-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor−α and matrix metalloproteinase expression. Circulation. 1998;98:300–7. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 27.Kirby AC, Meghji S, Nair SP, et al. The potent bone-resorbing mediator of Actinobacillus actinomycetemcomitans is homologous to the molecular chaperone GroEL. J Clin Invest. 1995;96:1185–94. doi: 10.1172/JCI118150. [DOI] [PMC free article] [PubMed] [Google Scholar]