Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease primarily affecting cartilaginous joints but also extra-articular tissues such as the nose and upper respiratory tract. We have investigated extra-articular cartilage involvement in two commonly used animal models for RA, collagen-induced and pristane-induced arthritis, by immunizing rats with different susceptibility to disease (LEW.1 A, LEW.1F and DA rats). We found that nasal and tracheolaryngeal cartilage is affected in LEW.1 A and DA rats to varying degrees in collagen-induced arthritis but not in any strain in the pristane-induced model. Antibodies to matrilin-1, a cartilage-specific protein expressed mainly in tracheolaryngeal and nasal cartilage but not in joints, were positively associated with the presence of inflammation in nasal cartilage. In contrast, no antibody response to matrilin-1 could be detected in pristane-induced arthritis. In addition, nasal vaccination with collagen type II prior to immunization in DA rats significantly decreased the antibody response to matrilin-1 at day 56, but not at earlier time points, indicating a late protective effect on extra-articular cartilage. We conclude that pristane-induced arthritis is a joint-specific model whereas collagen-induced arthritis affect joints as well as extra-articular cartilage. Furthermore, collagen immunization induces an antibody response to matrilin-1.
Keywords: arthritis, CIA, collagen, matrilin-1, PIA
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic disorder affecting approximately 1% of the population. The most obvious symptom related to the disease is inflammation and deformation of the joints, caused by an inflammatory attack of cartilage. However, symptoms originating from cartilage tissue in the nose and throat are also present. Joints that are located in the area around the larynx (the cricothyroid and cricolaryngeal joints) are affected in some patients and in a thorough examination 60% of RA patients were found to present signs of laryngeal involvement [1]. In addition, reports of destruction of nasal cartilage causing saddle nose deformity or septal perforation have been demonstrated in seronegative as well as seropositive patients [2–4]. Nasal tissue has in several cases been analysed for vasculitis with negative result, despite signs of Raynaud's phenomenon in a few. Cartilage tissue, however, was never investigated. Septal perforation has also been reported in related entities such as psoriatic arthritis, systemic lupus erythematosus, progressive systemic sclerosis and mixed connective tissue disease [4]. The nasal and tracheolaryngeal symptoms found in RA patients have been poorly investigated and the target antigen causing inflammation and/or cartilage destruction has not been defined. However, collagen type II (CII) has been proven in several publications to be involved in RA pathogenesis, as has collagen type IX (CIX) and type XI (CXI) [5–8]. In addition, antibodies to CIX and CXI, but not CII, have been found in patients with cartilage graft resorption in nasal surgery, indicating that CIX and CXI have a role in nasal cartilage destruction [9].
Relapsing polychondritis is another human disorder affecting joints, nose and tracheolaryngeal cartilage [10] in which CII [11–13], CIX and CXI [14–16] have been proven to be involved in the pathogenesis. Recent publications from our group also describe a prominent role for matrilin-1, both in animal models mimicking symptoms of relapsing polychondritis (MIRP – matrilin-1 induced relapsing polychondritis) [17] and in humans [18]. Matrilin-1 is a cartilage-specific matrix protein mainly expressed in tracheal, but not joint, cartilage [19] and thereby primarily involved in extra-articular cartilage inflammation. However, despite the fact that extra-articular symptoms are presented in RA, no antibody response to matrilin-1 has yet been detected during investigation of patient sera [18].
Collagen-induced arthritis (CIA) is a widely used animal model mimicking the joint inflammation seen in human RA. In recent years pristane, a mineral oil (2,6,10,14-tetramethylpentadecane), has also been used to induce arthritis in mice and rats [20,21]. It needs to be emphasized, however, that pristane-induced arthritis in mice and rats are two different models with different induction requirements and different biology and therefore cannot be compared directly [21]. The pristane-induced arthritis model (PIA) in the rat has many similarities to the phenotypes found in CIA but differences have also been reported. In both models severe paw arthritis is induced with similar histology [21,22]. T cells have been proven to be important in both models [23] as has the influence of both MHC and non-MHC genes; however, different MHC genes seem to be involved in disease susceptibility [21,24,25]. Anti-CII antibodies, a common feature of CIA, have so far not been detected in PIA. Similarly to PIA use of incomplete Freund's adjuvant (oil-induced arthritis), has been described to induce peripheral arthritis [26,27].
Earlier reports on arthritis models have focused mainly on the involvement of joint inflammation, the most noticeable sign of RA, and not on the other components containing cartilage. To further understand the pathogenesis of cartilage destruction in RA and related disorders and to describe the most accurate model of RA by considering the inflammation of different cartilage tissues we have analysed the rat models of CIA and PIA. Examination of nasal tissue is particularly interesting since nasal vaccination is commonly used as a therapeutic method for autoimmune diseases and allergic disorders in animal models and to some extent in humans.
We found that extra-articular cartilage is affected in the CIA model but not at any time point in the pristane-induced model. We did not find any antibody response against any cartilage protein investigated in the PIA rats. However, in the CIA rats, antibodies to matrilin-1 correlated positively with the presence of inflammation in the nasal cartilage. In addition, the antibody titre to matrilin-1 was significantly lower in the CII immunized rats nasally vaccinated with CII indicating that CII nasal vaccination has an effect on extra-articular, as well as joint, cartilage.
MATERIALS AND METHODS
Animals
Rats of three different strains, LEW.1 A, LEW.1F and DA, were used, originally obtained from ZFV (Zentralinstitut Fur Versuchstierzucht, Hanover, Germany). They were bred and kept in the animal department of Medical Inflammation Research in Lund. The animals were kept in a climate-controlled environment (temperature and humidity) with cycles of 12h light/dark and sound, housed two to three individuals in each polystyrene cage containing wood shavings. They were allowed water ad libitum and fed standard rodent chow. They were found to be free from common pathogens including Sendai virus, Hantaan virus, corona virus, reovirus, cytomegalovirus and mycoplasma pulmonis. All animals were immunized at an age of 8–13 weeks and were age-matched before the experiments.
Induction of disease and nasal vaccination procedure
Rat CII and bovine matrilin-1 were purified as previously described [28,29]. Rats were immunized intradermally (i.d.) at the base of the tail with 150 μg of protein emulsified with incomplete Freund's adjuvant (Difco, Detroit, IL, USA) or with 150 μl of pristane (Aldrich Inc., Milwaukee, WI, USA). The rats were evaluated for disease three times a week and scored according to an established protocol whereby each paw reaches a maximum of 15 points. The nasal vaccination protocol has been described previously [30]. Briefly, female DA rats were vaccinated by nasal installation of CII or acetic acid (control) prior to immunization with CII or pristane.
Antibody detection
Blood was collected from the vein of the tail and the sera were stored at – 20°C until assayed. To evaluate antibody responses ELISAs were performed. Plates (Costar, Corning Inc., NY, USA) were coated with 1 μg/ml of matrilin-1 or 10 μg/ml (1 μg/ml in Fig. 4) of CII in PBS + 0·02% sodium azide overnight at 4°C. They were washed in washing-buffer (0·1 m Tris-Cl + 0·05% Tween 20) and incubated for 2h at room temperature with sera diluted 1: 1000 (antibodies to CII) (1/100 in Fig. 4) and 1 : 100 (antibodies to matrilin-1) in PBS buffer (PBS + 0·05% Tween 20 + 0·02% sodium azide). Washing was repeated and the plates were then incubated for another 2 h with conjugates detecting IgG, donkey-α-rat (Jackson ImmunoResearch laboratories Inc., West Grove, PA, USA). The plates were developed with p-nitrophenol as the substrate and the amount of antibody was estimated as absorbency at 405 nm by using a Titertek Multiscan filterphotometer. A positive control consisting of a mixture of sera from DA, LEW and E3 rats immunized with the respective protein was used on all plates assayed.
Fig. 4.
Antibody levels of total IgG to CII and matrilin-1 at days 9, 12, 15 and 21 to CII and matrilin-1. Sera were diluted 1/100, prepared and analysed in the same assay. Titer expressed as OD level at 405 nm *P < 0·05. □, Anti-CII; ▪, anti-matrilin-1.
Western blot
Western blotting was performed according to established methods. Ready gels precast for polyacrylamide electrophoresis (Bio-Rad Laboratories, CA, USA) were used for protein loading and transferred onto a nitrocellulose membrane. Gels were blotted with monoclonal mouse-α-bovine antimatrilin-1 (AM5) antibodies (Hansson, unpublished) and detected with peroxidase conjugated goat-α-mouse IgG (H + l) (Jackson ImmunoResearch laboratories Inc., West Grove, PA, USA). Colour was developed by DAB. Silver staining of the investigated samples was performed as well.
Histological procedure
Tissue samples, tracheolaryngeal-, nasal-, ear- and joint cartilage were processed for light microscopy according to standard procedures. Briefly the tissue samples were fixed in 4% paraformaldehyde for 24h, dehydrated and embedded in paraffin, sectioned at 5 mm and stained with haematoxylin and erythrosine. Noses and paws were decalcified in EDTA solution for 2–3 weeks prior to staining. Sections were evaluated according to a scoring system previously described [17].
Statistic analysis
Antibody levels were analysed with Mann–Whitney U-test and correlation with simple regression. Unless otherwise indicated, P < 0·05 was considered significant.
RESULTS
Extra-articular cartilage is attacked in CIA but not in PIA
Three strains of rats (LEW.1 A, LEW.1F and DA) were immunized with CII according to established protocols. As expected from the expression of the MHC haplotypes, only the RT1a strains LEW.1 A and DA developed arthritis. No additional clinical signs or rheumatoid noduli were detected. When investigating sections of extra-articular cartilage structures (nose, trachea and ear) at different time points after immunization, inflammatory lesions of the nasal and tracheolaryngeal cartilage were detected in the acute phase (around onset day) (Table 1, Fig. 1a,b). Mild inflammation with tissue reorganization was found in some individuals in the late chronic phase (Table 1, Fig. 1c). The lesions consisted mainly of neutrophils and macrophages but also lymphocytes. In the acute phase eosinophils were present. Nasal cartilage was more severely affected than laryngeal, while ear cartilage was not affected in any rat. The CII preparation used for immunization and antibody detection was analysed by Western blotting for contamination of matrilin-1 but with a negative result (Fig. 2).
Table 1.
Female rats immunized with collagen type II or pristane
| Collagen type II immunized rats † | Pristane immunized rats † | |||||
|---|---|---|---|---|---|---|
| LEW.1 A | LEW.1 F | DA | LEW.1 A | LEW.1 F | DA | |
| Incidence | 4/11 | 0/11 | 9/15 | 4/5 | 3/3 | 15/16 |
| Day of onset | 30 ± 8 | 21 ± 5 | 36 ± 33 | 19 ± 3 | 17 ± 6 | |
| Max score | 14 ± 3 | 29 ± 17 | 19 ± 17 | 28 ± 5 | 30 ± 21 | |
| Histological score ‡ | ||||||
| Acute phase | ||||||
| Larynx | A2 (2/4) | N (3) | A2 (3/4) | N (2) | N (2) | N (11) |
| Nose | A2 (3/4) | N (3) | A2 (4/4) | N (2) | N (2) | N (11) |
| Chronic phase | ||||||
| Larynx | A1 (5/7) | N (8) | H1 (1/11) | N (3) | N (1) | N (5) |
| Nose | A1, H2 (4/7) | N (8) | H2 (2/11) | N (3) | N (1) | N (5) |
Rats immunized intradermally with 150μg of collagen type II + incomplete Freund's adjuvant or 150μl of pristane.
Mean histological score of histologically affected rats. Numbers of animals in parenthesis correspond to number of affected animals/total number of rats analysed or in cases with normal histology, number of rats analysed. Histological scores: A, acute inflammatory phase with possible scoring A1–A3; H, healing phase with possible scoring H1–H3; N, normal histology. For more detailed scoring description see Material and methods.
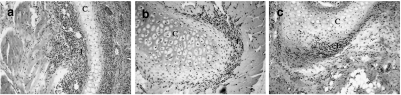
Fig. 1.
Sections from rats immunized with CII showing inflammatory infiltrations close to the cartilage in (a) nasal cartilage from DA at day 16 (b) tracheolaryngeal cartilage from LEW.1 A at day 27 and (c) nasal cartilage from LEW.1 A at day 146. C =cartilage, I =inflammatory infiltrate. Original magnification ×70.
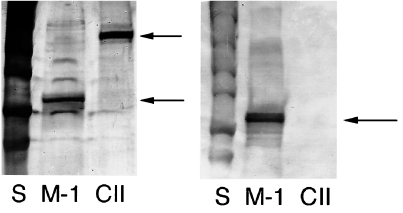
Fig. 2.
Western blot and silverstaining. (a) Silverstaining and (b) Western blot (reduced conditions) of the protein batches of CII and matrilin-1 that were used. S, standard; m-1, matrilin-1; CII, collagen type II. Arrows indicating positive signals from m-1 and CII, showing that no m-1 was found in the CII preparation.
Rats of the same strains and numbers were immunized with pristane. Despite severe arthritis, no sign of inflammation could be detected clinically or in the histological sections (days 16, 35, 75 and 146) of the nose, larynx, trachea or ear in any animal or at any time point of disease (Table 1, Fig. 3).
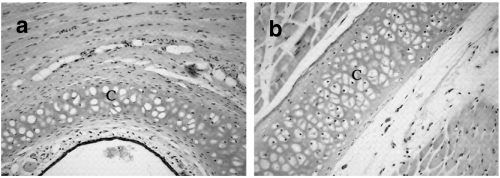
Fig. 3.
Sections from LEW.1 A rat immunized with pristane showing normal cartilage structure without any sign of inflammation (a) nasal septum and (b) tracheolaryngeal cartilage. C =cartilage, Original magnification ×70.
In order to investigate the same cartilage structures in mice we immunized QD mice. These mice are known to be very susceptible to MIRP (Hansson, unpublished observation) and CIA [31]. Two out of 10 QD mice showed mild signs of microscopical inflammation in the nose and in the respiratory tract (data not shown).
Matrilin-1 antibodies are produced in CIA but not in PIA
Rats of all strains immunized with CII were analysed for an IgG antibody response to matrilin-1 at various time points throughout the course of disease. LEW.1 A rats were found to have significantly higher titres than the LEW.1F and DA rats at day 35 (P < 0·05) and 75 (P < 0·001), while very late in the disease, on day 146, both LEW strains mounted higher titres (P < 0·05) than the DA strain (data not shown). All titres were approximately 100 times less than the ones found in rats immunized with matrilin-1 [17]. Antibody titres to CII differed from the pattern of matrilin-1 antibodies at day 35 as LEW.1F rats responded with lower titres compared with LEW.1 A and DA (P < 0·001) rats.
When analysing sera from the DA rats at short intervals before arthritis onset we could detect an increase in antibody levels to CII prior to an increase in antimatrilin-1 antibodies (Fig. 4). We sacrificed animals for histological analysis at the same time points as the sera analysis (days 9, 12, 15 and 21) and concluded that antibodies to both CII and matrilin-1 concur with the positive histological findings as mild inflammatory changes were detected on day 12 in 50% of the animals. On day 15 all rats showed mild to moderate nasal inflammation and mild laryngeal inflammation was seen in one rat.
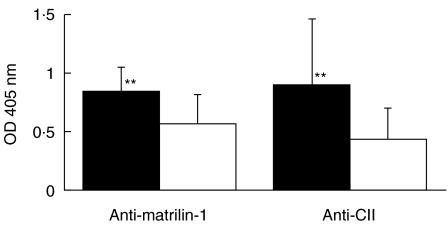
No clinical signs originating from the larynx or the nose could be detected in any animal. However, animals presenting microscopical nasal cartilage inflammation mounted significantly higher antibody titres to matrilin-1 as well as to CII on day 35 than rats with normal histological sections. This indicates that both proteins are engaged in nasal chondritis (Fig. 5).
Fig. 5.
Antibody levels of total IgG to CII and matrilin-1 at day 35. All rats (LEW.1 A, LEW.1F and DA) immunized with CII (not the nasally vaccinated ones) were included and divided into two groups regarding positive or negative sign of inflammation in sections of the nasal cartilage. Sera were diluted 1/100 for matrilin-1 and 1/1000 for CII. **P < 0·01. ▪, Positive histology; □, negative histology.
All rats immunized with pristane were analysed at various time points (days 35, 75 and 146) for antibodies to CII and matrilin-1, but with negative results. Also mice immunized with CII were investigated for antibody production to matrilin-1, but only a very weak response was detected (data not shown).
CII nasal vaccination induces a lower antibody response to matrilin-1 in CIA rats
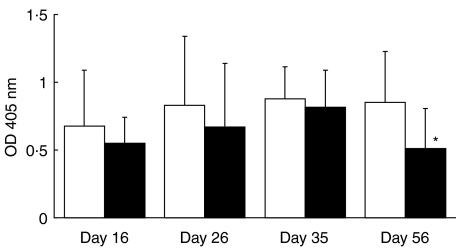
We have previously published data demonstrating amelioration in joint inflammation when nasally vaccinating with CII prior to CII immunization [30]. We used sera from this experiment [30] to investigate the nasal vaccination effect on extra-articular cartilage by measuring the antibody response to matrilin-1. Significant differences were found at day 56 after immunization but not at earlier time points (days 16, 26 or 36) (Fig. 6). The total IgG response to matrilin-1 was lower in the CII vaccinated group compared with the control group, which were nasally vaccinated with acetic acid, and a correlation with the arthritic max score was found (P < 0·05, R = 0·65).
Fig. 6.
Antibody levels of total IgG to matrilin-1 at days 16, 26, 36 and 56. All rats (DA) were nasally vaccinated with CII prior to CII immunization. Sera were diluted 1/100. *P < 0·05. □, Vaccinated with Hac; ▪, vaccinated with CII.
Cartilage tissue was investigated at days 16 and 36 in all nasally vaccinated rats but only a weak amelioration in nasal cartilage inflammation could be detected in the CII nasally vaccinated ones and only at day 36 (data not shown). These histological data support the antimatrilin-1 antibody results: that nasal vaccination has a stronger beneficial effect on extra-articular cartilage inflammation in the late phase of the disease course rather than in the acute one. Rats nasally vaccinated with CII and immunized with pristane did not produce any antibodies to any of the investigated cartilage proteins.
DISCUSSION
CIA and PIA are both established models for the human disease RA. However, some clinical and laboratory differences between these models have been reported. In this paper we present a major difference concerning target antigens and tissues. In CIA extra-articular cartilage as well as joint cartilage were affected, while pristane-induced cartilage inflammation was restricted to joints. In addition, rats with inflammation in the nasal cartilage generated significantly higher levels of antibodies to matrilin-1 and to CII than rats with no inflammation. We also show that nasal CII vaccination prior to CII immunization in rats affect extra-articular cartilage destruction as evidenced by lower antibody responses to matrilin-1.
The presence of nasal and laryngeal inflammation when immunizing with CII is most probably induced primarily by CII, since we did not detect any matrilin-1 in the CII preparation used for immunization. Secondary to induction, and caused by cartilage destruction and release of fragments, we propose that matrilin-1 becomes an important target antigen and that antibodies to matrilin-1 further nourish the inflammatory process in extra-articular cartilage. Thus, CII immunization induces matrilin-1 antibodies, which most probably participate in the subsequent inflammatory process detected in nasal and laryngeal cartilage. The lack of inflammation in ear cartilage could be explained by the fact that this is an elastic cartilage without any easily accessible CII in the perichondrium and, in addition, the expression of matrilin-1 in auricular cartilage is very low [19]. Of the three rat strains investigated, LEW.1A was found to respond with the highest antibody levels to matrilin-1 at all time points. Antibodies to matrilin-1 were also detected in the LEW.1F strain, despite the lack of arthritis and a low anticollagen response, indicating the CII response in CIA to be high enough to induce subclinical inflammation in laryngeal and/or nasal tissue not detected by histology but which initiates a secondary response to matrilin-1. This hypothesis is likely, as mild arthritis in the joints have been found in 20% of LEW.1F rats when immunized with CII in a similar experiment in our laboratory (Lu, unpublished observation). An alternative explanation could be that despite a negative Western blot, a minor contamination of matrilin-1 is present in the CII preparation. A transient antimatrilin-1 response was found in the DA rats supporting the role of matrilin-1 in the secondary phase of disease since DA rats do not respond clinically to matrilin-1 immunization while both LEW strains are susceptible [17]. The lack of significant findings in mice, histological or serological (antimatrilin-1 antibodies), could depend on species variations since different cartilage proteins might be present in different concentrations in the various cartilaginous tissues depending on the species examined.
A suggested method to treat autoimmune diseases is nasal vaccination whereby the candidate autoantigen is instilled in the nose. The method has proven successful in ameliorating arthritis in rats as well as mice [30,32]. However, according to our results, care should be taken not to induce a response to new target antigens, such as matrilin-1, by the locally induced nasal inflammation. We have shown recently that the level of antibodies to matrilin-1 is increased in a subgroup of patients with relapsing polychondritis, especially in those individuals with severe respiratory distress [18]. On the other hand, we show in this study that the antibody response to matrilin-1 is significantly lower in the CIA rats treated with nasal instillations of CII than in the controls. This indicates a decreased inflammatory activity to extra-articular cartilage, which is also most probably of benefit to the patients, particularly those with a predisposition to develop nasal or respiratory symptoms. Consequently, titration of the nasally instilled protein and a thorough control of its local effects are of great importance.
It is not known whether the pathogenesis of PIA involves a target antigen in the joints, or the identity of such antigens, as has been reviewed recently [33]. In the present experiments we could not detect any antibody response to CII or matrilin-1 in any animal immunized with pristane, despite very severe arthritis. The lack of a B-cell response in our study supports the role of T-cells in the PIA model that has been emphasized in previous publications [21].
The fact that PIA is a joint-specific model, which does not produce any antibodies to cartilage-specific proteins such as CII or matrilin-1, makes it questionable as an animal model for the subtypes of RA in which autoimmune responses to cartilage are involved, if concern is to be given to cartilage involvement. The CIA model on the other hand, mimics these subforms of the human disease by engaging joints as well as nasal cartilage and cartilage of the respiratory tract. We conclude that both models are valuable for arthritis and chondritis research, but awareness of their differences should be taken in account when interpreting results and correlating them to human disease.
Acknowledgments
We would like to thank Prof Dick Heinegård for providing matrilin-1 and Carlos Palestro for taking care of the animals. The work was supported by grants from the Anna Greta Crafoord Foundation for Rheumatological Research, King Gustaf V’s 80-year foundation, Greta and Johan Kock’s Foundations, Alfred Österlund's foundation, the Swedish Association against Rheumatism and the Swedish Medical Research Council.
REFERENCES
- 1.Geterud A, Bake B, Berthelsen B, et al. Laryngeal involvement in rheumatoid arthritis. Acta Otolaryngol. 1991;111:990–8. doi: 10.3109/00016489109138441. [DOI] [PubMed] [Google Scholar]
- 2.Mathews JL, Ward JR, Samuelson CO, et al. Spontaneous nasal septal perforation in patients with rheumatoid arthritis. Clin Rheumatol. 1983;2:13–8. doi: 10.1007/BF02032063. [DOI] [PubMed] [Google Scholar]
- 3.Harris BK, Tello R. Septal perforation with saddle nose deformity in rheumatoid arthritis. Arthritis Rheum. 1979;22:101–2. doi: 10.1002/art.1780220124. [DOI] [PubMed] [Google Scholar]
- 4.Willkens RF, Roth GJ, Novak A, et al. Perforation of nasal septum in rheumatic diseases. Arthritis Rheum. 1976;19:119–21. doi: 10.1002/art.1780190122. [DOI] [PubMed] [Google Scholar]
- 5.Andriopoulos NA, Mestecky J, Miller EJ, et al. Antibodies to human native and denatured collagens in synovial fluids of patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1976;6:209–12. doi: 10.1016/0090-1229(76)90112-4. [DOI] [PubMed] [Google Scholar]
- 6.Charriere G, Hartmann DJ, Vignon E, et al. Antibodies to types I, II, IX, and XI collagen in the serum of patients with rheumatic diseases. Arthritis Rheum. 1988;31:325–32. doi: 10.1002/art.1780310303. [DOI] [PubMed] [Google Scholar]
- 7.Morgan K, Clague RB, Collins I, et al. Incidence of antibodies to native and denatured cartilage collagens (types II, IX, and XI) and to type I collagen in rheumatoid arthritis. Ann Rheum Dis. 1987;46:902–7. doi: 10.1136/ard.46.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan K, Buckee C, Collins I, et al. Antibodies to type II and XI collagens: evidence for the formation of antigen specific as well as cross reacting antibodies in patients with rheumatoid arthritis. Ann Rheum Dis. 1988;47:1008–13. doi: 10.1136/ard.47.12.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bujia J, Alsalameh S, Naumann A, et al. Humoral immune response against minor collagens type IX and XI in patients with cartilage graft resorption after reconstructive surgery. Ann Rheum Dis. 1994;53:229–34. doi: 10.1136/ard.53.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAdam LP, O’Hanlan MA, Bluestone R, et al. Relapsing polychondritis. prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976;55:193–215. [PubMed] [Google Scholar]
- 11.Ebringer R, Rook G, Swana GT, et al. Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis. 1981;40:473–9. doi: 10.1136/ard.40.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foidart JM, Abe S, Martin GR, et al. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med. 1978;299:1203–7. doi: 10.1056/NEJM197811302992202. [DOI] [PubMed] [Google Scholar]
- 13.Terato K, Shimozuru Y, Katayama K, et al. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990;33:1493–500. doi: 10.1002/art.1780331006. [DOI] [PubMed] [Google Scholar]
- 14.Alsalameh S, Mollenhauer J, Scheuplein F, et al. Preferential cellular and humoral immune reactivities to native and denatured collagen types IX and XI in a patient with fatal relapsing polychondritis. J Rheumatol. 1993;20:1419–24. [PubMed] [Google Scholar]
- 15.Joliat T, Seyer J, Bernstein J, et al. Antibodies against a 30 kilodalton cochlear protein and type II and IX collagens in the serum of patients with inner ear diseases. Ann Otol Rhinol Laryngol. 1992;101:1000–6. doi: 10.1177/000348949210101207. [DOI] [PubMed] [Google Scholar]
- 16.Yang CL, Brinckmann J, Rui HF, et al. Autoantibodies to cartilage collagens in relapsing polychondritis. Arch Dermatol Res. 1993;285:245–9. doi: 10.1007/BF00371591. [DOI] [PubMed] [Google Scholar]
- 17.Hansson AS, Heinegard D, Holmdahl R. A new animal model for relapsing polychondritis, induced by cartilage matrix protein (matrilin-1) J Clin Invest. 1999;104:589–98. doi: 10.1172/JCI5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson A-S, Burkhardt H, Piette J-C, et al. The occurrence of autoantibodies to matrilin-1 reflects a tissue specific response to cartilage of the respiratory tract in patients with relapsing polychondritis. Arthritis Rheum. 2001;44:2402–12. doi: 10.1002/1529-0131(200110)44:10<2402::aid-art405>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Paulsson M, Heinegard D. Radioimmunoassay of the 148-kilodalton cartilage protein. Distribution of the protein among bovine tissues. Biochem J. 1982;207:207–13. doi: 10.1042/bj2070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter M, Wax JS. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. J Immunol. 1981;127:1591–5. [PubMed] [Google Scholar]
- 21.Vingsbo C, Sahlstrand P, Brun JG, et al. Pristane-induced arthritis in rats. a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–83. [PMC free article] [PubMed] [Google Scholar]
- 22.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–68. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldschmidt TJ, Holmdahl R. Anti-T cell receptor antibody treatment of rats with established autologous collagen-induced arthritis: suppression of arthritis without reduction of anti-type II collagen autoantibody levels. Eur J Immunol. 1991;21:1327–30. doi: 10.1002/eji.1830210536. [DOI] [PubMed] [Google Scholar]
- 24.Holmdahl R, Vingsbo C, Hedrich H, et al. Homologous collagen-induced arthritis in rats and mice are associated with structurally different major histocompatibility complex DQ-like molecules. Eur J Immunol. 1992;22:419–24. doi: 10.1002/eji.1830220220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths MM, DeWitt CW. Genetic control of collagen-induced arthritis in rats: the immune response to type II collagen among susceptible and resistant strains and evidence for multiple gene control. J Immunol. 1984;132:2830–6. [PubMed] [Google Scholar]
- 26.Cannon GW, Woods ML, Clayton F, et al. Induction of arthritis in DA rats by incomplete Freund's adjuvant. J Rheumatol. 1993;20:7–11. [PubMed] [Google Scholar]
- 27.Kleinau S, Erlandsson H, Holmdahl R, et al. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J Autoimmun. 1991;4:871–80. doi: 10.1016/0896-8411(91)90050-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmdahl R, Moran T, Andersson M. A rapid and efficient immunization protocol for production of monoclonal antibodies reactive with autoantigens. J Immunol Meth. 1985;83:379–84. doi: 10.1016/0022-1759(85)90260-1. [DOI] [PubMed] [Google Scholar]
- 29.Paulsson M, Heinegard D. Purification and structural characterization of a cartilage matrix protein. Biochem J. 1981;197:367–75. doi: 10.1042/bj1970367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu S, Holmdahl R. Different therapeutic and bystander effects by intranasal administration of homologous type II and type IX collagens on the collagen-induced arthritis and pristane-induced arthritis in rats. Clin Immunol. 1999;90:119–27. doi: 10.1006/clim.1998.4615. [DOI] [PubMed] [Google Scholar]
- 31.Jansson L, Holmdahl R. Oestrogen-induced suppression of collagen arthritis; 17 beta-oestradiol is therapeutically active in normal and castrated F1 hybrid mice of both sexes. Clin Exp Immunol. 1992;89:446–51. doi: 10.1111/j.1365-2249.1992.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers LK, Seyer JM, Stuart JM, et al. Suppression of murine collagen-induced arthritis by nasal administration of collagen. Immunology. 1997;90:161–4. doi: 10.1046/j.1365-2567.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmdahl R, Lorentzen JC, Lu S, et al. Arthritis induced in rats with nonimmunological adjuvants as models for rheumatoid arthritis. Immunol Rev. in press. [DOI] [PubMed]