Abstract
The overall role of complement in the host–pathogen relationship is now well understood. However, its involvement at a chronic stage of infection, such as chronic hepatitis C, is less well documented. Here, results are reported which point to the use of specific C4 monitoring in the follow-up of HCV patients. This study concerns 66 patients with chronic HCV infection, treated with interferon alpha 2b alone or with interferon alpha 2b + ribavirin, and 50 healthy adults as controls. Complement blood tests were performed to measure C1q, C3, C4, mannan binding lectin (MBL), C1s-C1 inhibitor complexes, total (CH50) and C4 (C4H) haemolytic activity; specific C4 activity was taken as the C4H/C4 protein ratio. Rheumatoid factor (RF) levels were also measured. A significant reduction in CH50 and specific C4 activity in HCV patients, compared with the healthy controls, was observed before the onset of treatment; the other parameters were not affected and no C1s-C1 inhibitor complexes were detected. At the same time, a significant reduction in specific C4 activity was observed in relapsers compared with sustained responders. These results point to a potential predictive function of C4 specific activity to monitor the response to therapy. Restoration of specific C4 activity at 6 months was better in responders than in non-responders. Complement activation in chronic hepatitis C does not seem to involve the C1 stage of the classical pathway. A negative correlation between specific C4 activity and RF titres suggests a possible involvement of RF in C4 activation, via the lectin pathway. Specific C4 monitoring appears to be a valuable tool for the follow-up of chronic hepatitis C treatment, together with the other conventional investigations.
Keywords: complement, HCV, specific C4, rheumatoid factor
INTRODUCTION
Hepatitis C virus (HCV) is the major aetiological agent of non-A, non-B viral hepatitis [1]. Chronic infection causes considerable morbidity due to a high percentage of patients progressing to cirrhosis, with a significant risk for developing hepatocellular carcinoma [2].
The mechanisms responsible for HCV persistence and disease pathogenesis are not well understood, although it is likely that interactions between HCV and the host immune system play an important role. HCV inhibits the protein kinase PKR, a mediator of interferon activity, with subsequent impairment of the ability of interferon to protect cells from viral infection [3,4].
Complement represents a significant non-specific host defence system involved in the protection of the host from virus infection [5]. To escape this protection, viruses are able to express host-homologous proteins, or to borrow cell membrane proteins from the host with complement regulatory activity, protecting viral particles from neutralization by complement [6].
The involvement of complement in the course of HCV infection has been poorly documented. A few studies show changes in the acute phase complement components in HCV patients receiving interferon alpha 2b (Dr L Varga, personal communication), or an association between HCV infection and a cold-dependent activation of the classical complement pathway [7,8] or hypocomplementemia associated with cryoglobulinemia [9].
The present work deals with sensitive functional assays of complement in blood to monitor chronic hepatitis C treatment. C4 specific activity appears as a valuable parameter for predic-ting and monitoring interferon alpha 2b or interferon alpha 2b + ribavirin therapy. Moreover, C4 activation seems to be associated with rheumatoid factor (RF). This activation, in an inflammatory context, is likely to play a role in hepatocyte proliferation.
MATERIALS AND METHODS
Patients
Sixty-six patients with chronic hepatitis C (mean age: 44 ± 10 years) were included in the study. Sera from all patients were anti-HCV antibody positive (first generation Elisa Axsym HCV 3·0, Abbott Diagnostic, Illinois, USA) and HCV RNA positive by PCR (Amplicor HCV Monitor assay 2·0; Roche Diagnostic, Meylan, France), and had alanine aminotransferase values above normal; liver biopsy showed histological features of chronic hepatitis (Knodell and Metavir scores). Thirty patients were treated with interferon alpha 2b alone (3 megaunits, three times a week, subcutaneously) for at least 3 months, and 36 patients were treated with a combination of interferon alpha 2b (3 megaunits, three times a week, subcutaneously) plus ribavirin (100–1200 mg/day) for at least 3 months [10]. At the end of this 3 month period, patients were classified into two groups: non-responders with positive HCV RNA and responders with negative HCV RNA. The definition of relapse was the negativity of HCV RNA from the 3 months until the end of treatment, followed by positivity afterwards.
Using these criteria, 28 patients were classified as non- responders, 20 as responders and, among patients belonging to this latter group, 10 as sustained responders and five as relapsers. Fifty healthy blood donors, age and sex matched, were chosen as the control group.
Determination of complement haemolytic activity
Total haemolytic complement (CH50) was measured with a kinetic assay in citrated plasma [11]. Haemolytic C4 was determined in citrated plasma as previously described by Gaither et al. [12]. The C4 specific activity value was defined as the ratio between haemolytic C4/C4 protein × 100 and expressed in arbitrary units (AU).
Determination of complement proteins and rheumatoid factor
C1q, C3 and C4. C1q, C3 and C4 concentrations were measured in sera by nephelometry using an automated analyser (BNII; Dade Behring, La Défense, France) and specific antisera (Dade Behring).
C1s-C1 inhibitor complexes. C1s-C1 inhibitor complexes were quantified using an ELISA method set up in the laboratory [13]. Microtitre plates (96 well; Maxisorp Nunc-immuno plate, Polylabo, Strasbourg, France) were coated with anti-C1s anti-body (Binding Site, St Egreve, France; dilution 1/3000) in 0·1 m NaHCO3 (pH 8·6) overnight at 4°C. After three washings with PBS-Tween (136 mm NaCl, 2·7 mm KCl, 1·45 mm KH2PO4, 8 mm Na2HPO4 anhydrous (pH 6·85) supplemented with 0·05% Tween 20 (w/v)), blocking was achieved with PBS-Tween-BSA containing 0·5% bovine serum albumin (BSA) (w/v). A C1s-C1 inhibitor complex standard was set up from purified C1s and a pool of normal human serum quantified for C1 inhibitor as described [14]. Serial dilutions of sera and of the standard were added to the wells and incubated overnight at room temperature. The wells were then washed three times with PBS-Tween, before addition of peroxidase-conjugated anti-C1 inhibitor antibody (Binding Site, St Egreve France; dilution 1/4000) and incubation for 180 min at room temperature. After three washings with PBS-Tween, o-phenylenediamine (OPD) (Sigma, Chesnes, France) in 0·1 m acetate buffer (pH 5) was added, before incubation for 10 min at room temperature and addition of 50 μl 4N H2SO4; absorbance was measured at 490 nm.
ELISA for mannan binding lectin (MBL). MBL concentration was assayed by a sandwich ELISA. Microtitre plates (96 well) were coated with 100 μl/well polyclonal rabbit anti-MBL total Ig (dilution 1/30 000) in 15 mm Na2CO3, 35 mm NaHCO3 (pH 9·6), with subsequent incubation overnight at 4°C. This polyclonal anti-MBL antibody was prepared using MBL purified in the laboratory and a three-step method (submitted for publication) modified from Tan et al. [15]. The plates were then washed four times with TBS-Tween-Ca (10 mm Tris-HCl, 150 mm NaCl (pH 7·5) containing 0·1% Tween 20 (w/v) and 6 mm CaCl2)), and blocked for 60 min at 37°C by 250 μl TBS-Tween-Ca-BSA containing 2% BSA (w/v). Samples (100 μl) and MBL standard sera, evaluated on purified MBL, were added to each well in duplicate; after 90 min incubation at 37°C and washing as above, biotinylated polyclonal anti-MBL total Ig (dilution 1/1000) was added to each well. After further incubation at 37°C for 90 min, the plates were washed four times with TBS-Tween-Ca, 100 μl/well streptavidin-horseradish peroxidase conjugate (Amersham- Pharmacia-Biotech, St Quentin-Yvelines, France; dilution 1/650) were added and the plates incubated at 37°C for 60 min. After four additional washes with TBS-Tween-Ca and subsequent addition of OPD, followed by 10 min incubation, the reaction was stopped by addition of H2SO4 as described for C1s-C1 inhibitor complexes; absorbance was measured at 490 nm.
Rheumatoid factor titration. The rheumatoid factor level was evaluated in patients by ELISA, adapted from Gioud-Paquet et al. [16]. Microtitre plates (96 well) were coated with 0·5 μg human IgG (ref. 14506, Sigma, Chesnes, France) in PBS (1·45 m NaCl, 0·074 m Na2HPO4 anhydrous, 0·024 M NaH2PO4.2H2O). After saturation with PBS-0·1%-Tween 20 (w/v) 1% BSA (w/v), 100 μl serial dilutions of sera from patients and controls were added to the wells and incubated for 60 min at 37°C. After washing with PBS containing 0·1% Tween 20 (w/v), wells were filled with 100 μl peroxydase-conjugated anti-human IgM (Dako, Trappes France; dilution 1/1000) and incubated for 45 min at room temperature. The last step involving OPD was as described above.
Statistical analysis
All results are expressed as means ± standard deviation (s.d.). Differences between datasets were calculated by Wilcoxon rank and Mann–Whitney non-parametric tests, and correlations were evaluated with the Spearman rank test. For all tests developed in this study, the results were considered significant at P < 0·05.
RESULTS
Activation of complement in patients with chronic hepatitis C
A total of 66 patients (42 men and 24 women) with chronic hepatitis C were included in this study, whatever the responder/non-responder state. Their serum and plasma were assayed for total complement and C4 haemolytic activities as well as protein content.
Compared with the healthy controls, the patients’ sera/plasma showed a decrease in CH50 and specific C4 levels (P < 0·0001, Table 1). The other parameters (C1q, C4, C3 and MBL) were nearly normal and no C1s-C1 inhibitor complex was detected. This suggests complement activation upon hepatitis C virus infection, without involvement of the classical pathway.
Table 1.
Complement classical pathway data in patients with chronic hepatitis C.
| Patients | Healthy controls | ||
|---|---|---|---|
| n =66 | n =50 | P | |
| CH50 (%)* | 66 ± 39 | 92 ± 10 | < 0·0001 |
| C1q protein (%)† | 116 ± 25 | 105 ± 14 | < 0·01 |
| C1s-C1inh complex (mg/l) | 11 ± 10 | 10 ± 10 | NS |
| C4 protein (%)† | 90 ± 40 | 106 ± 28 | < 0·01 |
| Haemolytic C4 (%)* | 55 ± 38 | 111 ± 36 | < 0·0001 |
| Specific C4 (AU)‡ | 58·7 ± 30 | 103 ± 10 | < 0·0001 |
| C3 protein (mg/ml) | 1078 ± 220 | 982 ± 157 | NS |
| MBL protein (ng/ml) | 1550 ± 1270 | 1500 ± 1150 | NS |
| RF (UI/ml) | 67 ± 75 | 10 ± 6 | < 0·0001 |
Ratio of sample relative to a pool of 20 normal citrated plasma.
Ratio of sample relative to a pool of 40 normal sera.
Calculated as described in Material and Methods.
Statistical significance between groups was determined by Mann–Whitney test. RF, Rheumatoid Factor; MBL, Mannan Binding Lectin; NS, Not significant.
Evaluation of the role of rheumatoid factor in the activation of complement
It has recently been shown that HCV can replicate in B cells, and it has been suggested that HCV could induce clonal selection of B cells producing monoclonal IgMk-type RF [17]. As formation of cryoprecipitate is generally considered to require the initial production of RF in patients with chronic hepatitis C infection [18], it was also of interest to investigate RF in our series. All 66 patient samples were assayed for RF and Table 1 shows that significantly higher titres were present in patients’ sera compared with controls (P < 0·0001). Of these 66 patients, RF was detected in 36 (54·5%).
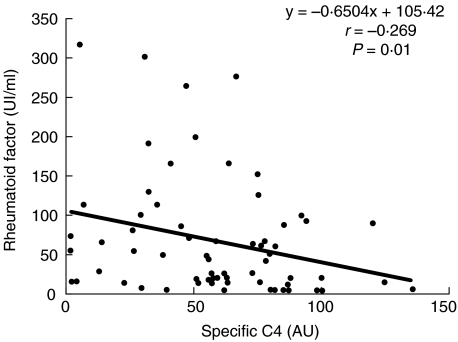
RF titres were compared with specific C4 activity data in this patient series before the initiation of therapy (M0) using non-parametric Spearman rank order correlation analysis. A significant negative correlation was observed between these two parameters (Fig. 1, P =0·01), suggesting a possible association of RF with C4 activation.
Fig. 1.
Correlation between specific C4 and rheumatoid factor (RF) in patients with hepatitis C before therapy. Specific C4 and RF titres were determined as described in Material and Methods. Correlation coefficients between variables and statistical significance of the associations were determined with Spearman rank order correlation analyses.
Complement in the monitoring of patients with chronic hepatitis C: specific C4 activity as an informative test
C4 in responders versus non-responders. Forty-eight patients (20 responders and 28 non-responders) were studied at 3 and 6 months after starting therapy (M0) with interferon alpha 2b or interferon alpha 2b + ribavirin. The same assays were performed as above and data from individuals in each responder and non-responder series were compared using the Wilcoxon rank test, a non-parametric paired statistic test.
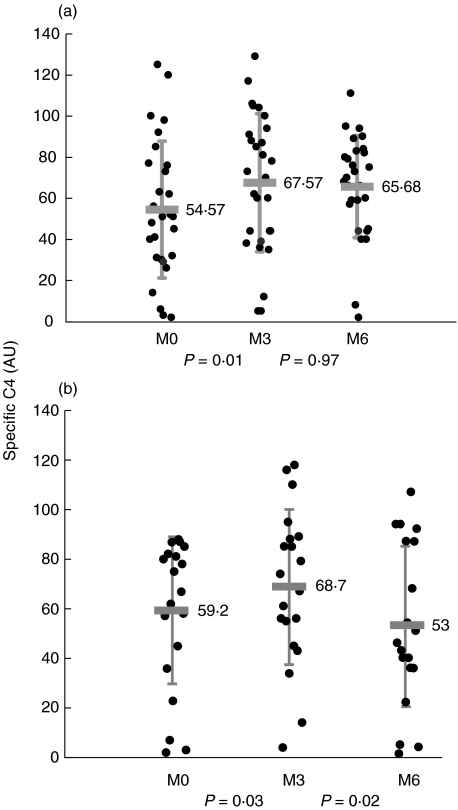
A significant increase in specific C4 activity was observed in the responder group after the first 3 months of therapy (54 ± 33% to 67 ± 38%, P =0·019), and this C4 restoration was durable for the 6 months of treatment (Fig. 2a).
Fig. 2.
Specific C4 in patients before (M0), after 3 months (M3) and after 6 months (M6) of therapy. Specific C4 was determined according to Material and Methods. The shaded bar corresponds to the mean and vertical lines correspond to the standard deviation. Statistical significance between groups was determined by the Wilcoxon test. (a) Responder group (n =28); (b) non-responder group (n =20).
C4 restoration was also observed in the non-responder group after the first 3 months (59 ± 29% to 68·7 ± 31%, P =0·03), but this was not sustained by month 6 (68·7 ± 31% to 53 ± 32%, P =0·02, Fig. 2b).
After 6 months of therapy, the results show restoration of specific C4 activity in the responder group (negative serum HCV RNA) and a decrease in specific C4 activity in the non-responder group (stable serum HCV RNA).
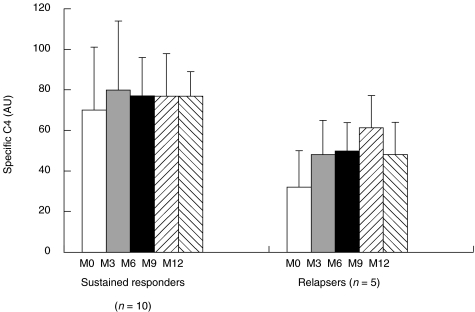
C4 in sustained responder versus relapser groups. The same patient groups as above were studied during the course of 12 months of therapy; the responder group was subdivided into two groups (sustained responders, n =10; relapsers, n =5) as described in Material and Methods.
Specific C4 activity data from these two subgroups were compared using the Mann–Whitney non-parametric statistical test. A significant decrease in specific C4 activity was observed in the relapser compared with the sustained responder groups before and during the first 6 months of therapy (31·8 ± 18 versus 70 ± 31 at MO, P =0·037; 48 ± 17 versus 80 ± 34 at M3, P =0·066; 49·8 ± 14 versus 77 ± 19 at M6, P =0·049, Fig. 3). However, no significant modification was detected statistically when comparing sustained responder and non-responder groups (data not shown). These results favour an association between specific C4 activity and the patient’s response to therapy at the onset of the treatment.
Fig. 3.
Follow-up of specific C4 in sustained responder and relapser groups during 12 months of therapy. Specific C4 was determined as described in Material and Methods.
Comparison of clinical liver parameters and specific C4
C4 specific activity values were compared with virology, enzymology and liver histological parameters using non-parametric Spearman rank order correlation analysis in the 66 patients. No significant correlations were found between specific C4 activity and HCV RNA, alanine aminotransferase, Knodell score, Metavir histological fibrosis and Metavir histological activity (P =0·29, P =0·9, P =0·48, P =0·96 and P =0·22, respectively) (data not shown).
DISCUSSION
We report here the first observation of complement consumption in chronic hepatitis C based on evaluation of specific C4 activity. Calibration of specific C4 activity, from protein and haemolytic activity data, sensitizes the evaluation of the biological activity of C4. C4 is a highly polymorphic protein with contrasting levels of activity between its different isotypes; specific C4 activity thus appears to be a valuable alternative to the classical isotyping of this protein [19]. Previous studies reported decreased C4 serum levels in patients with chronic hepatitis C [20,21].
Complement activation is observed in the sera of chronically HCV-infected patients, correlating with decreased specific C4 activity. In the same sera, nearly normal levels of C1s-Cinh complexes were detected, suggesting that the reduction in specific C4 activity is not dependent on complement classical pathway activation [22].
One explanation for this could be that activation goes through MBL and the MBL-associated serine protease MASP-2, leading to cleavage of C2 and C4 to form a C3 convertase [23,24] able to cleave C4 and so account for the observed decrease in specific C4 activity.
Moreover, in agreement with such a view, a negative correlation between specific C4 activities and RF titres in the patients’ sera was observed (Fig. 1). RF is likely to activate the classical pathway of complement by complexing with IgG, but this is probably not the case as no C1s-C1 inhibitor complex was detected; instead, it can be proposed that activation via MBL-MASP2 may occur from binding of MBL to underglycosylated RF, as suggested by Malhotra et al. [25] and Matsumoto et al. [26]. Further investigation into RF glycosylation is necessary.
Another explanation for the decreased C4 specific activity could involve cryoglobulins present in the patients’ sera, as cryoglobulins are detected in about 40% of HCV-infected patients [18]. These mixed cryoglobulins contain polyclonal anti-HCV IgG, HCV RNA and RF [27,28]. They are likely to support MBL binding with subsequent formation of complement C3 convertase. This is presently under laboratory investigation.
Evolution of specific C4 activity after 6 months of therapy appears to be an interesting basis for distinguishing between responder and non-responder patients. In non-responders, the persistence of viral RNA is associated with the presence of cryoglobulins, which have been found in 49% of patients before treatment and in 76% of non-responders [29]. The present view is that cryoglobulins are able to activate complement via the MBL + MASPs pathway, and contribute to a decrease in specific C4 activity in non-responder patients.
A significant decrease in specific C4 activity is observed in relapsers compared with sustained responders, before and during the first 6 months of therapy. The data reported in this first study on specific C4 activity point to a potential predictive function for this parameter to monitor a response to therapy. As a possible explanation, according to the inverse association with the RF titres, low specific C4 activity detected in the relapser group could depend on significantly higher RF titres (m=115 UI/ml ± 79, Fig. 1), and the ensuing C4 consumption via the lectin pathway. Specific C4 activities appear to be different depending on whether interferon alpha 2b monotherapy or bitherapy with ribavirin is used (data not shown), but these results have to be confirmed.
Decreased specific C4 activity, without C3 consumption, in chronic hepatitis C suggests complement activation leading to the N-terminal cleavage of C4 with production of C4a. In two post-transplant patients with hepatitis C infections, Pfeifer et al. showed an increased C4a level without a significant increase in the level of C3a [30]. C4a levels may represent a valuable test for monitoring the course of the viral infection. Whether this test is valid for the follow-up of interferon alpha 2b or interferon alpha 2b + ribavirin treatment in hepatitis C is now under consideration. Various biological effects of C4a are probably initiated by the binding of this fragment to cell receptors, similar to the cell interactions involving C5a [31,32]. Schieferdecker et al. reported that in vivo treatment of rats with the pro-inflammatory cytokine IL-6 caused the expression of functional C5a receptors on hepatocytes [33]. Another study reported a critical role for C5a receptor signaling in the early events, leading to hepatocyte proliferation [34]. Until now, there have only been a few reports on the role of C4a receptors, which may well play a role in hepatocyte proliferation during chronic hepatitis C treatment.
Overall, these data suggest that C4 specific activity can be a useful parameter in the follow-up of HCV patients, together with other conventional clinical, histological, viral and enzymic investigations.
Acknowledgments
This study was supported by the DRC (Délégation à la Recherche Clinique) (CHU–Grenoble). The authors thank F. Csopaki, A. Mabboux, N. Marchand and V. Reininger (Laboratory of Immunology) for expert technical assistance.
REFERENCES
- 1.Choo QL, Kuo G, Wriner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–9. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gales MJ, Korth MJ, Tang NM, et al. Evidence that hepatitis C resistance to interferon is mediated through repression of the PKR protein kinase by the non structural 5A protein. Virology. 1997;230:217–27. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 4.Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunol Today. 2000;21:447–55. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan BP, Harris CL. Complement regulators and micro-organisms. In: Morgan BP, Harris CL, editors. Complement regulatory proteins. San Diego: Academic Press; 1999. pp. 207–25. [Google Scholar]
- 6.Lubinski J, Nagashunmugam T, Friedman HM. Viral interference with antibody and complement. Semin Cell Dev Biol. 1998;9:329–37. doi: 10.1006/scdb.1998.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda K, Nakajima H, Nakagawa T, Shimizu A. The association of complement activation at a low temperature with hepatitis C virus infection in comparison with cryoglobulin. Clin Exp Immunol. 1995;101:284–7. doi: 10.1111/j.1365-2249.1995.tb08352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei G, Yano S, Kuroiwa T, Hiromura K, Maezawa A. Hepatitis C virus (HCV)-induced IgG-IgM rheumatoid factor (RF) complex may be the main causal factor for cold-dependent activation of complement in patients with rheumatic disease. Clin Exp Immunol. 1997;107:83–8. doi: 10.1046/j.1365-2249.1997.d01-882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh K, Tanaka H, Shiga J-I, et al. Hypocomplementemia associated with hepatitis C viremia in sera from voluntary blood donors. Am J Gastroenterol. 1994;89:2019–24. [PubMed] [Google Scholar]
- 10.Souvignet C, Zarski J-P. Combination treatment for chronic hepatitis C. What is the role of ribavirin? Fundam Clin Pharmacol. 2000;14:321–5. doi: 10.1111/j.1472-8206.2000.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 11.Abbal M, Tkaczuk J, Praud C, Msayeh F, Ohayon E. Computer-assisted kinetic assay for quantification of total complement activity. Complement Inflamm. 1991;8:92–103. doi: 10.1159/000463185. [DOI] [PubMed] [Google Scholar]
- 12.Gaither TA, Alling DW, Frank MM. A new one-step method for the functional assay of the fourth component (C4) of human and guinea pig complement. J Immunol. 1974;113:574–83. [PubMed] [Google Scholar]
- 13.Cooper NR, Nemerow GR, Mayes JT. Methods to detect and quantitate complement activation. Springer Semin Immunopathol. 1983;6:195–212. doi: 10.1007/BF00205873. [DOI] [PubMed] [Google Scholar]
- 14.Drouet C, Alibeu C, Ponard D, Arlaud GJ, Colomb MG. A sensitive method to assay blood complement C1 Inhibitor activity. Clin Chim Acta. 1988;174:121–30. doi: 10.1016/0009-8981(88)90379-8. [DOI] [PubMed] [Google Scholar]
- 15.Tan SM, Chung MCM, Kon OL, Thiel S, Lee SH, Lu J. Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem J. 1996;319:329–32. doi: 10.1042/bj3190329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gioud-Paquet M, Auvinet M, Raffin T, et al. IgM rheumatoid factor (RF), IgA RF, IgE RF and IgG RF detected by ELISA in rheumatoid arthritis. Ann Rheum Dis. 1987;46:65–71. doi: 10.1136/ard.46.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornasieri A, Bernasconi P, Ribero ML, et al. Hepatitis C virus (HCV) in lymphocyte subsets and in B lymphocytes expressing rheumatoid factor cross-reacting idiotype in type II mixed cryoglobulinaemia. Clin Exp Immunol. 2000;122:400–3. doi: 10.1046/j.1365-2249.2000.01396.x. 10.1046/j.1365-2249.2000.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt WN, Stapleton JT, LaBrecque DR, et al. Hepatitis C virus (HCV) infection and cryoglobulinemia: analysis of whole blood and plasma HCV-RNA concentrations and correlation with liver histology. Hepatology. 2000;31:737–44. doi: 10.1002/hep.510310326. [DOI] [PubMed] [Google Scholar]
- 19.Lambris JD, Sahu A, Wetsel RA. The chemistry and biology of C3, C4 and C5. In: Volanakis JE, Frank MM, editors. The human complement system in health and disease. New York: Marcel Dekker; 1998. pp. 83–118. [Google Scholar]
- 20.Farkas H, Csepregi A, Nemesanszky E, et al. Acquired angioedema associated with chronic hepatitis C. J Allergy Clin Immunol. 1999;103:711–2. doi: 10.1016/s0091-6749(99)70248-4. [DOI] [PubMed] [Google Scholar]
- 21.Poljacki M, Gajinov Z, Ivkov M, Matic M, Golusin Z. Skin diseases and hepatitis virus C infection. Med Pregl. 2000;53:141–5. [PubMed] [Google Scholar]
- 22.Sim RB, Arlaud GJ, Colomb MG. C1 inhibitor-dependent dissociation of human complement component C1 bound to immune complexes. Biochem J. 1979;179:449–57. doi: 10.1042/bj1790449a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita M, Fujita T. Activation of the classical component pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gadjeva M, Thiel S, Jensenius JC. The mannan-binding-lectin pathway of the innate immune response. Curr Opin Immunol. pp. 74–8. [DOI] [PubMed]
- 25.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nature Medicine. 1995;1:237–43. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto A, Shikata K, Takeuchi F, Kojima N, Mizuochi T. Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J Biochem. 2000;128:621–8. doi: 10.1093/oxfordjournals.jbchem.a022794. [DOI] [PubMed] [Google Scholar]
- 27.Sasso EH. The rheumatoid factor response in the etiology of mixed cryoglobulins associated with hepatitis C virus infection. Ann Med Interne. 2000;151:30–40. [PubMed] [Google Scholar]
- 28.Obermayer-Straub P, Manns MP. Hepatitis C and D, retroviruses and autoimmune manifestations. J Autoimmun. 2001;16:275–85. doi: 10.1006/jaut.2000.0488. 10.1006/jaut.2000.0488. [DOI] [PubMed] [Google Scholar]
- 29.Tridon A, Abergel A, Kuder P, et al. Cryoglobulines mixtes et auto-immunité au cours de l’hépatite C. Path Biol. 1997;45:291–7. [PubMed] [Google Scholar]
- 30.Pfeifer PH, Brems JJ, Brunson M, Hugli TE. Plasma C3a and C4a levels in liver transplant recipients: a longitudinal study. Immunopharmacology. 2000;46:163–74. doi: 10.1016/s0162-3109(99)00167-8. [DOI] [PubMed] [Google Scholar]
- 31.Kacani L, Banki Z, Zwirner J, et al. C5a and C5adesArg enhance the susceptibility of monocyte-derived macrophages to HIV infection. J Immunol. 2001;166:3410–5. doi: 10.4049/jimmunol.166.5.3410. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia M, Saluja AK, Singh VP, et al. Complement factor C5a exerts an anti-inflammatory effect in acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2001;280:G974–8. doi: 10.1152/ajpgi.2001.280.5.G974. [DOI] [PubMed] [Google Scholar]
- 33.Schieferdecker HL, Schlaf G, Koleva M, Götze O, Jungermann K. Induction of functional anaphylatoxin C5a receptors on hepatocytes by in vivo treatment of rats with Il-6. J Immunol. 2000;164:5453–8. doi: 10.4049/jimmunol.164.10.5453. [DOI] [PubMed] [Google Scholar]
- 34.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–86. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]