Abstract
The purpose of this study was to identify autoantigens that are recognized by human sera and are associated with a speckled cytoplasmic fluorescent staining pattern on tissue culture cells, and to determine clinical features associated with specific autoantibodies. A serum from a patient with systemic lupus erythematosus was used to identify a 3·7-kb cDNA insert from a HeLa cell expression library. The purified cDNA (VLK2·1) encoded a peptide of 1051 amino acids that shared 98·4% similarity with the carboxyl terminal portion of a previously reported 170 kD protein named cytoplasmic linker protein-170 (CLIP-170). Antibodies affinity purified with the recombinant CLIP-170 protein, the prototype human serum and a monoclonal antibody raised against CLIP-170 exhibited identical speckled staining of the cytoplasm in HEp-2 cells. The human autoantibodies reacted with the purified recombinant protein in a Western immunoblot and immunoprecipitated the in vitro translated recombinant protein. Three additional human sera also immunoprecipitated the recombinant CLIP-170 protein. The clinical diagnoses in these patients were limited scleroderma, glioblastoma and idiopathic pleural effusion. This is the first report that identifies CLIP-170 as a human autoantigen.
Keywords: autoantibody, autoantigen, cytoplasmic linker protein, endosome, systemic lupus, erythematosus
INTRODUCTION
Autoantibodies have proved to be valuable serological markers of autoimmune diseases and have been used successfully to identify their cognate cellular antigens. These autoantigens include protein–nucleic acid complexes in the nucleus [1–3] and cytoplasm [4] and protein–protein complexes in mitochondria [5–7], endosomes [8,9] and the Golgi complex [10]. These cytoplasmic antigens have a diverse inherent function within the cell and are different in their subcellular localization.
Cytoplasmic linker proteins (CLIPs) are a class of proteins that help in serving to facilitate the interaction of cellular organelles with microtubules [11]. CLIP-170, a 170-kD protein that was originally discovered in HeLa cells [12], serves as a linker between endosomal vesicles and cytoplasmic microtubules [11]. CLIP-170 was localized to the plus ends of microtubules, a feature that is facilitated by its recognition of newly polymerized tubulin, and prometaphase kinetochores [13–15]. In vivo, CLIP-170 is a homodimer with non-helical domains at both the amino and carboxyl terminals that are separated by a long α-helical coiled-coil domain [16]. The amino terminal domain is responsible for the binding to microtubules [17], while the carboxyl terminal is implicated in binding to kinetochores in prometaphase cells [15].
We identified a human serum that produced a unique speckled cytoplasmic fluorescent staining pattern on HEp-2 cells and reacted with unidentified proteins in immunoblotting. We used this serum to screen a cDNA expression library and isolate a cDNA that encodes CLIP-170. This is the first report that identifies CLIP-170 as a human autoantigen.
MATERIALS AND METHODS
Patients and antibodies
The index serum was from a patient who fulfilled the American College of Rheumatology criteria for SLE [18] and also had a transiently elevated creatine phosphofructose kinase but no evidence of muscle weakness, myocardial infarction or myopathy as assessed by electromyography. The autoantibody specificity was identified first on the basis of indirect immunofluorescence (IIF) on HEp-2 cells. Other sera were obtained from serum banks in the Advanced Diagnostics Laboratory at the University of Calgary, Calgary, Alberta and the University of Montreal, Montreal, Quebec. Control sera were randomly selected from a bank of healthy volunteers. The serum samples were stored at −20°C or −70°C. Clinical data were obtained by retrospective chart review.
Indirect immunofluorescence (IIF)
IIF was performed on commercially prepared HEp-2 cell substrates (HEp-2000; Immuno Concepts Inc., Sacramento, CA, USA) using sera diluted at 1/80 and a fluorescein-conjugated goat antihuman IgG (light and heavy chain-specific) as previously described [19,20]. Slides were viewed with a Zeiss Universal microscope fitted with epifluorescence (Carl Zeiss Inc., Thornwood, NY, USA) and images were recorded with a Sony digital camera.
SDS-PAGE and Western blotting
Proteins or cellular preparations were solubilized in SDS sample buffer, separated by discontinuous SDS-PAGE [21], and then transferred to nitrocellulose membranes as described previously [22]. After transfer, nitrocellulose strips were blocked with 5% nonfat milk in PBS containing 0·05% Tween-20 (PBS-T), overlaid with primary antibody, and then washed with PBS-T to remove any unbound antibody. Bound antibody was traced with horseradish peroxidase conjugated polyvalent goat antihuman immunoglobulin (Calbiochem-Behring Corp., La Jolla, CA, USA). Bound antibodies were visualized by incubating the washed nitrocellulose strips in Enhanced ChemiLuminescence (ECL) substrate solution (Amersham Life Science Ltd, UK), and then exposing the nitrocellulose strips to X-OMAT AR film (Eastman Kodak Co., Rochester, NY, USA).
cDNA library screening
Clones from a HeLa UniZAP cDNA library (Stratagene, La Jolla, CA, USA) were identified by an immunological method described originally by Young and Davis [23] and modified in our laboratory [24,25]. All cDNA screening was performed on duplicate filters and positive clones were subsequently purified to 100%. Bound antibodies were detected using an adaptation of the Western blotting described above.
DNA subcloning and sequence determination
Positive clones were isolated and subcloned in vivo into the pBluescript plasmid using R408 helper phage as described in the manufacturer’s instructions (Stratagene). The nucleotide sequences of a single clone of interest, designated VLK2·1, were determined using dye terminator sequencing and an automated sequencer from Applied Biosystems Inc. (Foster City, CA, USA). Nucleic acid and protein sequences were analysed by the University of Wisconsin Genetics Computer Group (GCG) Sequence Analysis Software Package [26]. Comparisons to known sequences were performed by BLAST on the Internet server. Secondary structure analysis for coiled-coil motifs was conducted with the software programs coils[27] and paracoils[28].
Recombinant protein production
Plasmids were transformed in E. coli strain XL-1 Blue cells (Stratagene). The recombinant protein was prepared from 200 ml cultures of the recombinant cells grown to O.D. = 0·6 at 37°C and induced with 10 mm IPTG. The bacterial suspension was cultured overnight and harvested by the method of Adam et al.[29]. The final pellet of inclusion bodies was washed with 1 m urea in 0·1m Tris buffer (pH 7·3) on ice for 5 min to remove residual bacterial proteins. The inclusion bodies were then extracted with 8m urea in 0·1m Tris buffer containing 0·2%β-mercaptoethanol, for 15 min on ice. Reactivity of recombinant proteins was confirmed by immunoblotting described above.
In vitro RNA transcription and translation
One microgram of purified plasmid DNA served as a template for a 50-μl in vitro transcription and translation reaction, containing rabbit reticulocyte lysate, T3 RNA polymerase, [S35]-methionine (trans-S35 label, ICN Biochemicals, Irvine, CA, USA) and RNasin (Promega, Madison, WI, USA), as suggested by the manufacturer (Promega). The reaction was carried out at 30°C for 1h, followed by SDS-PAGE of a 5-μl aliquot to confirm the presence of the translation products. Samples were stored at –70°C until required.
Immunoprecipitation
Immunoprecipitation of [S35]-labelled in vitro translation products was performed using Protein A-Sepharose beads as described previously [24]. Briefly, 10 μl of human serum and 2–5 μl of translation product were incubated with Protein A-Sepharose beads overnight at 4°C. After incubation, the Protein A-Sepharose beads were washed four times with buffer and resuspended in SDS sample buffer. Samples were then analysed by SDS-PAGE and autoradiography.
Affinity purification of antibodies
Affinity purified antibodies were prepared by eluting absorbed antibodies from recombinant proteins of phage plaques induced by isopropyl thiogalactosepyranoside (IPTG) and blotted onto nitrocellulose filters as described previously [24,25]. A mouse monoclonal antibody directed to CLIP-170 was kindly provided by Dr Franck Perez (Institut Curie, Paris, France).
RESULTS
Indirect immunofluorescence
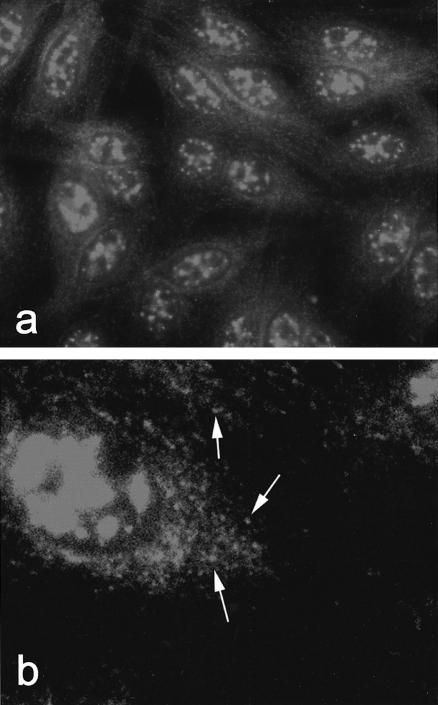
The prototype serum demonstrated a nuclear matrix and speckled cytoplasmic pattern when tested by IIF on HEp-2 cells (Fig. 1a). The cytoplasmic component was characterized by punctate staining dispersed uniformly throughout the cytoplasm (Fig. 1b).
Fig. 1.
Indirect immunofluorescence detection of CLIP-170 autoantibodies on HEp-2 cells. (a) IIF staining pattern of index human serum demonstrating reactivity in the cytoplasm as well as the nuclear matrix. Original magnification 100×. (b) Higher power (500x) of Hep-2 cells stained with the prototype sera demonstrating the dispersed and polymorphous cytoplasmic speckled staining pattern. In some areas the staining appears more punctate (arrows).
Cloning and characterization of CLIP-170 as the cytoplasmic autoantigen
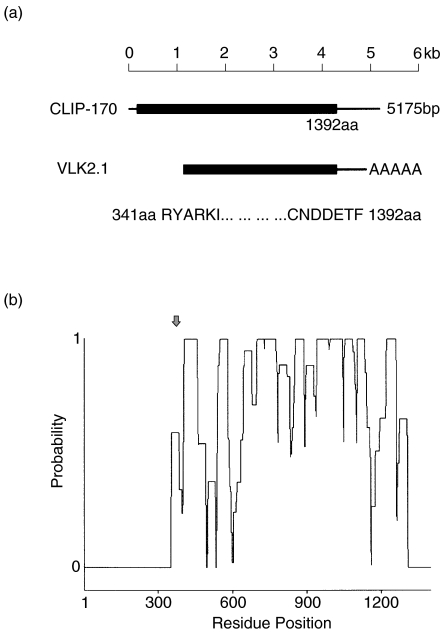
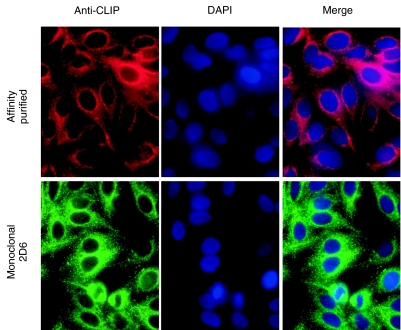
When the index serum was used to screen 5 × 105 plaques from a HeLa UniZAP cDNA expression library, one clone (VLK2·1) remained reactive throughout subsequent screenings until 100% purity was attained. The nucleotide sequence of the VLK2·1 cDNA was 98·4% identical to the carboxyl terminal portion of a previously characterized 170 kD protein known as CLIP-170 (GenBank accession no. M97501·1) [11]. The purified VLK2·1 cDNA was 3766 base pairs long, and it encoded a recombinant protein of 1051 amino acids with a predicted molecular weight of 126 kD (Table 1). Thus, the isolated VLK2·1 cDNA encoded 75·5% of the full length 170 kD protein (Fig. 2a). Secondary structure analysis identified coiled- coil motifs in the central domain of VLK2·1 (Fig. 2b). The conclusion that CLIP-170 was the reactive autoantigen was supported by observations that the antibodies affinity purified by binding to the IPTG-induced VLK2·1 phage plaques and the mouse monoclonal antibody to CLIP-170 showed similar cytoplasmic staining patterns (Fig. 3). Neither the affinity purified human antibody or the murine monoclonal antibody stained Hep-2 nuclei (Fig. 3).
Table 1.
Attributes of the cloned CLIP-170 (VLK2.1) and the previously reproted CLIP-170
| VLK2.1 | CLIP-170 | |
|---|---|---|
| Sequence data | ||
| DNA length (bp) | 3766 | 5175 |
| No. of amino acids | 1051 | 1392 |
| Physical measurements | ||
| In vitro TnT product (kD) | 126.2 | 167.2 |
| Native HeLa cell protein (kD) | 170 | 170 |
Fig. 2.
Analysis of the VLK2·1 cDNA clone. (a) Comparison of the VLK2·1 cDNA and the previously reported CLIP-170 cDNA. The VLK2·1 cDNA is 1·2kb shorter at the 5′ terminus then the previously published sequence. (b) Probability plots for the determination of coiled-coil domains of CLIP-170/VLK2·1 as calculated by the COILS and PRACOILS programs. CLIP-170 has multiple coiled-coil domains except at the amino and carboxy terminus. Arrow indicates the beginning of the VLK2·1 sequence.
Fig. 3.
Indirect immunofluorescence of Hep-2 cells stained with the antibodies from the index patient affinity purified with the CLIP-170 phage VLK 2·1 and murine monoclonal antibody 2D6 to CLIP-170. The staining patterns of these two antibodies are similar in cellular distribution. Nuclei are counterstained with DAPI. Note the absence of nuclear staining with both the affinity purified and monoclonal probes.
Immunoblotting
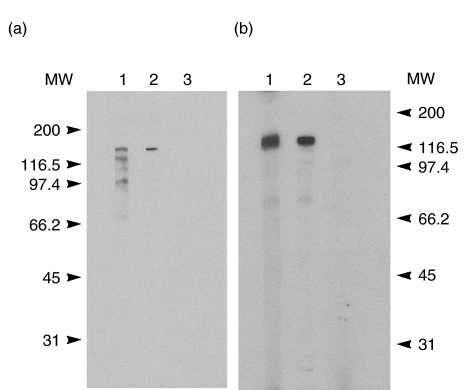
Immunoblotting of HeLa whole cell extracts with the index serum displayed strong reactivity with a 170-kD protein (Fig. 4a, lanes 1 and 2). Several other bands representing lower MW proteins were also observed using the index serum. This observation is consistent with the reactivity of the prototype serum to other nuclear components observed by IIF. Affinity purified antibodies to the VLK2·1 phage proteins also displayed reactivity to the 170 kD protein (Fig. 4a, lane 2) but not with the other bands observed with the index serum, suggesting that these lower molecular weight bands are not degradation products of the native 170 kD protein. In addition, these results are consistent with the expected molecular weight of CLIP-170. Normal human serum did not demonstrate any reactivity (Fig. 4a, lane 3).
Fig. 4.
Immunoblotting profile of anti CLIP-170 serum. (a) HeLa whole cell extracts were separated by 10% gel SDS-PAGE and transferred to nitrocellulose. Lane 1 shows the reactivity of the index human serum with a polypeptide of approximately Mr 170. Lane 2 shows the reactivity with antibodies from the index patient serum affinity purified on recombinant phage protein. Lane 3 shows the lack of reactivity of normal human serum. (b) Reactivity with the VLK2·1 recombinant protein produced in Escherichia coli was evident using the index serum (lane 1) and with affinity purified antibodies (lane 2), but normal human serum (lane 3) did not react with the recombinant protein. All sera were diluted 1/100, except affinity purified antibodies that were used undiluted. Molecular weight markers are shown on the left (a) and right (b).
The reactivity of the recombinant protein encoded by the VLK2·1 cDNA was tested against affinity purified antibodies as well as the index human serum. In immunoblotting analysis (Fig. 4b), the index serum (lane 1) and the antibodies affinity purified from the VLK2·1 phage protein (lane 2), but not normal human serum (lane 3) were reactive with the VLK2·1 recombinant protein.
In vitro TnT and immunoprecipitation
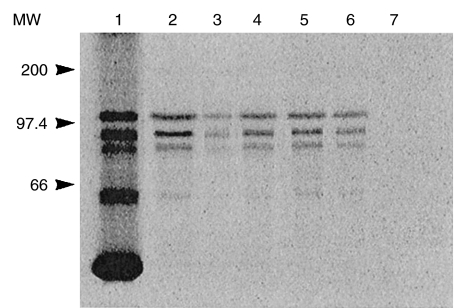
The reactivity with the recombinant protein was confirmed by parallel experiments that used the in vitro transcription and translation (TnT) product derived from the VLK2·1 cDNA (Fig. 5) The [S35]-labelled in vitro TnT product produced by the VLK2·1 cDNA migrated in SDS-PAGE at approximately 120 kD (Fig. 5, lane 1). Several other bands corresponding to lower molecular weight translation products were also observed. The in vitro TnT product was immunoprecipitated by the index human anti-CLIP 170 serum (lane 2) and by antibodies affinity purified from the VLK 2·1 phage protein (lane 3).
Fig. 5.
Immunoprecipitation of the in vitro TnT product of VLK2·1 (CLIP-170). The VLK2·1 TnT product migrated at ~120kD (lane 1) with smaller translation products also observed. The index anti-CLIP-170 human serum immunoprecipitated the in vitro translation product (lane 2), as did affinity purified antibodies to the recombinant phage protein (lane 3). Three additional human sera also immunoprecipitated the CLIP-170 TnT product (lanes 4–6), whereas normal human serum did not (lane 7). Molecular weight markers are indicated on the left.
Human sera displaying IIF patterns of cytoplasmic staining similar to the index serum were also tested for immunoprecipitation of the VLK2·1 TnT product. Reactivity with the recombinant protein was observed with three additional sera (Fig. 5, lanes 4–6). Several lower molecular weight translation products were also immunoprecipitated by the anti-CLIP 170 sera, suggesting that these products are the result of either translation initiated at internal methionine sites or degradation of the recombinant protein.
Clinical features of patients with CLIP-170 autoantibodies
In the Advanced Diagnostics Laboratory at the University of Calgary, 57 sera out of approximately 7500 obtained over a 3-year period, demonstrated an IIF cytoplasmic staining pattern that was similar to that observed with the index serum. These sera were tested for reactivity by immunoblotting and when a band of ~170 kD was observed, reactivity with the VLK2·1 TnT product was confirmed by immunoprecipitation. Three (5·3%) bound the CLIP-170 recombinant protein (Fig. 4, lanes 5–7). An additional serum that bound recombinant CLIP-170 was identified in a cohort of 173 systemic sclerosis sera from the Autoimmune Research Laboratory in Montreal. The isotype of all CLIP-170 autoantibodies was IgG as demonstrated by the isotype specificity of IIF, immunoblotting and protein A-Sepharose immunoprecipitation of CLIP-170 (data not shown).
The clinical features of the four patients who displayed this punctate cytoplasmic vesicular staining and immunoprecipitated the recombinant CLIP-170 protein are shown in Table 2. All four were females with an average age of 35 years (range 21–66). Three of the patients were less than 30 years of age. There was no uniform diagnosis in this small group of patients: the index patient had SLE, a persistent pleural effusion and an elevated CPK, one patient had the limited form of systemic sclerosis, one had a glioblastoma and one had an idiopathic pleural effusion with no other diagnostic criteria for the classification of SLE.
Table 2.
Clinical features of patients with CLIP-170 autoantibodies
| Patient | Gender | Age | Diagnosis |
|---|---|---|---|
| 1 | F | 66 | SLE, pleural effusion |
| 2 | F | 21 | Limited cutaneous scleroderma, Raynaud’s, telangiectasia, oesophageal dysmobility |
| 3 | F | 27 | Glioblastoma |
| 4 | F | 24 | Pleural effusion |
DISCUSSION
Historically, autoantibodies directed to nuclear antigens have been the primary focus of clinicians and scientists interested in systemic rheumatic diseases [2]. In the past decade, however, considerably more attention has been given to cytoplasmic autoantigens. Among these are antibodies localized in mitochondria [5–7[, ribosomes [4], the Golgi complex [10,24,25] and endosomes [8,9]. In addition, the molecular identity of these autoantigens is expanding. In this report we are the first to describe human autoantibodies to CLIP-170, a protein that function to tether endosomes and other vesicles to cytoplasmic microtubules [11,17].
Although our studies suggest that this not a common autoantibody, it is interesting that two of the patients identified in this study were considered to be ‘ANA negative’ and thus the attending clinicians presumed that they did not have autoantibodies at all. The prevalence of ANA-negative rheumatic diseases ranges from up to 60% in RA, 25% in Sjögren’s syndrome, 20% in scleroderma and 5% in SLE [30,31]. These numbers are based on conventional autoantibody screening tests using IIF performed on commercially prepared HEp-2 cells or ELISA using native or recombinant antigens coated on microtitre plates. If other techniques such as Western immunoblotting are used, it is apparent that many of the so-called ANA negative sera do indeed express autoantibodies that react with intracellular antigens, including extracellular constituents, cell surface antigens and the cytoplasmic antigens referred to above. The frequency of antibodies to CLIP-170 is not known in any disease group. Studies are under way to test sera from large cohorts of patients to derive more accurate frequency data for the major autoimmune diseases seen in clinical practice.
The CLIP-170 autoantigen is of particular interest because it joins a group of other cytoplasmic antigens that have extensive coiled-coil domains. Other autoantigens in this category include all Golgi complex autoantigens identified to date [10], early endosome antigen 1 (EEA1) [8], the 52 kD SS-A/Ro [32], the 70/80 kD ku autoantigens [33,34]and the Purkinje cell antigen APCA-1/Yo) [35]. The significance of coiled-coils in the context of the autoimmune response is not understood. Based on the studies reported thus far, it seems highly unlikely that the immune response is directed merely to cross-reactive coiled-coils in these autoantigens.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Cheryl Hanson and Joan Miller. This research was supported by a grant from the Canadian Institutes for Health Research.
REFERENCES
- 1.Tan EM. Autoantibodies in pathology and cell biology. Cell. 1991;67:841–2. doi: 10.1016/0092-8674(91)90356-4. [DOI] [PubMed] [Google Scholar]
- 2.von Muhlen CA, Tan EM. Autoantibodies in the diagnosis of systemic rheumatic disease. Semin Arthritis Rheum. 1995;24:323–58. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 3.Fritzler MJ. Clinical relevance of autoantibodies in systemic rheumatic diseases. Mol Biol Rep. 1996;23:133–45. doi: 10.1007/BF00351161. [DOI] [PubMed] [Google Scholar]
- 4.Teh LS, Isenberg DA. Antiribosomal P protein antibodies in systemic lupus erythematosus. Arthritis Rheum. 1994;37:307–15. doi: 10.1002/art.1780370303. [DOI] [PubMed] [Google Scholar]
- 5.McMillan SA, Alderdice JM, McKee CM, et al. Diversity of autoantibodies in patients with antimitochondrial antibody and their diagnostic value. J Clin Pathol. 1987;40:232–6. doi: 10.1136/jcp.40.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung PSC, Coppel RL, Gershwin ME. Mitochondrial antibodies. In: Peter JB, Shoenfeld Y, editors. Autoantibodies. New York: Elsevier; 1996. pp. 494–500. [Google Scholar]
- 7.Caldwell SH, Leung PS, Spivey JR, et al. Antimitochondrial antibodies in kindreds of patients with primary biliary cirrhosis: antimitochondrial antibodies are unique to clinical disease and are absent in asymptomatic family members. Hepatology. 1992;16:899–905. doi: 10.1002/hep.1840160408. [DOI] [PubMed] [Google Scholar]
- 8.Mu FT, Callaghan JM, Steele-Mortimer HS, et al. EEA1, an early endosomal protein. J Biol Chem. 1995;270:13503–11. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 9.Selak S, Scheonroth L, Senécal J-L, Fritzler MJ. Early endosome antigen 1: an autoantigen associated with neurological diseases. J Invest Med. 1999;47:311–8. [PubMed] [Google Scholar]
- 10.Chan EKL, Fritzler MJ. Golgins: coiled-coil-rich proteins associated with the Golgi complex. Electronic J Biotechnol. http://ejb.ucv.cl/content/vol1/issue2/full/1. [Google Scholar]
- 11.Pierre P, Scheel J, Rickard JE, Kreis TE. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- 12.Rickard JE, Kreis TE. Identification of a novel nucleotide-sensitive microtubule-binding protein in HeLa cells. J Cell Biol. 1990;110:1623–33. doi: 10.1083/jcb.110.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamantopoulos GS, Perez F, Goodson HV, et al. Dynamic localization of CLIP-170 to microtubule plus ends is coupled to microtubule assembly. J Cell Biol. 1999;144:99–112. doi: 10.1083/jcb.144.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez F, Diamantopoulos GS, Stalder R, Kreis TE. CLIP-170 highlights growing microtubule ends in vivo. Cell. 1999;96:517–27. doi: 10.1016/s0092-8674(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 15.Dujardin D, Wacker UI, Moreau A, Schroer TA, Rickard JE, DeMey JR. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J Cell Biol. 1998;141:849–62. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheel J, Pierre P, Rickard JE, et al. Purification and analysis of authentic CLIP-170 and recombinant fragments. J Biol Chem. 1999;274:25883–91. doi: 10.1074/jbc.274.36.25883. [DOI] [PubMed] [Google Scholar]
- 17.Pierre P, Pepperkok R, Kreis TE. Molecular characterization of two functional domains of CLIP-170 in vivo. J Cell Sci. 1994;107:1909–20. doi: 10.1242/jcs.107.7.1909. [DOI] [PubMed] [Google Scholar]
- 18.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 19.Fritzler MJ, Etherington J, Sokoluk C, Kinsella TD, Valencia DW. Antibodies from patients with autoimmune disease react with a cytoplasmic antigen in the Golgi apparatus. J Immunol. 1984;132:2904–8. [PubMed] [Google Scholar]
- 20.Fritzler MJ. Autoantibody testing. Procedures and significance in systemic rheumatic diseases. Meth Achiev Exp Pathol. 1986;12:224–60. [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Gordon J. Immunoblotting and dot immunobinding – current status and outlook. J Immunol Meth. 1984;72:313–40. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 23.Young RA, Davis RW. Efficient isolation of genes using antibody probes. Proc Natl Acad Sci USA. 1983;80:1194–8. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritzler MJ, Lung C-C, Hamel JC, Griffith K, Chan EKL. Molecular characterization of golgin-245: a novel Golgi complex protein containing a granin signature. J Biol Chem. 1995;270:31262–8. doi: 10.1074/jbc.270.52.31262. [DOI] [PubMed] [Google Scholar]
- 25.Griffith K, Chan EKL, Hamel JC, Miyachi K, Fritzler MJ. Molecular characterization of a novel 97 kDa Golgi complex autoantigen recognized by autoimmune antibodies from patients with Sjögren’s syndrome. Arthritis Rheum. 1997;40:1693–702. doi: 10.1002/art.1780400920. [DOI] [PubMed] [Google Scholar]
- 26.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucl Acids Res. 1984;12:387–95. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger B, Wilson D, Wolf E, Tonchev T, Milla M, Kim P. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–63. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–4. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 29.Adam SA, Nakagawa T, Swanson MS, Woodruff TK, Dreyfuss G. mRNA polyadenylate binding protein. gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986;6:2932–43. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes GRV, Asherson RA. Atypical lupus with special reference to ANA negative lupus and lupus subsets. Adv Nephrol. 1985;14:333–46. [PubMed] [Google Scholar]
- 31.Reichlin M. ANA negative systemic lupus erythematosus sera revisited serologically. Lupus. 2000;9:116–9. doi: 10.1191/096120300678828091. [DOI] [PubMed] [Google Scholar]
- 32.Buyon JP, Slade SG, Reveille JD, Hamel JC, Chan EKL. Autoantibody responses to the ‘native’ 52-kDa SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus, and Sjogren’s syndrome. J Immunol. 1994;152:3675–84. [PubMed] [Google Scholar]
- 33.Chou C-H, Reeves WH. Recognition of multiple epitopes in the coiled-coil domain of lamin B by human autoantibodies. Mol Immunol. 1992;29:1055–64. doi: 10.1016/0161-5890(92)90037-x. [DOI] [PubMed] [Google Scholar]
- 34.Reeves WH, Pierani A, Chou CH, et al. Epitopes of the p70 and p80 (ku) lupus autoantigens. J Immunol. 1991;146:2678–86. 35. [PubMed] [Google Scholar]
- 35.Furneaux HM, Dropcho EJ, Barbut D, et al. Characterization of a cDNA encoding a 34kd Purkinje neuron protein recognized by sera from patients with paraneoplastic cerebellar degeneration. Proc Natl Acad Sci USA. 1989;86:2837–77. doi: 10.1073/pnas.86.8.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]