Abstract
Plasma levels of soluble CD27 (sCD27) are elevated in diseases characterized by T cell activation and are used as a marker of immune activation. We assessed the usefulness of determining plasma sCD27 as a marker for monitoring immune activation in HIV-1-infected patients treated with highly active antiretroviral therapy (HAART). A first cross-sectional examination of 68 HIV-1-infected and 18 normal subjects showed high levels of sCD27 in HIV-1 infection; plasma sCD27 was correlated to HIV-1 viraemia and inversely correlated to CD4+ T cell count. Twenty-six HIV-1-infected patients undergoing HAART were studied at baseline and after 6, 12, 18 and 24 months of therapy. Seven additional patients under HAART were analysed at baseline, during and after interruption of therapy. In the total population, HAART induced a significant and progressive reduction, but not a normalization, of plasma levels of sCD27 after 24 months. A full normalization of plasma sCD27 was observed in the virological responders (undetectable HIV-1 RNA at months 18 and 24) and also in patients with moderate immunodeficiency at baseline (CD4+ T cell count >200 cells/mm3). Changes in plasma neopterin paralleled the changes in sCD27 but only baseline sCD27 levels were predictive of a greater increase in CD4+ T cell count during the follow-up. Discontinuation of therapy resulted in a rapid increase of sCD27 plasma levels associated with viraemia rebound and drop in CD4+ T cell count. Our findings suggest that plasma sCD27 may represent an alternative and simple marker to monitor immune activation during potent antiretroviral therapy. HIV-1-induced immune activation can be normalized by HAART in successfully treated patients where the disease is not advanced.
Keywords: HIV-1, HAART, immune activation, neopterin, soluble CD27
INTRODUCTION
The CD27 molecule is a transmembrane protein belonging to the tumour necrosis factor (TNF) receptor family which is expressed by peripheral T lymphocytes and memory B lymphocytes [1]. Antigen-specific T cell stimulation up-regulates the expression of membrane-bound CD27 [2,3] and induces the release of a soluble form of CD27 (sCD27), probably as a result of proteolytic cleavage mediated by a yet unknown metalloprotease [4–6]. In vitro, recombinant sCD27 has been shown to affect both T and B cell function, inhibiting T cell proliferation as well as pokeweed mitogen (PWM)-induced IgG secretion [1,7]. High levels of sCD27 have been detected in biological fluids from patients with autoimmune disorders involving T cell hyperactivity, such as rheumatoid arthritis and systemic lupus erythematosus [8,9]. Patients with multiple sclerosis also have increased levels of sCD27 in the cerebrospinal fluid (CSF) [10]. Moreover, high levels of sCD27 have been reported in plasma and CSF of patients with B cell malignancies [11,12] and AIDS-associated lymphoma [13]. These findings suggest that sCD27 may be a useful marker in disorders where immune activation plays an important role.
Chronic immune activation is also a hallmark of HIV-1 infection and plays a relevant role in the pathogenesis of AIDS. The elevated expression of lymphocyte activation molecules, mainly on T cells, is the major indication of the chronic immune activation [14–17]. In addition, high levels of soluble activation molecules such as neopterin, TNFα, TNFR-II, sIL-2R and Fas are found in biological fluids of HIV-1-infected subjects [18–20]. As shown recently, these plasma activation markers have prognostic significance for disease progression [21,22] and are being evaluated for monitoring the effects of antiretroviral treatment on immune activation [23–27].
Patients with HIV-1 infection are currently being treated with highly active antiretroviral therapy (HAART), a combination therapy using two reverse transcriptase inhibitors and at least one protease inhibitor. Such a therapeutic regimen effectively suppresses viral replication and increases CD4+ T cell count in the majority of patients. The therapy has also been shown to restore immune functions partially and reduce the status of immune activation [28–31]. However, full recovery and normalization of immune functions is not achieved during HAART, especially in patients with advanced disease [29,30,32]. In addition to being a useful marker of immune activation, sCD27 has been recently shown to be a significant predictor of CD4+ T cell count decline in a cohort of HIV-1-infected subjects [33]. We evaluated the possibility of using the plasma levels of sCD27 as a marker for therapy monitoring in a cohort of HIV-1-infected patients undergoing HAART.
MATERIALS AND METHODS
Patients and samples
Thirty-two HIV-1-infected subjects attending the Division of Infectious Diseases at the Huddinge University Hospital (Stockholm, Sweden) and 18 age-matched healthy volunteers were enrolled in the study after informed consent was given. The HIV-1-infected population was subdivided as follows: Group A included 26 patients undergoing HAART and followed-up for 24 months with samples collected at baseline and every 6 months. Subjects under HAART received a combination therapy made of two inhibitors of the viral transcriptase and at least one inhibitor of the viral protease. Group B was a control group consisting of six patients not receiving any therapy. The immunological and virological characteristics of patients in Groups A and B are summarized in Table 1. In addition, archival plasma samples stored at the Department of Molecular Biology, University of Siena (Siena, Italy) were obtained from seven patients at baseline (median 2 months before initiation of HAART, range 0–6), during HAART (median 14 months, range 5–25) and after the discontinuation of therapy (median 4 months, range 2–6).
Table 1.
Immunological and virological characteristics of patients in groups A and B at baseline and after 24 months of therapy
| Therapy | CD40 | CD424 | HIV RNA0 | HIV RNA24 | |
|---|---|---|---|---|---|
| Group A | HAART | 110 (10–660) | 390 (50–1010) | 5·06 (2·95–6·15) | 2·6 (1·7–4·17) |
| Group B | None | 385 (340–450) | 420 (260–700) | 3·78 (3–4·68) | n.d. |
CD4+ and CD8+ T cell counts are given as cells/mm3. HIV-1 RNA is expressed as log10 (copies/ml). Values are indicated as median (range).
Another group of 36 patients was included for a cross-sectional analysis of the levels of sCD27 in plasma. Twenty-one subjects were receiving HAART and 15 were drug-naive. The median CD4+ T cell count in this group was 390 cells/mm3 (range 110–910).
Quantification of HIV-1 RNA
Determination of HIV-1 RNA load in plasma was performed by using the ultrasensitive HIV Amplicor Monitor test (Roche Molecular Systems, Branchburg, NJ, USA). In the seven patients who interrupted therapy HIV-1 viraemia was measured by an in-house competitive RT-PCR as described elsewhere [34].
ELISA for sCD27 quantification
Soluble CD27 in plasma was quantified by a commercially available ELISA (CLB, Amsterdam, the Netherlands) according to the manufacturer’s instructions. Microtitre 96-well plates (Costar, Cambridge, MA, USA) were coated overnight at room temperature with 50 μl/well of anti-CD27 MoAb diluted 1:100 in PBS. CD27 standard curve and samples diluted 1:10 were added in duplicate wells and incubated for 1 h at RT. Detection of bound antigen was performed by adding biotinylated anti-CD27 MoAb and conjugated streptavidin-HRP. After incubation with tetramethylbenzidin (Pharmingen, San Diego, CA, USA) the optical densities were recorded at 450 nm.
Neopterin quantification
Neopterin was measured by commercially available ELISA (BRAHMS, Berlin, Germany) according to the manufacturer’s instructions. The lower detection limit of the test is 2 nmol/l, the 95th percentile in healthy controls was 8·7 nmol/l neopterin [35].
Statistical analysis
Statistical analyses were performed by using the software Prophet 6·0 (AbTech Corporation, VA, USA). Normal distribution of the data was checked by the Shapiro–Wilk test. Student’s t-test was used to analyse the differences in sCD27 levels between groups. Changes in CD4+ T cell counts, HIV-1 RNA, sCD27 and neopterin were evaluated using the Wilcoxon signed-rank test or a paired t-test, as appropriate. Linear regression or Spearman’s rank tests were used to analyse the correlation between variables. Data in the text are presented as mean ± s.e.m.
RESULTS
Plasma levels of sCD27 during potent antiretroviral therapy
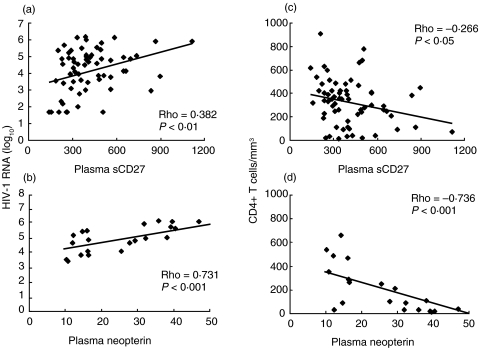
We analysed the plasma levels of sCD27 in the population of 68 HIV-1-infected and 18 uninfected subjects. The cross-sectional examination showed that in HIV-1-infected patients the sCD27 plasma levels are elevated compared to healthy donors (401 ± 23 versus 164 ± 8 U/ml, P < 0·001). As shown in Fig. 1 (upper panel), the plasma levels of sCD27 were correlated to HIV-1 plasma viraemia (P < 0·01) and inversely correlated to CD4+ T cell count (P < 0·05). These findings are in agreement with a recent study on HIV-1-infected Ethiopians reported by Messele et al.[33]. The sensitivity of plasma sCD27 was calculated in order to assess its use as an immunodeficiency marker. The cut-off value of 200 U/ml was calculated as the mean value plus one standard deviation of the sCD27 levels in the plasma of healthy individuals. Three of 68 patients had sCD27 plasma levels below the cut-off value with the sensitivity being 95%.
Fig. 1.
Correlation analysis between HIV viral load (a,b), CD4+ T cells (c,d) and plasma sCD27 (n = 68, a,c) and neopterin (n = 26, b,d). The analysis was performed with the Spearman rank correlation test.
In order to evaluate the reliability of sCD27 as a prognostic marker for disease progression and therapy monitoring, we also analysed the plasma concentration of neopterin, another immune activation and prognostic marker of HIV-1 infection. Cross-sectional analysis of the 26 HAART-treated subjects revealed a correlation between plasma sCD27 and neopterin (correlational coefficient = 0·534, P = 0·008). Similarly to plasma sCD27, neopterin levels were correlated to HIV-1 RNA load (P < 0·001) and inversely correlated to CD4+ T cell count (P < 0·001) (Fig. 1, lower panel).
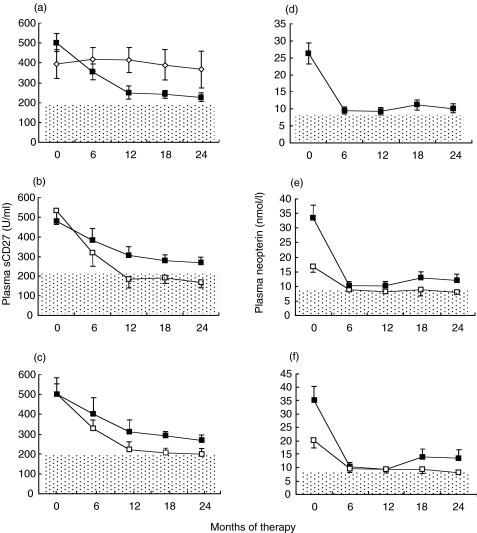
We analysed the variation of plasma sCD27 in the 26 HIV-1-infected subjects undergoing HAART. Longitudinal analysis showed that HAART induced a significant decrease of sCD27 already detectable 6 months after start of therapy (Fig. 2a). The levels of sCD27 after 24 months of therapy were significantly lower than at baseline (P < 0·001) but still higher than levels observed in the healthy subjects (P = 0·007). The sCD27 levels in untreated subjects remained constant throughout the follow-up period (Fig. 2a). Neopterin levels dropped dramatically up to 1 year after initiation of HAART (Fig. 2d) but remained higher than normal levels after 2 years of therapy.
Fig. 2.
Longitudinal analysis of the sCD27 (a,b,c) and neopterin (d,e,f) plasma levels in 26 patients undergoing HAART. In panels (a) and (d) six untreated patients (◊) are shown in addition to patients undergoing HAART (■). Panels (b) and (e) show patients grouped as moderately (MOD, n = 11) □, or severely (SEV, n = 15) ■, immunocompromised at baseline. In panels (c) and (f) patients were grouped into virological responders (n = 16) □, and non-responders (n = 10) ■, to HAART. Data are indicated as mean ± s.e.m. The dotted areas indicate the cut-off value of 200 U/ml for plasma sCD27 and 8·7 nmol/l for neopterin.
Plasma sCD27 and neopterin levels in relation to virological and immunological factors
To investigate which factors influenced the decrease of sCD27 levels we divided the treated group in two subgroups on the basis of the CD4+ T cell count at baseline. Patients were defined as severely (SEV, n = 15) or moderately (MOD, n = 11) immunodeficient if their CD4+ T cell count at baseline was below or above 200 cells/mm3, respectively. As illustrated in Fig. 2b, similar sCD27 plasma levels were detected in the two populations at baseline. In the MOD group the plasma levels of sCD27 were already fully normalized after 12 months from the onset of therapy and remained below the cut-off value of 200 U/ml until the end of the follow-up (Fig. 2b). On the contrary, such a normalization was not observed in the SEV subjects whose sCD27 levels were reduced by treatment but remained significantly higher than in control subjects (268 ± 26 U/ml versus 164 ± 8 U/ml, respectively, P = 0·002). The CD4= T cell count increased from 380 ± 43 to 583 ± 59 cells/mm3 in the MOD patients (P = 0·01) and from 53 ± 9 to 273 ± 38 cells/mm3 (P = 0·001) in the SEV group. Baseline plasma HIV-1 RNA was significantly higher in the SEV group compared to the MOD group (5·48 ± 0·17 and 4·17 ± 0·22 log copies/ml, respectively, P = 0·005). After 24 months, HAART induced a significant reduction in HIV-1 RNA load in both groups, although MOD patients had lower viraemia compared to SEV patients (1·84 ± 0·15 vs. 2·78 ± 0·27, respectively, P = 0·02).
Patients were then stratified in two groups on the basis of prolonged suppression of viral replication from month 18 to month 24 after therapy. Subjects were defined as virological responders to HAART if the viral load at months 18 and 24 was below the detection limit of 50 copies/ml. The baseline levels of sCD27 were similar in the two groups. As shown in Fig. 2c, the plasma levels of sCD27 in the group of virological responders (n = 16) were significantly lower than in the non-responders group (n = 10) at both months 18 and 24 (P < 0·05) and were comparable to levels in healthy subjects.
Detailed analysis of the changes in neopterin in relation to virological and immunological factors revealed a similar trend to the one observed for sCD27. As shown in Fig. 2e,f, the neopterin levels at end of follow-up were lower in both the MOD and responders groups compared to the SEV and non-responders groups, respectively.
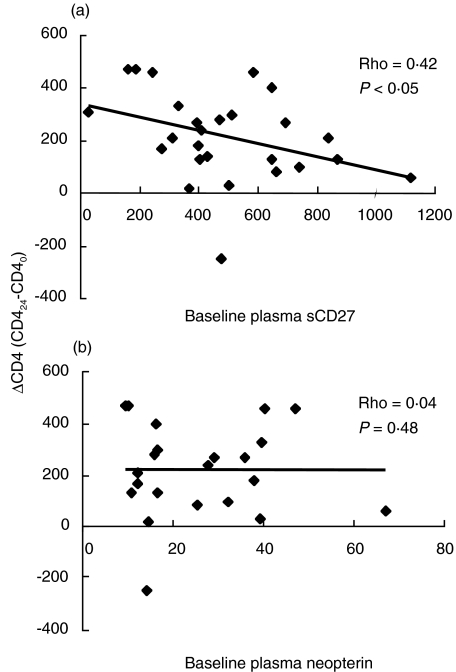
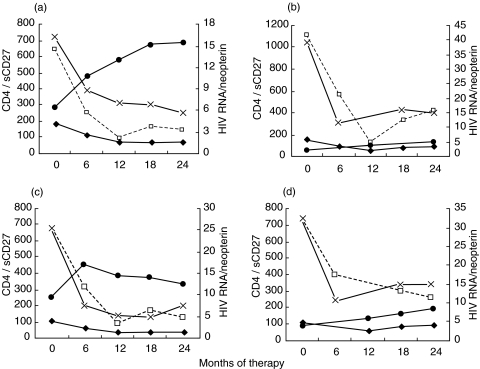
Correlation analysis indicated that the CD4+ T cell count at baseline was predictive of the absolute change of sCD27 (ΔsCD27 = sCD270−sCD2724) after 24 months (correlational coefficient = 0·44, P = 0·029) but it did not correlate to the change of neopterin concentration (correlational coefficient = 0·35, P = 0·108). In addition, we observed that low baseline levels of sCD27 were predictive of a greater increase in the CD4+ T cell count after 2 years of therapy (Fig. 3), although this correlation was not statistically very strong. Conversely, no correlation was observed between baseline plasma neopterin and the change in CD4+ T cell count after 24 months therapy in our settings (P = 0·48, Fig. 3). The changes in virological and immunological factors during therapy are shown in four representative patients in Fig. 4.
Fig. 3.
Correlation analysis between absolute change of CD4+ T cells after 24 months of therapy and plasma sCD27 (a) and plasma neopterin (b). Plasma sCD27 is measured as U/ml, neopterin as nmol/l and CD4+ T counts as cells/mm3. Analysis was performed with Spearman’s rank correlation test.
Fig. 4.
Changes in HIV-1 RNA (copies/ml,  ), CD4+ T cell count (cells/mm3, –•–), plasma sCD27 (U/ml,□) and plasma neopterin (nmol/l, ×) during therapy in four patients representative of the MOD group (a), SEV group (b), responders (c) and non-responders (d).
), CD4+ T cell count (cells/mm3, –•–), plasma sCD27 (U/ml,□) and plasma neopterin (nmol/l, ×) during therapy in four patients representative of the MOD group (a), SEV group (b), responders (c) and non-responders (d).
Effects of discontinuation of HAART on plasma sCD27
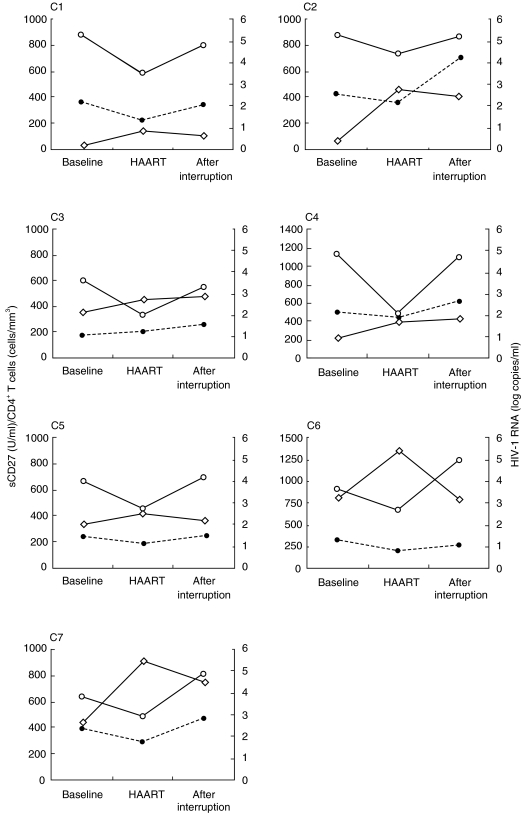
We next analysed the plasma levels of sCD27 in longitudinal samples from seven patients who discontinued HAART (Fig. 5). After a median of 14 months of HAART, the CD4+ T cell count had increased from 324 ± 100 cells/mm3 to 593 ± 153 cells/mm3 and HIV-1 RNA load had dropped from 4·4 ± 0·3 to 2·7 ± 0·3logcopies/ml. After discontinuation of therapy the viral load returned to pre-HAART levels (4·6 ± 0·2 log copies/ml) in parallel with a drop in CD4+ T cell count (480 ± 88 cells/mm3). Plasma levels of sCD27 followed the same kinetics as HIV-1 RNA, decreasing significantly after 14 months of therapy (from 341 ± 42 to 272 ± 36 U/ml, P = 0·01) and increasing above to pretherapy levels (408 ± 72 U/ml) after HAART discontinuation. Similarly, plasma neopterin dropped from a baseline value of 17·8 ± 10–6·6 ± 0·8 nmol/l during the therapy, with an increase to 10·8 ± 1·1 nmol/l after therapy interruption.
Fig. 5.
Longitudinal analysis of plasma sCD27 after interruption of HAART. Seven patients (C1-C7) were analysed at baseline, after 14 months of HAART and 4 months after interrupting therapy. Plasma sCD27 (U/ml, •) and CD4+ T cells (cells/mm3, ◊) are plotted on the left y axis and HIV-1 RNA (log copies/ml, ○) on the right y axis.
DISCUSSION
Immune activation is a major determinant in the pathogenesis of HIV-1 infection and several immune activation markers have been shown to correlate with disease progression. The use of immune activation markers has been suggested for the monitoring of HIV-1 infection and its outcome after antiretroviral therapy [23–27,33,36]. In the present study we have evaluated the effect of potent antiretroviral therapy on the plasma levels of soluble CD27, a marker of T cell activation. A first cross-sectional examination of 68 HIV-1-infected patients indicated that plasma sCD27 levels are increased in HIV-1 infection, and are correlated to HIV-1 viraemia and inversely correlated to CD4+ T cell count, indicating that sCD27 can be used as a marker of disease progression. In a cohort of 26 patients undergoing HAART and followed-up for 24 months the treatment induced a significant decrease, but not a normalization of the plasma concentration of sCD27. This result is well in line with the findings that also the levels of soluble activation molecules such as Fas, TNFα, TNFRs and neopterin are reduced but not normalized after administration of HAART [23–27].
However, we detected normal sCD27 levels in certain subgroups of patients. In the patients who were moderately immunosuppressed at the initiation of therapy (CD4+ T cell count >200 cells/mm3), the plasma levels of sCD27 were already normalized after 12 months of HAART and remained as low as in normal controls up to 2 years after the onset of therapy. The same effect was also found in the patients with optimal antiretroviral effect, as determined by undetectable plasma HIV-1 RNA for up to 24 months. On the other hand, in patients with more severe immunodeficiency at initiation of therapy the sCD27 plasma levels remained significantly above the levels detected in healthy individuals. The early treatment of asymptomatic or acutely HIV-1 infected subjects seems to induce both a quantitative and qualitative recovery of immune functions and a better control of viral replication [37–39]. In fact, the recovery of the immune system is often a function of the immunodepression status before commencing therapy[ 32,37–38,39[,. In agreement with these observations, we found that the CD4+ T cell count at baseline was predictive of the absolute change of plasma sCD27 after 24 months of HAART. The normalization of the plasma levels of sCD27 indicates a reduction of immune activation, although it does not provide information on the functional recovery of the immune system. In view of the importance of immune activation for HIV-1 pathogenesis, our findings suggest clearly that successful anti-HIV-1 therapy, possibly initiated early, may restore important immunological functions.
A dramatic drop in CD4+ T cell count associated with viraemia rebound occurs rapidly after discontinuation of HAART [40–42]. To our knowledge, the majority of studies on patients who interrupted HAART have focused on HIV-1 RNA dynamics and no study has evaluated the consequences on the immune activation status [40–43]. In seven treated subjects who discontinued HAART, we observed that the sCD27 plasma levels increased rapidly after therapy interruption, paralleling the kinetics of viral rebound and the drop in CD4+ T cell count. This observation indicates that discontinuation of therapy promotes viral replication and concomitantly a high degree of immune activation, further confirming the close relationship between immune activation and viral replication. The parallel kinetics of HIV viraemia and plasma sCD27 levels observed in our study supports the use of plasma sCD27 as a valuable marker to monitor immune activation during antiretroviral therapy. Our suggestion is also supported by the fact that in patients with low plasma levels of sCD27 at baseline HAART induced a greater increase of the CD4+ T cell counts, although this observation needs to be confirmed on a larger population. The analysis of the changes in plasma neopterin further supports the preferential use of sCD27 in therapy monitoring. In fact, although neopterin changes paralled the changes of sCD27 during the therapy, in our settings the baseline neopterin concentrations were not predictive of the changes in CD4+ T cell count. This finding is in agreement with a recent study performed on HIV-1-infected subjects in Ethiopia and showing that among several plasma markers of immune activation, the sCD27 was the best independent marker for disease progression [33]. Recently, Leng et al. have showed that immune activation is a major determinant of CD4+ T cell decline [36] suggesting that monitoring of immune activation might be more useful to evaluate the response to antiretroviral therapy. These findings provide the rationale and also the support for the assessment of sCD27 as a marker to monitor immune activation during therapy.
CD27 plays an important role in the regulation of B and T cell activation [1,7,44]. Cells expressing CD27 undergo apoptosis through interaction with the ligand CD70 [45]. The increased expression of CD70 on T cells during HIV-1 infection [46,47] may lead to the autocrine suicide of cells expressing CD27. Thus, during HIV-1 infection, the release of CD27 from activated T cells may also represent a strategy interfering with CD27-mediated apoptosis [45]. Recombinant sCD27 has also been shown to inhibit T cell proliferation induced by various stimuli in vitro and to reduce PWM-induced IgG secretion [7]. The presence of high levels of circulating sCD27 may therefore interfere with the normal regulation of CD70–CD27 interaction in B and T lymphocytes. The reduced plasma levels of sCD27 induced by antiretroviral therapy may represent a co-factor in restoring lymphocyte functions. The possibility that this phenomenon may be due to a direct effect of protease inhibitors (PIs) may not be excluded. Indeed, sCD27 is produced by the cleavage of the membrane-bound form mediated by a yet unknown metalloprotease and HIV-1 PIs might interfere with cellular protease activity. Plasma levels of sCD27 were reduced in humans by in vivo treatment with the metalloprotease inhibitor GI5204 [6].
Immune activation measured on T cells has been reported to correlate better than HIV-1 viraemia with the loss of CD4+ T cells [36], suggesting that assessment of immune activation (rather than HIV-1 viral load) may improve to monitor the response to antiviral treatment [22,36,48,49]. In our study we evaluated the effect of therapy on immune activation as measured by soluble CD27, a marker of T cell activation. Our findings suggest that the monitoring of the plasma levels of sCD27 may give important information about the immunological benefit of the therapy. An increasing number of studies have suggested the use of circulating markers of immune activation as an alternative and/or additional tool to evaluate the efficacy of antiretroviral therapies [22,48,49]. Our study shows for the first time that sCD27 plasma levels parallel the kinetics of HIV-1 viraemia and CD4+ T cell count during HAART, suggesting the use of plasma sCD27 not only as a prognostic marker [33] but also as a marker to monitor antiretroviral therapy. So far, the clinical monitoring of patients undergoing antiretroviral treatment has included evaluation of therapy efficacy through the measurements of CD4+ T cell count, viral load and cellular markers of immune activation. The availability of reliable soluble markers of immune activation easily measurable by ELISA may represent a feasible, cost-effective and simple complement and/or alternative to currently used virological–immunological markers, especially in places with poor hospital settings [49].
Acknowledgments
This work was supported by the Swedish Medical Research Council, the Swedish Physicians Against AIDS Research Foundation and the National Board of Health and Welfare. Angelo De Milito is a recipient of an AIDS fellowship from Istituto Superiore di Sanita’, Italy. The authors thank Dr Anna Nilsson for critical reading of the manuscript.
REFERENCES
- 1.Lens SMA, Tesselaar K, van Oers MHJ, van Lier RAW. Control of lymphocyte function through CD27–CD70 interactions. Semin Immunol. 1998;10:491–9. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 2.van Lier RAW, Borst J, Vroom TM, et al. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987;139:1589–96. [PubMed] [Google Scholar]
- 3.Bigler RD, Bushkin Y, Chiorazzi N. S152 (CD27). A modulating disulfide-linked T cell activation antigen. J Immunol. 1988;141:21–8. [PubMed] [Google Scholar]
- 4.Hintzen RQ, de Jong R, Hack CE, et al. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 5.Loenen WAM, de Vries E, Gravestein LA, et al. The CD27 membrane receptor, a lymphocytic-specific member of the nerve growth factor family, gives rise to a soluble form by protein processing that does not involve receptor endocytosis. Eur J Immunol. 1992;22:447–55. doi: 10.1002/eji.1830220224. [DOI] [PubMed] [Google Scholar]
- 6.Dekkers PP, ten Hove T, Lauw FN, et al. The metalloproteinase inhibitor GI5402 inhibits endotoxin-induced soluble CD27 and CD16 release in healthy human. Infect Immun. 2000;68:3036–9. doi: 10.1128/iai.68.5.3036-3039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agematsu K, Kobata T, Sugita K, et al. Role of CD27 in T cell immune response. Analysis by recombinant soluble CD27. J Immunol. 1994;153:1421–9. [PubMed] [Google Scholar]
- 8.Font J, Pallares L, Martorell J, et al. Elevated soluble CD27 levels in serum of patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1996;81:239–43. doi: 10.1006/clin.1996.0184. [DOI] [PubMed] [Google Scholar]
- 9.Tak PP, Hintzen RQ, Teunissen JJ, et al. Expression of the activation antigen CD27 in rheumatoid arthritis. Clin Immunol Immunopathol. 1996;80:129–38. doi: 10.1006/clin.1996.0106. [DOI] [PubMed] [Google Scholar]
- 10.Hintzen RQ, Paty D, Oger J. Cerebrospinal fluid concentrations of soluble CD27 in HTLV-I associated myelopathy and multiple sclerosis. J Neurol Neurosurg Psychiatry. 1999;66:791–3. doi: 10.1136/jnnp.66.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Oers MH, Pals ST, Evers LM, et al. Expression and release of CD27 in human B-cell malignancies. Blood. 1993;82:3430–6. [PubMed] [Google Scholar]
- 12.Kersten MJ, Evers LM, Dellemijn PL, et al. Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies. Blood. 1996;87:1985–9. [PubMed] [Google Scholar]
- 13.Widney D, Gundapp G, Said JW, et al. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol. 1999;93:114–23. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 14.Salazar-Gonzalez JF, Moody DJ, Giorgi JV, et al. Reduced ecto-5′-nucleotidase activity and enhanced OKT10 and HLA-DR expression on CD8 (T suppressor/cytotoxic) lymphocytes in the acquired immune deficiency syndrome: evidence of CD8 cell immaturity. J Immunol. 1985;135:1778–85. [PubMed] [Google Scholar]
- 15.Giorgi JV, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989;52:10–8. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 16.Prince HE, Jensen ER. Three-color cytofluorometric analysis of CD8 cell subsets in HIV-1 infection. J Acquir Immune Defic Syndr. 1991;4:1227–32. [PubMed] [Google Scholar]
- 17.Gehri R, Hahn S, Rothen M, et al. The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS. 1996;10:9–16. [PubMed] [Google Scholar]
- 18.Aukrust P, Liabakk NB, Muller F, et al. Activation of tumor necrosis factor-alpha system in HIV-1 infection: association with markers of immune activation. Infection. 1995;23:9–15. doi: 10.1007/BF01710050. [DOI] [PubMed] [Google Scholar]
- 19.Fadeel B, Samuelsson A, Hachiya T, Brostrom C, Chiodi F. Elevated serum levels of soluble Fas/APO-1 in human immunodeficiency virus-infected individuals. Blood. 1996;88:4727–30. [PubMed] [Google Scholar]
- 20.Sabri F, De Milito A, Ritva M, et al. Elevated levels of soluble Fas and Fas ligand in cerebrospinal fluid of patients with AIDS dementia complex. J Neuroimmunol. 2001;114:197–206. doi: 10.1016/s0165-5728(00)00424-0. [DOI] [PubMed] [Google Scholar]
- 21.Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–72. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 22.Fahey JL. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aukrust P, Muller F, Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 24.Stylianou E, Aukrust P, Kvale D, Muller F, Froland SS. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression – down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol. 1999;116:115–20. doi: 10.1046/j.1365-2249.1999.00865.x. 10.1046/j.1365-2249.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Milito A, Hejdeman B, Albert J, et al. High plasma levels of soluble Fas in HIV-1-infected subjects are not normalized during highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2000;16:1379–84. doi: 10.1089/08892220050140928. 10.1089/08892220050140928. [DOI] [PubMed] [Google Scholar]
- 26.Amirayan-Chevillard N, Tissot-Dupont H, Obadia Y, et al. Highly active antiretroviral therapy (HAART) and circulating markers of immune activation: specific effect of HAART on neopterin. Clin Diagn Lab Immunol. 2000;7:832–4. doi: 10.1128/cdli.7.5.832-834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroianni CM, Lichtner M, Mengoni F, et al. Changes in circulating levels of soluble cell adhesion molecules following highly active antiretroviral treatment of HIV-1-infected patients. Clin Immunol. 2000;95:212–7. doi: 10.1006/clim.2000.4865. 10.1006/clim.2000.4865. [DOI] [PubMed] [Google Scholar]
- 28.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann GR, Zaunders JJ, Cunningham P, Cooper DA. Phenotypic analysis of CD8+ T lymphocytes in a cohort of HIV type 1-infected patients treated with saquinavir, ritonavir, and two nucleoside analogs for 1 year, and association with plasma HIV type 1 RNA. AIDS Res Hum Retroviruses. 1999;15:963–72. doi: 10.1089/088922299310476. 10.1089/088922299310476. [DOI] [PubMed] [Google Scholar]
- 30.Zaunders JJ, Cunningham PH, Kelleher AD, et al. Potent antiretroviral therapy of primary human immunodeficiency virus type 1 (HIV-1) infection: partial normalization of T lymphocyte subsets and limited reduction of HIV-1 DNA despite clearance of plasma viraemia. J Infect Dis. 1999;180:320–9. doi: 10.1086/314880. [DOI] [PubMed] [Google Scholar]
- 31.Vigano A, Saresella M, Rusconi S, et al. Expression of CD38 on CD8 T cells predicts maintenance of high viraemia in HAART-treated HIV-1-infected children. Highly active antiretroviral therapy. Lancet. 1998;352:1905–6. doi: 10.1016/s0140-6736(05)60396-0. [DOI] [PubMed] [Google Scholar]
- 32.Mezzaroma I, Carlesimo M, Pinter E, et al. Long-term evaluation of T-cell subsets and T-cell function after HAART in advanced stage HIV-1 disease. AIDS. 1999;13:1187–93. doi: 10.1097/00002030-199907090-00006. 10.1097/00002030-199907090-00006. [DOI] [PubMed] [Google Scholar]
- 33.Messele T, Brouwer M, Girma M, et al. Plasma levels of viro-immunological markers in HIV-infected and noninfected Ethio1pians: correlation with cell surface activation markers. Clin Immunol. 2001;98:212–9. doi: 10.1006/clim.2000.4958. [DOI] [PubMed] [Google Scholar]
- 34.Zazzi M, Romano L, Catucci M, Venturi G, De Milito A, Valensin PE. Clinical evaluation of an in-house reverse transcription-competitive PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1999;37:333–8. doi: 10.1128/jcm.37.2.333-338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious and malignant diseases. Crit Rev Clin Lab Sci. 1992;29:307–41. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- 36.Leng Q, Borkow G, Weisman Z, Stein M, Kalinkovich A, Bentwich Z. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27:389–97. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]
- 37.Bisset LR, Cone RW, Huber W, et al. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities. Swiss HIV Cohort Study. AIDS. 1998;12:2115–23. doi: 10.1097/00002030-199816000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Plana M, Garcia F, Gallart T, et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS. 2000;14:1921–33. doi: 10.1097/00002030-200009080-00007. [DOI] [PubMed] [Google Scholar]
- 39.Al-Harti L, Siegel J, Spritzler J, Pottage J, Agnoli M, Landay A. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS. 2000;14:761–70. doi: 10.1097/00002030-200005050-00001. 10.1097/00002030-200005050-00001. [DOI] [PubMed] [Google Scholar]
- 40.Garcia F, Plana M, Vidal C, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 41.Harrigan PR, Whaley M, Montaner JSG. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS. 1999;12:F29–F62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 42.Hatano H, Vogel S, Yoder C, et al. Pre-HAART HIV burden approximates post-HAART viral levels following interruption of therapy in patients with sustained viral suppression. AIDS. 2000;14:1357–63. doi: 10.1097/00002030-200007070-00008. 10.1097/00002030-200007070-00008. [DOI] [PubMed] [Google Scholar]
- 43.Orenstein JM, Bhat N, Yoder C, et al. Rapid activation of lymph nodes and mononuclear cell HIV expression upon interrupting highly active antiretroviral therapy in patients after prolonged viral suppression. AIDS. 2000;14:1709–15. doi: 10.1097/00002030-200008180-00004. 10.1097/00002030-200008180-00004. [DOI] [PubMed] [Google Scholar]
- 44.Kobata T, Jacquot S, Kozlowski S, et al. CD27–CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci USA. 1995;92:11249–53. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad KV, Ao Z, Yoon Y, et al. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci USA. 1997;94:6346–51. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolthers KC, Otto SA, Lens SM, et al. Increased expression of CD80, CD86 and CD70 on T cells from HIV-infected individuals upon activation in vitro: regulation by CD4+ T cells. Eur J Immunol. 1996;26:1700–6. doi: 10.1002/eji.1830260806. [DOI] [PubMed] [Google Scholar]
- 47.De Milito A, Mörch C, Sönnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15:957–64. doi: 10.1097/00002030-200105250-00003. 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 48.Marchant A. Serological markers of disease activity in tubercolosis and HIV infection. Clin Exp Immunol. 2000;122:10–2. doi: 10.1046/j.1365-2249.2000.01371.x. 10.1046/j.1365-2249.2000.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosp M, Lisse IM, Quigley M, et al. An evaluation of low-cost progression markers in HIV-1 seropositive Zambians. HIV Med Res. 2000;1:125–7. doi: 10.1046/j.1468-1293.2000.00016.x. [DOI] [PubMed] [Google Scholar]