Abstract
Myeloid and plasmacytoid dendritic cells (DC) are present in cerebrospinal fluid (CSF) in non-inflammatory neurological diseases (NIND) and elevated in clinically definite multiple sclerosis (MS) and in early MS–acute monosymptomatic optic neuritis (ON). Here, we show that expression of CCR5, a chemokine receptor for regulated on activation, normal T cell expressed and secreted (RANTES) and macrophage inflammatory protein (MIP)-1α/β, is elevated on blood myeloid (CD11c+) DC in MS and ON compared to non-inflammatory controls. In contrast, expression of CXCR4, a receptor for stromal cell-derived factor (SDF)-1α, is similar in all groups. Blood myeloid DC from MS patients respond chemotactically to RANTES and MIP-1β, which are expessed in MS lesions. In active MS and ON, expression of CCR5 by myeloid DC in blood correlates with numbers of these cells in CSF. Thus, elevation of CCR5 may contribute to recruitment of myeloid DC to CSF in MS and ON. Recruitment of plasmacytoid DC to CSF appears to be CCR5-independent.
Keywords: cerebrospinal fluid, CCR5, dendritic cells, multiple sclerosis, optic neuritis
INTRODUCTION
Dendritic cells (DC) are professional antigen presenting cells (APC) that possess the exclusive ability to activate naive T cells. Human DC are usually divided into myeloid (CD11c+), plasmacytoid (CD11c–) and Langerhans cells [1]. All three subsets are capable of inducing primary T cell responses [1,2]. In addition, plasmacytoid DC can produce large amounts of type I interferons, potent antiviral and immunomodulatory cytokines [3,4].
The presence of professional APC in the central nervous system (CNS) has been debated, although certain cell types in the CNS have long been known to act as non-professional APC and to support secondary T cell responses [5,6]. Recent studies have shown that in the normal CNS, DC are located in meninges and choroid plexus, but are absent from the brain tissue [7–9]. In experimental inflammatory diseases of the CNS, DC appear in perivascular space and in parenchyma of the brain [7–11]. At least in mice, brain DC have a myeloid phenotype and are able to activate naive T cells [9,11].
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS associated with autoimmune response against myelin [12–14]. The reason for chronicity of MS is unknown. If DC can migrate to the CNS in MS, take up myelin antigens and present them to T cells either locally or in regional (e.g. deep cervical) lymph nodes, this could contribute to perpetuation of the autoimmune process. The ability of DC to induce autoimmunity has been demonstrated both in experimental and in clinical conditions [15,16]. As yet, the presence of DC in MS plaques has not been demonstrated, although CD68+CCR5+ cells found in the plaques and usually regarded as monocytes/macrophages [17] are phenotypically compatible with DC as well. We have recently described myeloid and plasmacytoid DC in human cerebrospinal fluid (CSF), which mirrors CNS inflammation [18]. While only a few DC were present in non-inflammatory CSF, there was a significant accumulation of myeloid and plasmacytoid DC in CSF from patients with MS, especially with early MS – acute monosymptomatic optic neuritis (ON). Phenotypically, myeloid and plasmacytoid DC in CSF were similar to corresponding DC subsets in blood [19,20]: myeloid DC in blood and CSF were CD4+, CD11c+, CD45RO+, CD83–, CD123dim, CCR5+, HLA-DR++, while plasmacytoid DC were CD4+, CD11c–, CD45RO–, CD83–, CD123high, CCR5+, HLA-DR+. Thus, it is likely that blood DC, similarly to other mononuclear cells (MNC), can enter the CNS, the process being enhanced in case of inflammation.
Recent studies have identified mechanisms of recruitment of immature DC to inflamed tissues. Immature monocyte-derived DC (moDC), frequently used as prototypic myeloid DC, respond chemotactically to a set of inflammatory chemokines, including monocyte chemotactic protein (MCP)-3, regulated on activation, normal T cell expressed and secreted (RANTES), and macrophage inflammatory protein (MIP)-1α/β (ligands of the chemokine receptors CCR1 and CCR5) [21–23]. Data for primary blood DC are more limited. Myeloid blood DC express high levels of CCR5 and CXCR4 [24], and have been shown to respond chemotactically to ligands of CCR2 (MCP-1), CCR5 (RANTES) and CXCR4 (SDF-1) [25]. Plasmacytoid DC express CCR5, CXCR4 and CXCR3 (a receptor for interferon-γ-inducible protein of 10 kDa [IP-10] and monokine induced by interferon-γ [Mig]) [3], and have been shown to respond to SDF-1 [25]. Importantly, ligands of CCR2 (MCP-1–3), CCR5 (RANTES, MIP-1α/β) and CXCR3 (IP-10, Mig) are expressed in MS plaques and/or elevated in MS CSF [26–30].
In this study, we attempted to approach the mechanisms underlying increased recruitment of DC from blood to CSF in MS and ON. First, we examined expression of CCR5, CXCR3 and CXCR4 by myeloid and plasmacytoid blood DC in MS, ON, non-inflammatory neurological diseases (NIND) and healthy donors. Secondly, we examined the ability of CSF from patients with MS, ON and NIND to chemoattract immature moDC from a healthy donor. Correlations of all these variables with numbers of DC in CSF were analysed.
MATERIALS AND METHODS
Patients and donors
Two groups of patients were included in the study. The first group included 18 patients with clinically definite MS (median age 43, range 17–68; 11 females; duration from onset 1–35 years), 13 patients with acute monosymptomatic ON (median age 32, range 22–46; seven females), 15 patients with NIND (median age 43, range 35–82; eight females) and 15 healthy donors (median age 40, range 27–65; 10 females). Venous blood samples (20 ml) were obtained from this group and used for determination of chemokine receptors on DC. From 10 MS and 6 ON patients, CSF samples were obtained at the same time as blood, and numbers of myeloid and plasmacytoid DC per 1 ml CSF were determined using flow cytometry.
The second group consisted of 15 patients with clinically definite MS (median age 35, range 17–67; 10 females; duration from onset 1–41 years), 10 with ON (median age 35, range 25–46; eight females) and 12 with NIND (median age 49, range 21–65; seven females). Age differences between groups were not significant. Nine of the MS patients, eight of the ON and none of the NIND patients had mononuclear CSF pleocytosis (>5 cells per 1 μl CSF). Blood and CSF samples (10–20 ml each) were obtained from all patients. Blood and CSF MNC were isolated and used for enumeration of myeloid and plasmacytoid DC by flow cytometry. CSF supernatants were used for chemotactic assays.
As far as the whole study is concerned, MS and ON were diagnosed according to accepted criteria [31,32]. None of the patients had ever received immunomodulatory therapy, including corticosteroids and interferon-β. All patients with MS and ON had oligoclonal IgG bands in CSF [33]. As this CSF finding in acute monosymptomatic ON indicates a 60–70% risk of developing clinically definite MS [34], we considered our ON patients as having early MS. Patients with NIND had no clinical evidence for CNS inflammation and no pleocytosis in CSF (total cell count ≤ 5 cells per 1 μl CSF).
Antibodies, recombinant chemokines and cytokines
Unlabelled anti-CXCR3 MoAb was from R&D Systems (Minneapolis, MA, USA). Fluorescein isothiocyanate (FITC)-labelled lineage cocktail (lin) and anti-CD1a, phycoerythrin (PE)-labelled anti-CD11c, anti-CD83 and anti-CD123, peridinin chlorophyll protein (PerCP)-labelled anti-HLA-DR, unlabelled anti-CCR5, anti-CXCR4, irrelevant IgG1 and IgG2a, streptavidine– peroxidase conjugate were from Becton Dickinson (Mountain View, CA, USA). PE-labelled IgG1 was from Dako (Copenhagen, Denmark). PE-labelled goat antimouse IgG Ab was from Serotec (Oxford, UK). Recombinant human IL-4, IP-10, MIP-1β and RANTES were from R&D Systems, recombinant human GM-CSF was from Novartis (Basel, Switzerland).
Isolation of blood and CSF MNC
Blood MNC were isolated by centrifugation against Lymphoprep density gradient (Nycomed, Oslo, Norway), washed twice, resuspended and counted. CSF was centrifuged at low speed, supernatants were collected, and cells gently resuspended in 1% BSA/PBS. CSF supernatants were stored at –70°C until use.
Detection and enumeration of DC in blood and CSF
Blood or CSF MNC were stained with FITC-lineage cocktail (lin), PE-anti-CD11c and PerCP-anti-HLA-DR (15 min, 4°C). Lineage cocktail (Becton Dickinson) consisted of FITC-labelled MoAbs against CD3, CD14, CD16, CD19, CD20 and CD56. Control samples were stained with irrelevant PE-IgG1 instead of PE-anti-CD11c. Cells were washed and analysed by FACScan flow cytometer and CellQuest software (both from Becton Dickinson). At least 5000 events per test in case of CSF were acquired. Corresponding blood samples served as positive controls (50 000 events per test). Myeloid DC were identified as lindim/–CD11c+HLA-DR++ MNC, and plasmacytoid DC as lindim/–CD11c–HLA-DR+ MNC. When anti-CD11c MoAb was replaced by anti-CD123, myeloid DC stained as CD123dim, and plasmacytoid as CD123high. To calculate numbers of DC subtypes per 1 ml of CSF, total CSF cell counts were multiplied by percentages of DC subtypes determined by flow cytometry.
Detection of chemokine receptors on blood DC
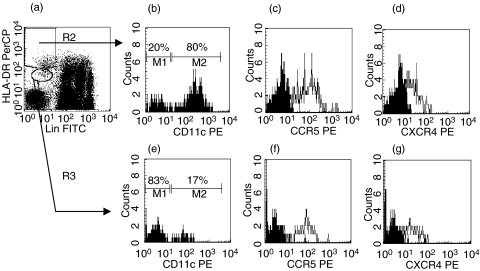
Blood MNC were first incubated with unlabelled antichemokine receptor mAbs or isotype controls, then with secondary PE-labelled goat antimouse antibody, and finally with FITC-lineage cocktail and PerCP-anti-HLA-DR, each step followed by a wash. A total of 100 000 events per test were acquired by FACScan/CellQuest, in order to have ≥100 DC of each subset in a sample. Myeloid DC were identified as lindim/–HLA-DR++ and plasmacytoid DC as lindim/–HLA-DR+ MNC (Fig. 1) with light scatter characteristics in between those of lymphocytes and monocytes. Thus, only two fluorescence channels (FITC and PerCP) were used for the discrimination between DC subsets [18,20], while the third one (PE) was used for detection of chemokine receptors. Levels of chemokine receptors on DC subsets were expressed as mean fluorescence intensity (MFI). In each case, background MFI was determined by staining with isotype-matched control MoAbs and subtracted from MFI values obtained with specific antichemokine receptor MoAbs.
Fig. 1.
Detection of CCR5 and CXCR4 on myeloid and plasmacytoid blood DC (three-colour flow cytometry). Blood MNC are distinguished from debris by light scatter properties (R1, not shown). (a) lindim/−HLA-DR++ cells (myeloid DC) and lindim/−HLA-DR+ cells (plasmacytoid DC) are gated by R2 and R3, respectively. (b) 80% of lindim/−HLA-DR++ express CD11c. (e) In contrast, most of lindim/−HLA-DR+ cells do not express CD11c. Based on this staining pattern, gates R2 and R3 were adjusted and kept through all tests from a given patient. Both myeloid and plasmacytoid DC express CCR5 (c,f) and CXCR4 (d,g). In c, d, f and g, empty histograms represent staining with antichemokine receptor MoAbs, and filled histograms represent staining with irrelevant IgG2a.
Generation of moDC
Immature moDC were generated from adherent blood MNC of a healthy donor by culture for 7 days in RPMI medium supplemented with 2 mml-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 10% fetal calf serum (all from Life Technologies, Paisley, UK), 800 U/ml GM-CSF and 500 U/ml IL-4. By flow cytometry, immature moDC on day 7 contained <10% lin+, >70% CD1a+ and <2% CD83+ cells.
Transwell chemotaxis assays
MoDC. CSF-and chemokine-induced chemotaxis of ‘indicator’ immature moDC from a healthy donor was assessed using 24-well transwell plates with porous (5 μm) inserts (Corning Costar Corporation, Cambridge, MA, USA), essentially as described for DC [23] and for CSF [35]. moDC were washed, resuspended in RPMI with 1% FCS and placed into upper chambers of transwells (5 × 104/100 μl/well), while lower chambers contained 600 μl of either medium or CSF supernatants diluted 1/2 (v/v) in the same medium. Recombinant RANTES (50 ng/ml) was used as a positive control. After a 90-min incubation at 37°C, inserts were lifted, washed on their upper surface with PBS, and Giemsa-stained on their lower surface, so as to visualize migrated DC. Migrated cells in 10 consecutive high-power (×250) fields along diameter of each insert were counted. Assays were performed in duplicate. Chemotactic effects of chemokines or CSF supernatants were expressed as chemotactic factors (CF):
Blood DC. Blood DC were enriched by centrifugation of blood MNC over a discontinuous (34%: 47·5%: 60%) gradient of Percoll (Pharmacia, Uppsala, Sweden). Low density MNC containing DC and monocytes, and depleted of lymphocytes were collected from the 34%: 47·5% interphase, resuspended in medium (RPMI with 2% human AB serum), and placed in upper chambers of transwells in triplicate (3 × 105/100 μl/well). Lower chambers contained 600 μl of medium or diluted chemokines. After a 2-h incubation at 37°C, inserts were lifted, and cells migrated to their lower surface were washed off into lower chamber by 2 ml cold PBS, collected (triplicate wells were pooled), spun down, resuspended in 100 μl PBS, and counted. Initial and migrated cell populations were stained with FITC-lin, PE-anti-CD123 and PerCP-anti-HLA-DR. Percentages of myeloid (lindim/–CD123dimHLA-DR++) and plasmacytoid (lindim/–CD123highHLA-DR+/++) DC among initial and migrated cells were determined by flow cytometry, and absolute numbers of myeloid and plasmacytoid DC among initial and migrated populations were calculated. Results of the assay were presented as (1) chemotactic factors (calculated as for moDC); and (2) percentages of migrated DC in relation to input.
Mixed leucocyte reaction (MLR)
To evaluate the functional importance of DC in CSF, irradiated (30 Gy) blood or CSF MNC from six MS patients (5 × 104/well) were co-cultured for 72 h in triplicate with 5 × 104 allogeneic T cells (this would give a DC:T ratio in the order of 1:100). T cells were isolated from blood MNC of a healthy donor using nylon wool columns. Co-cultures were pulsed with 0·5 μCi/well of [3H]thymidine for another 18h. [3H]thymidine uptake was analysed by a β-counter. As the degree of HLA mismatch between stimulators and responders was unknown, the following correction procedure was used. First, the degree of HLA mismatch was estimated for each patient as a ratio between cpm of the T cell/blood MNC co-culture and cpm of T cells alone (stimulation index). Then, cpm of the T cell/CSF MNC co-cultures were divided by this stimulation index, and these corrected cpm values were used for analysis.
Statistics
Several groups were compared by non-parametric anova (Kruskal–Wallis test). If P < 0·05 was obtained, differences between pairs of groups were further tested by Mann–Whitney U-test. Correlations between two variables were tested by Spearman rank correlation test.
RESULTS
CCR5 is elevated on myeloid blood DC in MS and ON
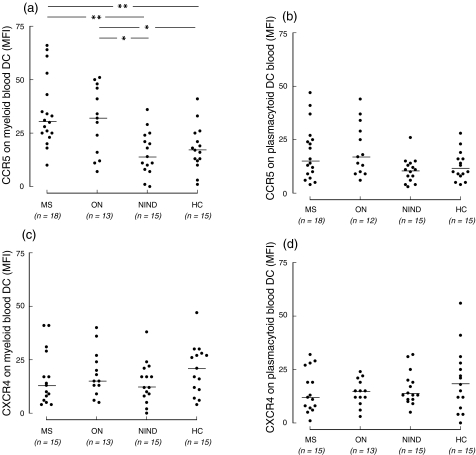
In the first part of the study, expression of CCR5, CXCR3 and CXCR4 by blood DC in MS, ON, NIND and healthy donors was examined by flow cytometry. The principle of chemokine receptor detection on blood DC subsets is shown in Fig. 1. Expression of CCR5 by myeloid blood DC was increased in patients with MS and ON, compared to patients with NIND or healthy donors (Fig. 2a). In contrast, there was no difference between healthy donors and patients with NIND. A slight increase of CCR5 on plasmacytoid blood DC was observed only in patients with ON, compared to patients with NIND and healthy donors (Fig. 2b). There was no correlation between duration of MS and expression of CCR5 by plasmacytoid and myeloid DC. Levels of CXCR4 on myeloid or plasmacytoid blood DC did not differ between the groups (Fig. 2d,c). We could not detect any significant expression of CXCR3 by blood DC subsets.
Fig. 2.
Expression of CCR5 and CXCR4 by myeloid (a,c) and plasmacytoid (b,d) blood DC in multiple sclerosis (MS), acute monosymptomatic optic neuritis (ON), non-inflammatory neurological diseases (NIND) and in healthy controls (HC), as determined by flow cytometry. Myeloid and plasmacytoid DC were identified as described in Materials and methods and Fig. 1. Levels of CCR5 and CXCR4 on myeloid and plasmacytoid DC are presented as mean fluorescence intensity (MFI) of entire cell populations. *P < 0·05; **P < 0·01. Horizontal bars are medians.
Myeloid blood DC responds to RANTES and MIP-1β
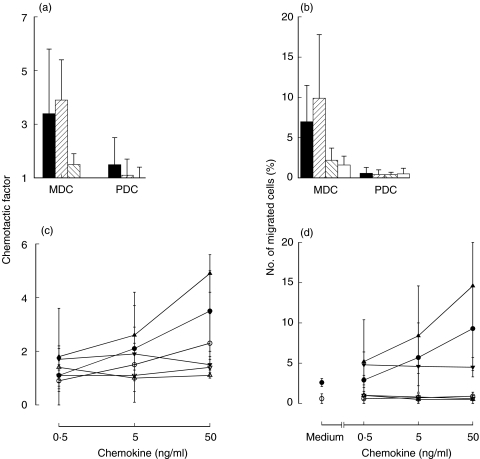
To test whether CCR5 on blood DC subsets is functional, we studied chemotactic responses of myeloid and plasmacytoid blood DC to two CCR5 ligands, RANTES and MIP-1β (see Materials and methods). DC were enriched from blood of nine MS patients. Both RANTES and MIP-1β induced chemotaxis of myeloid DC, but had little effect on plasmacytoid DC (Fig. 3). IP-10 was chemotactically inactive towards both DC subsets (Fig. 3)
Fig. 3.
Chemotactic responses of myeloid and plasmacytoid blood DC to recombinant RANTES, MIP-1β and IP-10, studied by transwell assay. Low-density fraction of MNC containing all DC and monocytes was isolated from blood of MS patients using Percoll density gradients. By flow cytometry, percentage of either DC subset in this fraction varied between 1 and 3%. The low density MNC were applied to wells in triplicates, 3 × 105 cells/well (thus, 3000–9000 cells of either DC subset per well). Cells migrated during 120 min were collected (triplicate wells were pooled) and analysed by flow cytometry as described in Materials and methods. (a,b) Chemotactic responses of DC from nine MS patients. All chemokines at 50 ng/ml. Mean ± s.d. ■, RANTES; , MIP-1β;
, MIP-1β; , IP-10, □, medium. MDC, myeloid DC; PDC, plasmacytoid DC. (c,d) Dose–response curves for each of the three chemokines and two DC subsets, studied in three MS patients. Closed symbols, myeloid DC; open symbols, plasmacytoid DC. Mean ± s.d. (a) and (c), chemotactic factors; (b) and (d), % of migrated cells in relation to the initial number.
, IP-10, □, medium. MDC, myeloid DC; PDC, plasmacytoid DC. (c,d) Dose–response curves for each of the three chemokines and two DC subsets, studied in three MS patients. Closed symbols, myeloid DC; open symbols, plasmacytoid DC. Mean ± s.d. (a) and (c), chemotactic factors; (b) and (d), % of migrated cells in relation to the initial number.
Expression of CCR5 by myeloid DC in blood correlates with their numbers in CSF
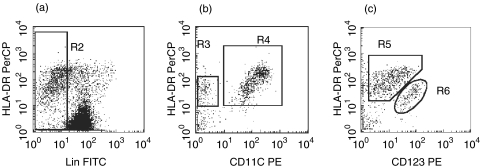
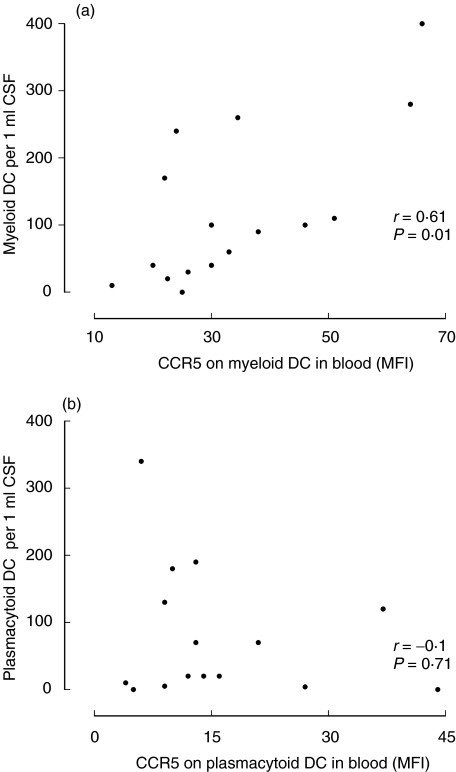
Although expression of CCR5 by myeloid DC was elevated in MS and in ON – early MS (Fig. 2a), it nevertheless varied between patients, which could influence the responsiveness of DC to ligands of CCR5 (RANTES and MIP-1α/β) expressed in MS brain[26,28,30] and, hence, recruitment of myeloid DC to the CNS. We therefore analysed correlation between expression of CCR5 by myeloid DC in blood and numbers of myeloid DC in CSF. Such data were available in 10 patients with MS (eight of them had CSF pleocytosis and thus active MS) and six with acute ON (all with CSF pleocytosis). Numbers of myeloid DC in CSF of these 16 patients varied between 5 and 520 per 1 ml (a CSF picture from an ON patient with high numbers of CSF DC is shown in Fig. 4) and correlated strongly with MFI of CCR5 on myeloid DC in corresponding blood samples (r = 0·61, P = 0·01, Fig. 5a). In contrast, no such correlation was observed for plasmacytoid DC (r =–0·1, P =0·7, Fig. 5b). Numbers of myeloid or plasmacytoid DC in CSF did not correlate with expression of CXCR4 by corresponding DC subsets in blood (data not shown).
Fig. 4.
Myeloid and plasmacytoid DC in the CSF from a patient with acute monosymptomatic optic neuritis (three-colour flow cytometry). CSF MNC are distinguished from debris by light scatter characteristics (R1, not shown). (a) Lindim/− cells are gated in R2. (b) Lindim/− cells are plotted against CD11c and HLA-DR. Myeloid DC are HLA-DR++CD11c+ cells (R4, around 4% of CSF MNC), plasmacytoid DC are HLA-DR+CD11c– cells (R3, around 1% of CSF MNC). (c) In another test from the same CSF sample, lindim/− cells are plotted against CD123 and HLA-DR. Myeloid DC are HLA-DR++CD123dim (R5), plasmacytoid DC are HLA-DR+CD123high (R6).
Fig. 5.
(a) Correlation between expression of CCR5 by myeloid DC in blood and numbers of myeloid DC in CSF. (b) Absence of such correlation for plasmacytoid DC.
To evaluate functional significance of DC in CSF, we examined the ability of CSF MNC to induce allogeneic MLR. Four of six CSF MNC samples induced a higher proliferation of allogeneic T cells than did blood MNC from the same patients. MLR values obtained with CSF MNC and corrected for HLA mismatch correlated with percentages of myeloid DC (r =0·89, P < 0·05, n =6), but not with percentages of plasmacytoid DC, all lin– cells, all HLA-DR+ cells, or lin+CD11c+ cells. Given low total numbers of DC in CSF samples, we could not sort these cells and study their allostimulatory capacity directly.
Chemotactic activity of the CSF for DC is not increased in MS and ON
In the second part of the study, we analysed whether factors present in the CSF itself could contribute to increased recruitment of myeloid and plasmacytoid DC to CSF in MS and ON. CSF was obtained from 15 patients with MS, 10 with ON and 12 with NIND, and CSF DC were quantified by flow cytometry (Table 1). Compared to NIND, numbers of myeloid and plasmacytoid DC were significantly elevated in ON, and to a lesser extent in clinically definite MS. We next directly evaluated the ability of CSF supernatants from these patients to induce chemotaxis of ‘indicator’ immature moDC from a healthy donor. MoDC were used instead of blood DC, as it was technically difficult to obtain blood DC at necessary amounts. In transwell assays, practically all CSF supernatants induced chemotaxis of immature moDC (Table 1). The responses of moDC were truly chemotactic but not chemokinetic, since placing CSF supernatants into both chambers of transwells completely abolished transmembrane migration (not shown). However, chemotactic activity (CFmoDC) of CSF from patients with MS and ON was not increased compared to that of patients with NIND (Table 1). It also did not correlate with numbers of DC in CSF, when analysed separately in MS, ON and NIND (by Spearman rank correlation test).
Table 1.
Median (min–max) numbers of myeloid and plasmacytoid DC per 1 ml CSF and chemotactic activity of CSF (CFmoDC)
| MS (n =15) | ON (n =10) | NIND (n =12) | |
|---|---|---|---|
| CD11c+ DC (per 1 ml) | 40 (0–400) | 105 (30–520)*** | 10 (0–60) |
| CD11c− DC (per 1 ml) | 20 (0–340)* | 125 (0–200)*** | 10 (0–30) |
| CFmoDC | 3·8 (1·8–9·2) | 3·8 (2·5–11·6) | 2·8 (0·9–7·4) |
aPercentages of DC subsets among CSF MNC were determined by flow cytometry (see Materials and methods), and numbers per 1 ml CSF were calculated by multiplying percentages of DC by total CSF cell count. CSF-induced chemotaxis of immature moDC was assessed by transwell chemotaxis assay and expressed as chemotactic factor (CFmoDC), calculated as number of moDC migrated in response to CSF divided by number of moDC migrated in response to medium alone.
P < 0·05;
P < 0·001 compared to NIND.
DISCUSSION
Recent studies in animal models have shown that DC, being absent from normal brain, appear in this organ during inflammation [8–11,36],. This is an important change within the immunological environment of the brain, since unlike other brain APC, DC are able to activate naive T cells and, potentially, to migrate from the CNS to deep cervical lymph nodes [37] and present antigens outside the CNS. Perivascular localization of DC in experimental allergic encephalomyelitis and toxoplasmic encephalitis[8–11,36], indicates that these cells may arise from blood precursors, although development from a CNS-resident precursor has also been suggested [9,38]. The two DC subsets observed by us in human CSF are phenotypically similar to myeloid and plasmacytoid DC in blood [18]. Therefore, we propose that myeloid and plasmacytoid DC in the CSF are recruited from the circulating DC. It has been reported that myeloid DC can pass through endothelium regardless of its activation state, and plasmacytoid DC can pass through activated endothelium [39]. Circulating myeloid DC are able to home to the sites of inflammation [40]. Thus, both cell types may cross the blood–brain or blood–CSF barrier, the process being enhanced in MS and ON.
Although nearly all CSF supernatants induced chemotaxis of moDC in vitro, this phenomenon appeared not to account for increased recruitment of DC in MS and ON (Table 1). Chemotactic activity of the CSF may contribute to basal recruitment of DC that may occur independently of inflammation [41] and give rise to the small numbers of DC seen in non-inflammatory CSF (Table 1). In additional experiments with neutralization of chemokines in CSF (not shown), we could not attribute this chemotactic activity to the presence of IP-10, MCP-1, MIP-1β, RANTES or SDF-1α. Increased numbers of CSF DC observed in MS and ON are probably due to shedding of DC from plaques located close to the CSF space (e.g. in periventricular white matter, optic nerves and corpus callosum), or from meningeal infiltrates, which are characteristic for active MS and probably represent the same inflammatory process as in MS lesions [42]. Thus, the numbers of DC in CSF may reflect DC infiltration in inflammatory lesions, and may therefore depend on the levels of chemokines within the plaques and on expression of cognate chemokine receptors by DC in blood.
The role of chemokines and chemokine receptors in MS has been investigated extensively. In particular, expression of CCR5 ligands (RANTES and MIP-1α/β) and presence of CCR5+ cells has been demonstrated in MS plaques [26,28–30],. In the present study, we found that myeloid blood DC in MS and MS-type ON express increased levels of CCR5, compared to NIND and healthy controls (Fig. 2a). Myeloid DC show a clear chemotactic response to RANTES and MIP-1β (Fig. 3), and the expression of CCR5 by myeloid DC in blood in MS and ON correlates with numbers of these cells in CSF (Fig. 5a). This suggests that the higher the expression of CCR5 by myeloid blood DC, the more actively do they respond to RANTES and MIP-1α/β present in MS brain. It is unclear whether elevated expression of CCR5 by myeloid blood DC is a primary abnormality in MS, or is induced by inflammatory mediators released from demyelinating lesions. Overexpression of CCR5 by T cells in MS has already been reported [43]. Thus, similar mechanisms may contribute to recruitment of both T cells and DC to MS lesions, resulting in enhancement of inflammation. Our data do not exclude that other chemokines expressed in MS plaques also participate in recruitment of myeloid DC to CSF.
Plasmacytoid DC marginally responded to RANTES and MIP-1β (Fig. 3), and expression of CCR5 by these cells in blood did not correlate with their numbers in CSF (Fig. 5b), suggesting that recruitment of plasmacytoid DC is CCR5 independent. We have observed previously that numbers of plasmacytoid DC in CSF are very high in Lyme meningoencephalitis and Behçet syndrome [18]. CSF of these patients contains high levels of SDF-1α, while blood plasmacytoid DC show a strong chemotaxis towards recombinant SDF-1α[44]. Although CSF levels of this chemokine in MS and ON are not changed compared to non-inflammatory controls (our unpublished observation), SDF-1α may be produced locally in MS plaques, contributing to plasmacytoid DC recruitment via the CXCR4 receptor. In addition, recruitment of plasmacytoid DC may depend on expression of certain molecules by activated brain endothelium, particularly on l-selectin ligands. l-selectin is highly expressed by plasmacytoid DC in blood [3], and a recent paper reported that accumulation of plasmacytoid DC in allergic nasal mucosa correlates with increased expression of peripheral lymph node addressin (PNAd), a ligand for l-selectin, by nasal endothelium [45]. Immunohistochemical studies of these molecules in MS plaques will be required to understand better plasmacytoid DC recruitment.
The roles of DC in the CNS, and particularly in the CSF, remain to be established both in normal and inflammatory conditions. A strong correlation between percentages of myeloid DC among CSF MNC and the ability of CSF MNC to induce allogeneic MLR (r =0·89, P < 0·05) indicates that myeloid DC may be a major type of APC in the CSF. Accumulation of DC in CSF in ON – early MS (Table 1) suggests a role for CSF DC in early events of MS pathogenesis. An intriguing possibility is a recently described migration of mature DC from CSF to deep cervical lymph nodes [37]. Presentation of myelin antigens by such DC in the lymph nodes may restimulate the autoimmune response at early stages of MS, resulting in a chronic disease.
In conclusion, expression of CCR5 by myeloid blood DC is elevated in MS and its early form, acute monosymptomatic optic neuritis, which may contribute to recruitment of myeloid DC to CNS/CSF. Recruitment of plasmacytoid DC appears to be CCR5-independent.
Acknowledgments
This study was supported by the Swedish Medical Research Council, the Swedish Association of the Neurologically Disabled (NHR), Karolinska Institute, and by an unrestricted research grant from Biogen Inc., Cambridge, MA, USA.
REFERENCES
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, Liu Y-J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 4.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 5.Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984;307:273–6. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- 6.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 7.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–62. 10.1002/(sici)1096-9861(19990322)405:4<553::aid-cne8>3.0.co;2-6. [PubMed] [Google Scholar]
- 8.Suter T, Malipiero U, Otten L, et al. Dendritic cells and differential usage of the MHC class II transactivator promoters in the central nervous system in experimental autoimmune encephalitis. Eur J Immunol. 2000;30:794–802. doi: 10.1002/1521-4141(200003)30:3<794::AID-IMMU794>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–26. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 10.Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74:599–608. doi: 10.1016/0306-4522(96)00160-1. 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 11.Fischer H-G, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–34. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 12.Link H, Baig S, Jiang Y-P, et al. B cells and antibodies in MS. Res Immunol. 1989;140:219–26. doi: 10.1016/0923-2494(89)90091-6. [DOI] [PubMed] [Google Scholar]
- 13.Hohlfeld R, Londei M, Massacesi L, Salvetti M. T-cell autoimmunity in multiple sclerosis. Immunol Today. 1995;16:259–61. doi: 10.1016/0167-5699(95)80176-6. [DOI] [PubMed] [Google Scholar]
- 14.Martino G, Hartung H-P. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr Opin Neurol. 1999;12:309–21. doi: 10.1097/00019052-199906000-00010. 10.1097/00019052-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–9. [PubMed] [Google Scholar]
- 16.Mackensen A, Herbst B, Chen JL, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34 (+) hematopoietic progenitor cells. Int J Cancer. 2000;86:385–92. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- 18.Pashenkov M, Huang Y-M, Kostulas V, Söderström M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–92. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 19.Kohrgruber N, Halanek N, Groger M, et al. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol. 1999;163:3250–9. [PubMed] [Google Scholar]
- 20.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. 10.1002/(sici)1521-4141(199909)29:09<2769::aid-immu2769>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Lin C-L, Suri RM, Rahdon RA, Austyn JM, Roake JA. Dendritic cell chemotaxis and transendothelial migration are induced by distinct chemokines and are regulated on maturation. Eur J Immunol. 1998;28:4114–22. doi: 10.1002/(SICI)1521-4141(199812)28:12<4114::AID-IMMU4114>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. 10.1002/(sici)1521-4141(199809)28:09<2760::aid-immu2760>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 26.Hvas J, McLean C, Justesen J, et al. Perivascular T cells express the pro-inflammatory chemokine RANTES mRNA in multiple sclerosis lesions. Scand J Immunol. 1997;46:195–203. doi: 10.1046/j.1365-3083.1997.d01-100.x. [DOI] [PubMed] [Google Scholar]
- 27.McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical in situ hybridization study. J Neuroimmunol. 1998;86:20–9. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 28.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5 (+) and CXCR3 (+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–15. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boven LA, Montagne L, Nottet HS, De Groot CJ. Macrophage inflammatory protein-1alpha (MIP-1alpha), MIP-1beta, and RANTES mRNA semiquantification and protein expression in active demyelinating multiple sclerosis (MS) lesions. Clin Exp Immunol. 2000;122:257–63. doi: 10.1046/j.1365-2249.2000.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 32.Perkin GD, Rose FC. Oxford, UK: Oxford University Press; Optic neuritis and its differential diagnosis. [Google Scholar]
- 33.Kostulas VK, Link H. Agarose isoelectric focusing of unconcentrated CSF and radioimmunofixation for detection of oligoclonal bands in patients with multiple sclerosis and other neurological diseases. J Neurol Sci. 1982;54:117–7. doi: 10.1016/0022-510x(82)90224-6. [DOI] [PubMed] [Google Scholar]
- 34.Söderström M, Jin Y-P, Hillert J, Link H. Optic neuritis: prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology. 1998;50:708–14. doi: 10.1212/wnl.50.3.708. [DOI] [PubMed] [Google Scholar]
- 35.Lahrtz F, Piali L, Nadal D, et al. Chemotactic activity on mononuclear cells in the cerebrospinal fluid of patients with viral meningitis is mediated by interferon-γ inducible protein-10 and monocyte chemotactic protein-1. Eur J Immunol. 1997;27:2484–9. doi: 10.1002/eji.1830271004. [DOI] [PubMed] [Google Scholar]
- 36.Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol. 1999;154:481–94. doi: 10.1016/S0002-9440(10)65294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer H-G, Bielinsky AK. Antigen presentation function of brain-derived dendriform cells depends on astrocyte help. Int Immunol. 1999;11:1265–74. doi: 10.1093/intimm/11.8.1265. [DOI] [PubMed] [Google Scholar]
- 39.Pettit AR, Thomas R. Dendritic cells: the driving force behind autoimmunity in rheumatoid arthritis? Immunol Cell Biol. 1999;77:420–7. doi: 10.1046/j.1440-1711.1999.00855.x. [DOI] [PubMed] [Google Scholar]
- 40.Robert C, Fuhlbrigge RC, Kieffer JD, et al. Interaction of dendritic cells with skin endothelium: a new perspective on immunosurveillance. J Exp Med. 1999;189:627–36. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: a potential reservoir of HIV in the central nervous system. Hum Pathol. 1998;29:88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- 42.Guseo A, Jellinger K. The significance of perivascular inflitrations in multiple sclerosis. J Neurol. 1975;211:51–60. doi: 10.1007/BF00312463. [DOI] [PubMed] [Google Scholar]
- 43.Zang Y-C, Samanta AK, Halder JB, et al. Aberrant T cell migration toward RANTES and MIP-1alpha in patients with multiple sclerosis. Overexpression of chemokine receptor CCR5. Brain. 2000;123:1874–82. doi: 10.1093/brain/123.9.1874. [DOI] [PubMed] [Google Scholar]
- 44.Pashenkov M, Teleshova N, Kouwenhoven M, et al. Recruitment of dendritic cells to cerebrospinal fluid in bacterial neuroinfections. J Neuroimmunol. 2002;122:106–16. doi: 10.1016/s0165-5728(01)00451-9. [DOI] [PubMed] [Google Scholar]
- 45.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062–8. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]