Abstract
We previously demonstrated a specific gluten-induced response in the rectal mucosa of coeliac patients. In the present study, we have evaluated the immune response to local gliadin challenge in the nasal mucosa of coeliac patients preliminary to exploring the feasibility of immune modulation by the nasal route. The local response to gliadin was evaluated on non-invasive scrapings of nasal mucosa. Cells harvested from the nasal scrapings of 21 coeliac patients and 12 healthy controls were counted after immunohistochemical staining. Six hours after gliadin challenge, the total number of cells was increased in coeliacs but not in controls. The increase was due principally to lymphoid cells and granulocytes. CD3+ cells doubled after gliadin challenge, but not after albumin control challenge. There was a similar rise in CD25+ cells, whereas the number of ICAM-expressing cells did not increase significantly. In control subjects, both gliadin and albumin induced a moderate but not significant increase in total cell number. In conclusion, the gliadin antigen provokes a mild inflammatory response in coeliac nasal mucosa.
Keywords: coeliac disease, gliadin challenge, mucosal scraping, nasal mucosa, immune modulation, human response
INTRODUCTION
Coeliac disease (CD) is a permanent intolerance to dietary wheat gliadin and related proteins [1]. It is characterized by a breakdown in immune tolerance to gluten, and the main target is the jejunal mucosa. Indeed, histological damage of the small intestinal mucosa, caused by a T-cell-mediated dysregulated local immune response to gliadin [2], is the hallmark of the disease.
Loft and colleagues [3] and our group [4] evaluated the local immune response of mucosa-associated lymphoid tissue other than jejunum, and found that local instillation of digested gliadin on the rectal mucosa triggers a specific inflammatory response that is not observed with other proteins. Similarly, Lahteenoja et al.[5] recently reported that local instillation of gliadin provokes a specific immune response in the oral mucosa of CD patients but not in that of controls; subsequent to gliadin challenge, CD4+ and CD8+ lymphocytes were increased in the epithelium, and CD4+ cells were increased in the lamina propria.
Nasal mucosa could be the elective site for the induction of tolerance to respiratory allergens [6,7]. In the mouse, nasal mucosa has been used to induce systemic tolerance in experimental autoimmune diseases [8–13]. Local exposure to the antigen stimulates, via Th1 priming, a local inflammatory response and increases IFN-γ production. This results in IL-4 production by the nasal mucosa T cells in the local draining lymph nodes. Here, CD4 and CD8 cells start to produce TGF-β, which is a potent anti-inflammatory and tolerogenic factor. These two cell types become Th3 cells, which can systemically migrate and colonize the intestinal Peyer’s patches [14]. This strategy could be relevant to CD because it is highly unlikely that tolerance is induced through the intestinal mucosa.
We recently showed that repeated nasal exposure to digested gliadin significantly suppresses the systemic response in previously immunized mice [15]. With the ultimate aim of inducing tolerance to the gliadin antigen in nasal mucosa, we investigated the local immune response to the specific gliadin antigen in nasal scrapings from CD patients. This non-invasive method has been validated for the study of allergic responses [16]. Here, we report the results obtained in CD patients and control subjects.
MATERIALS AND METHODS
Patients
The study population consisted of 21 CD patients (median age 13·4, range 7–22 years) with a diagnosis confirmed according to ESPGHAN criteria, and 12 healthy controls recruited among students. The controls were not age-matched with patients.
Antigen and nasal challenge
For the first 14 cases and 10 controls, gliadin (Sigma, St Louis, Missouri, USA) was dissolved in 10% ethanol at a concentration of 40 mg/ml, and instilled locally in the nasal mucosa by a halved dental roll, which absorbed about 5 ml gliadin solution; the solution was left in situ for 15 min. The same procedure was used for the control test with bovine albumin (Sigma). Subsequently, we used a gel formulation for the other seven cases and two controls. Gliadin (2·5 g) was suspended in 10 ml propylene glycol and added to carboxymethyl cellulose (5 g) previously soaked with 90 ml water in a mortar. Carbopol gels were prepared in the same fashion and their pH was adjusted to 6·0 with a NaOH solution. The polymer concentration was 5% w/v and the antigen concentration 2·5% w/v. Gliadin was instilled in the right nostril and albumin, as a control, in the left. Nasal mucosa scrapings from patients and controls were obtained at time 0 (before antigen exposure) and 6 h after antigen exposure.
Cell harvest and immunocytochemistry
Cells were harvested by gently rotating a cotton-tipped stick over the epithelial layer of the middle third of the inferior turbinate. Samples were shaken and resuspended for 3 min in 2 ml NaCl 0·9% solution; 20 μl of this solution were resuspended with 20 μl Trypan blue for 5 min, and the total cell count was obtained in a Burker’s chamber by two independent observers in a blind fashion. The cell suspension was then cytospun at 800 r.p.m. for 3 min. Slides were fixed-stained in acetone for 5 min; one slide was stained with May-Grumwald Giemsa solution for the assessment of epithelial cells, granulocytes and lymphoid cells, and six slides were stored at –20°C until required for immunocytochemical studies.
The expression of CD3 (1:200 in Tris-buffered saline, TBS, Dako, Denmark), CD25 (IL2R) (1:20 in TBS, Dako) and ICAM1 (CD54) (1:200 in TBS, Dako) was assessed by a routine immunocytochemical technique. Briefly, slides were repeatedly washed at room temperature in TBS, then incubated for 30 min in normal rabbit serum (1:100 in TBS, Dako) and stained using the alkaline phosphatase/anti-alkaline phosphatase (APAAP) method. The sections were individually tested with the specific monoclonal antibodies for 1 h at room temperature, then incubated for 30 min with rabbit anti-mouse serum (1:25 in TBS, Dako) and finally, for a further 30 min with the APAAP complex (1:40 in TBS, Dako). Slides were washed in TBS for 10 min after each antibody incubation. The immune reaction product was developed under continuous stirring for 3 min with a 40 ml solution of TBS, pH 8·7, comprising: 20 mg naphtol-AS-biphosphate in 0·5 ml N-N-dimethylformalmide (Sigma), 0·2 ml sodium nitrite (Sigma) with 0·08 ml New Fucsin (Merck, Darmstadt Germany) and 17·5 mg Levamisole (Sigma). The primary antibody was omitted in control tests for non-specific antibody binding. Lastly, the sections were stained with Mayer’s haematoxylin, mounted on slides and evaluated under the light microscope at a magnification of ×400. Four fields were observed, the total number of cells was counted and the immunostained cells were referred to as the total cell count.
Statistics
Neither the variables under evaluation (total cell counts and subset of lymphocytes) nor the immunocytochemistry results showed a normal distribution (Kolmogorov-Smirnoff test). Therefore, we used a decimal log transformation to reduce skewness so that differences between mean values could be evaluated with a parametric Student t-test. All means shown are an antilog of means, and 95% confidence intervals of decimal log data were also calculated.
Ethics
The study protocol was approved by the Ethics Committee of the School of Medicine, University of Naples ‘Federico II’, and informed consent was given by patients or guardians.
RESULTS
Cell population in nasal scrapings
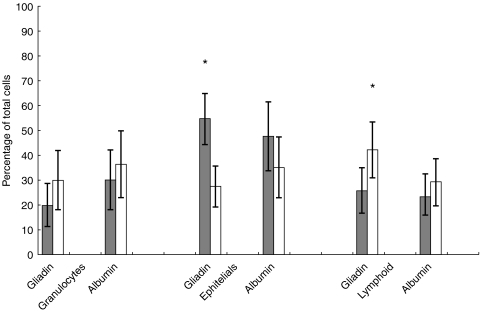
At time 0, the total cell count did not differ significantly between patients and controls. Before gliadin, the total cell number in patients was 40·5 cells/mm3 and in controls it was 36·2 cells/mm3 (t = 0·68, P = 0·5); before albumin it was 43·1 cells/mm3 in patients and 56·1 cells/mm3 in controls (t = 0·28, P = 0·78). As shown in Table 1, in patients, gliadin induced an increase in the total number of cells, i.e. from 40·5 to 74·2 cells/mm3 (t = 2·73, P = 0·014); no increase was seen with albumin (from 43·1 to 41·8 cell/mm3, t < 0·1, P > 0·05). Also, in controls, there was a slight but not significant increase in the total cell number (from 36·2 to 42·8 cells/mm3, t < 0·1, P > 0·05 with gliadin, and 56·1 to 51·8 cells/mm3, t < 0·1, P > 0·05 with albumin).
Table 1.
Total cell count, granulocytes, epithelial and lymphoid cells before and after nasal challenge with gliadin and albumin in coeliac patients and controls
| Coeliacs (n = 21) | Controls (n = 12) | |||
|---|---|---|---|---|
| Gliadin | Albumin | Gliadin | Albumin | |
| Cells | 40·5 (29–56·6)* | 43·1 (29·6–62·6) | 36·2 (24–54·6) | 56·1 (41·3–76·3) |
| Time 0 (cell/mm3) | ||||
| Cells | 74·2 (51·5–106·9) | 41·8 (27·7–63·1) | 42·8 (24·5–75·1) | 51·8 (27·4–79·9) |
| Time 6 h (cell/mm3) | t = 2·73* | t < 0·1 | t < 0·1 | t < 0·1 |
| P = 0·014 | P > 0·05 | P > 0·05 | P > 0·05 | |
| Granulocytes | 19·7 (11·1–28·3) | 30 (17·4–42·6) | 21·7 (12·4–30·9) | 20·2 (9·2–31·2) |
| Time 0 (%) | ||||
| Granulocytes | 30 (18–42) | 36·3 (22·9–49·7) | 25·2 (13·3–37·1) | 30 (14·5–45·4) |
| Time 6 h (%) | t = 1·75 | t < 0·1 | t < 0·1 | t < 0·1 |
| P = 0·097 | P > 0·05 | P > 0·05 | P > 0·05 | |
| Epithelial cells | 54·5 (44·2–64·8) | 47·6 (33·8–61·3) | 49 (37–60·9) | 47·4 (37·3–57·4) |
| Time 0 (%) | ||||
| Epithelial cells | 27·3 (19–35·5) | 35 (22·8–47·2) | 37·9 (26·7–49) | 39 (24·6–53·3) |
| Time 6 h (%) | t = 4·86 | t < 0·1 | t < 0·1 | t < 0·1 |
| P = 0·0001 | P > 0·05 | P = 0·032 | P > 0·05 | |
| Lymphoid cells | 25·7 (16·6–34·7) | 23 (15·7–32·3) | 30 (21·9–38) | 32·2 (23·3–40·8) |
| Time 0 (%) | ||||
| Lymphoid cells | 42 (30·6–53·3) | 29 (19·7–38·3) | 37·4 (23·8–50·9) | 31 (17·5–44·5) |
| Time 6 h (%) | t = 2·98 | t < 0·1 | t < 0·1 | t < 0·1 |
| P = 0·008 | P > 0·05 | P > 0·05 | P > 0·05 | |
Mean and 95% confidence intervals. Paired Student’s t-test was used to compare time 0 values with post-challenge values (6 h).
Gliadin challenge resulted in significant changes in the cell population (Table 1). In patients, total granulocytes were greatly increased (from 19·7% to 30%, t = 1·75, P = 0·097), epithelial cells were reduced by half (from 54·5% to 27·3%, t = 4·86, P = 0·0001), and lymphoid cells were significantly increased (from 25·7% to 42%, t = 2·98, P = 0·008). There was a similar but less significant trend in controls; epithelial cells decreased from 49% to 37·9% (t < 0·1, P = 0·032), but changes in the other cell subpopulations were not significant (granulocytes increased from 21·7% to 25·2%, t < 0·1, P > 0·05; lymphoid cells from 30% to 37%, t < 0·1, P > 0·05).
Albumin challenge did not significantly affect the cell population in patients; granulocytes increased from 30% to 36·3% (t < 0·1, P > 0·05), epithelial cells decreased from 47·6% to 35% (t < 0·1, P > 0·05), and lymphoid cells increased from 23% to 29% (t < 0·1, P > 0·05). Similarly, in controls, albumin did not result in significant changes in the cell populations investigated (data not shown).
T-cell activation markers
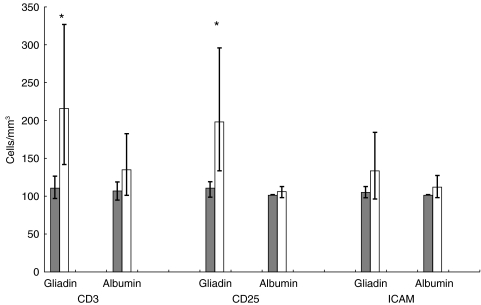
As shown in Table 2, CD3+ cells significantly increased in patients exposed to gliadin, i.e. from 110 cells/mm3 to 216 cells/mm3 (t = 3·97, P = 0·001); they also increased after albumin exposure, but the difference was not significant (from 107 to 135 cells/mm3, t = – 1·62, P > 0·05). CD3+ cells remained at the pre-challenge value of 101 cells/mm3 after both gliadin and albumin exposure in controls.
Table 2.
CD3, CD25 and ICAM positive cells before and after nasal challenge with gliadin and albumin in coeliac patients
| Coeliacs (n = 21) | ||
|---|---|---|
| Gliadin | Albumin | |
| CD3 | 110 (97–126)* | 107 (95–119) |
| Time 0 (cell/mm3) | ||
| CD3 | 216 (142–327) | 135 (101–183) |
| Time 6 h (cell/mm3) | t = 3·97* | t = – 1·62 |
| P = 0·001 | P > 0·05 | |
| CD25 | 108·5 (98–119) | 101 (98–104) |
| Time 0 (cell/mm3) | ||
| CD25 | 198 (133–295) | 105 (97–112) |
| Time 6 h (cell/mm3) | t = 3·32 | t = 1·0 |
| P = 0·004 | P > 0·05 | |
| ICAM | 104 (97–112) | 101 (86–110) |
| Time 0 (cell/mm3) | ||
| ICAM | 132 (96–184) | 111 (97–126) |
| Time 6 h (cell/mm3) | t = 1·38 | t = 1·41 |
| P > 0·05 | P > 0·05 | |
Mean and 95% confidence intervals. Paired Student t-test was used to compare time 0 values with post-challenge values (6 h).
CD25+ cells increased significantly only in patients after gliadin exposure (from 108·5 to 198 cells/mm3, t = 3·32, P = 0·004; after albumin, from 101 to 105 cells/mm3, t = 1·0, P > 0·05) (Fig. 3). In controls, CD25+ cells remained at a level of 101 cells/mm3 after both gliadin and albumin exposure
Fig. 3.
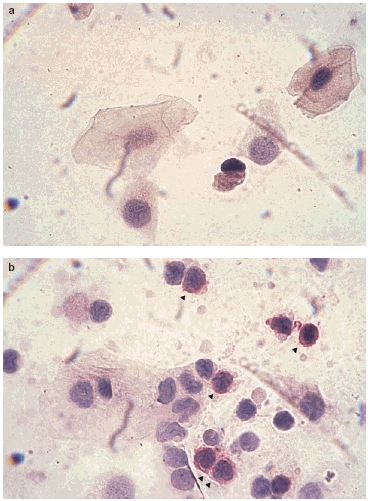
The cytopreps from a coeliac patient before (a) and after gliadin instillation (b). See the CD25 positive cells as the red cells indicated
Gliadin caused an increase in ICAM-expressing cells in patients, but the difference between pre- and post-challenge values was not significant (104 cells/mm3versus 132 cells/mm3, t = 1·38, P > 0·05). Similarly, the difference after albumin instillation was not significant (from 101 to 111 cells/mm3, t = 1·41, P > 0·05). No significant increase was observed with either antigen in control specimens.
To conclude, nasal exposure to gliadin, but not to albumin, induced a local immune response in CD patients. In fact, total cell count, the number of lymphoid cells and the number of cells expressing the IL-2 receptor (CD25+ cells) all increased (Figs 1 and 2). Significant changes were not observed in controls, although the differences between time 0 and 6 h are suggestive of the trend seen in patients. This finding could reflect an immune response to albumin, although it is far below the magnitude of the response to gliadin observed in CD patients.
Fig. 1.
Cell populations in nasal scrapings from coeliac patients before ( ) and 6 h after (□) local instillation of gliadin or albumin. Bars indicate means +95% CI. Asterisk indicates P < 0·01.
) and 6 h after (□) local instillation of gliadin or albumin. Bars indicate means +95% CI. Asterisk indicates P < 0·01.
Fig. 2.
CD3+, CD25+ and ICAM+ cells in nasal scrapings from coeliac patients before ( ) and 6 h after (□) local instillation of gliadin or albumin. Bars indicate means + 95% CI. Asterisk indicates P < 0·01.
) and 6 h after (□) local instillation of gliadin or albumin. Bars indicate means + 95% CI. Asterisk indicates P < 0·01.
DISCUSSION
Nasal challenge has been used with a large measure of success in animals. Nasal-associated lymphoid tissue (NALT) is a lymphoid structure peculiar to the mouse. It is located under the nasal mucosa, which plays an important role in the immune response and in immune tolerance induction. In fact, nasal mucosa is the respiratory tract’s first line of defence against foreign antigens. The cellular activation cascade suggests that NALT is an induction site of the immunological response to such effector sites as the salivary glands and the intestinal lamina propria. Cytological data support this notion; NALT lymphoid cells are smaller and less granular than effector tissue lymphocytes, probably because they are the less differentiated precursors [7].
Antigenic stimulation of the mucosal immune system induces both a local specific immune response and systemic specific immune tolerance, as witnessed by a decrease in serum IgE and IgA titres. Moreover, a single intranasal inoculation of a soluble antigen provokes no response, but a second inoculation with the same antigen is sufficient to induce a specific response, both systemic and local, which is mediated by an increase in IgA-producing B cells. It is tempting to compare the mouse NALT with Waldeyer’s ring in humans. However, the differences in the size and localization of the olfactory systems in mammals precludes a rigorous comparison between the two structures.
The aim of this study was to investigate the nasal immune response to gliadin in CD patients in order to verify whether mucosae outside the gastrointestinal tract recognize the gliadin antigen, with a view of using the nasal route to re-induce tolerance. Our results show that a nasal response is indeed present and that it appears to be antigen-specific and unique to CD patients.
In coeliac patients, gliadin, but not albumin, induced a significant increase in activated mucosal lymphocytes. Also, the nasal mucosa of healthy controls responded to local exposure to gliadin and albumin antigens, but the response was ambiguous and neither consistent nor statistically significant. Since we evaluated the immune response on mucosal scrapings rather than on biopsy samples, it is not surprising that gliadin did not induce a strong response in coeliac patients. However, the response was constant and specific, which indicates that the coeliac nasal mucosa exerts specific immune recognition. Therefore, the nasal mucosa, like the rectal and the oral mucosae, is able to mount an immune response. After the pivotal investigation by Loft et al.[3], we and others [4,17] showed that exposure of the coeliac rectal mucosa to gliadin induces T-cell infiltration in the epithelium and lamina propria. More recently, Lahteenoja et al.[5] reported that the oral mucosa of CD patients responded to locally-instilled gliadin with T-cell recruitment and mast cell degranulation. However, the gliadin-induced response was not constant among patients and across the methods used to evaluate it. Here, we demonstrate a significant increase in T cells that express activation markers in the nasal scrapings from CD patients exposed to gliadin. Although nasal scraping is less informative than nasal mucosal biopsy, it is non-invasive, and we did not feel justified in submitting patients or controls to a procedure that can cause bleeding and some pain. Nasal challenge was very well tolerated by all patients. None suffered clinically-relevant acute or delayed reactions. An ENT specialist did not identify any local reaction in either the patients or the controls. Scraping is thus confirmed to be a valid, non-traumatic method for the collection of nasal mucosal samples and for the evaluation of the inflammatory response in nasal mucosa [18]. The technique yields a relatively small number of cells and consequently, we were unable to explore the cell population in greater detail. Work is underway to optimize the technique using ELISPOT methods [19].
Having demonstrated a local mucosal response to gliadin, and consequent to the finding of gliadin-primed cells in blood after oral challenge [5], we plan to evaluate the systemic response to prolonged exposure using microencapsulated gliadin antigen to improve the exposure at nasal mucosa.
We had not intended to use our observations for diagnostic purposes, which is one of the reasons why we did not enrol untreated patients. Nonetheless, it might be revealing to investigate other CD patients and their first-degree relatives, in whom there is a significant prevalence of positive responses of rectal mucosa to locally-instilled gliadin [4].
In conclusion, our results support the hypothesis that the nasal mucosa recognizes gliadin locally and may prime an effector immune response in the draining lymph nodes. Consequently, the nasal mucosa becomes a target for procedures to re-induce immune tolerance.
Acknowledgments
This study received financial support from the Ministero della Sanità, Ricerca Finalizzata 1998: ‘Nuove terapie per il morbo celiaco’. Support from the Commission of the European Communities, specific RTD programme ‘Quality of Life and Management of Living Resources’, QLK1-CT-1999-00037, Evaluation of the prevalence of coeliac disease and its genetic components in the European population, is also gratefully acknowledged. This study does not necessarily reflect the Commission’s views and in no way anticipates the Commission’s future policy in this area. We are indebted to Jean Ann Gilder for revising and editing the text.
REFERENCES
- 1.Auricchio S, Maurano F, Troncone R. Coeliac disease in the year 2000. Ital J Gastroenterol Hepatol. 1999;31:773–80. [PubMed] [Google Scholar]
- 2.Maiuri L, Picarelli A, Boirivant M, Coletta S, et al. Definition of the initial immunologic modification upon in vitro gliadin challenge in the small intestine of celiac patients. Gastroenterology. 1996;110:1368–78. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- 3.Loft DE, Marsh MN, Sandle GI, et al. Studies of intestinal lymphoid tissue. XII. Epithelial lymphocyte and mucosal responses to rectal challenge in celiac sprue. Gastroenterology. 1989;97:29–37. doi: 10.1016/0016-5085(89)91411-x. [DOI] [PubMed] [Google Scholar]
- 4.Troncone R, Greco L, Mayer M, et al. In siblings of celiac children, rectal gluten challenge reveals gluten sensitization not restricted to celiac HLA. Gastroenterology. 1996;111:318–24. doi: 10.1053/gast.1996.v111.pm8690196. [DOI] [PubMed] [Google Scholar]
- 5.Lahteenoja H, Maki M, Viander M, Toivanen A, Syrjanen S. Local challenge of oral mucosa with gliadin in patients with coeliac disease. Clin Exp Immunol. 2000;120:38–45. doi: 10.1046/j.1365-2249.2000.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Passalacqua G, Albano M, Riccio AM, Scordamaglia A, Canonica GW. Local nasal immunotherapy: experimental evidences and general considerations. Allergy. 1997;52:10–6. doi: 10.1111/j.1398-9995.1997.tb04798.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu HY, Nguyen HH, Russel MW. Nasal Lymphoid Tissue (NALT) as a mucosal immune inductive site. Scand J Immunology. 1997;46:506–13. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 8.Bayrak S, Mitchinson NA. Bystander suppression of murine collagen-induced arthritis by long-term nasal administration of a self type II collagen peptide. Clin Exp Immunol. 1998;113:92–5. doi: 10.1046/j.1365-2249.1998.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick AD, Cheng YF, Liversidge J, Forrester JV. Intranasal administration of retinal antigens suppresses retinal antigen-induced experimental autoimmune uveoretinitis. Immunology. 1994;82:625–31. [PMC free article] [PubMed] [Google Scholar]
- 10.Wraith DC. Antigen-specific immunotherapy of autoimmune disease: a commentary. Clin Exp Immunol. 1996;103:349–52. doi: 10.1111/j.1365-2249.1996.tb08286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi FD, Li H, Wang H, et al. Mechanisms of nasal tolerance induction in experimental autoimmune myasthenia gravis: identification of regulatory cells. J Immunol. 1999;162:5757–63. [PubMed] [Google Scholar]
- 12.Karachunski PI, Ostile NS, Okita DK, Conti-Fine BM. Prevention of experimental myasthenia gravis by nasal administration of syn-thetic acetylcholine receptor T epitope sequences. J Clin Invest. 1997;12:3027–35. doi: 10.1172/JCI119857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian BJ, Atkinson MA, Clare-Salzler M, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces TH2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561–7. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–43. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 15.Rossi M, Maurano F, Caputo N, et al. In mice, intravenous or intranasal administration of gliadin is able to down-regulate the specific immune response. Scand J Immunol. 1999;50:177–82. doi: 10.1046/j.1365-3083.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 16.Passalacqua G, Scordamaglia A, Canonica GW. Immunoterapia specifica non iniettiva e infiammazione allergica: evidenze sperimentali. Not Allergol. 1997;16:15–22. [Google Scholar]
- 17.Mauriño E, Niveloni S, Dezi R, et al. Evidence of gluten sensitivity in the rectal mucosa of first degree relatives of coeliac disease patients. Am J Gastroenterol. 1997;92:1326–30. [PubMed] [Google Scholar]
- 18.Piacentini GL, Kaulbach H, Scott T, Kaliner MA. Evaluation of nasal cytology a comparison between methods. Allergy. 1998;53:326–8. doi: 10.1111/j.1398-9995.1998.tb03898.x. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RP, Degano P, Godkin AJ, et al. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nature Med. 2000;3:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]