Abstract
Vulnerability to Streptococcus pneumoniae is most pronounced in children. The microbial virulence factors and the features of the host immune response contributing to this phenomenon are not completely understood. In the current study, the humoral immune response to separated Strep. pneumoniae surface proteins and the ability to interfere with Strep. pneumoniae adhesion to cultured epithelial cells were analysed in adults and in children. Sera collected from healthy adults recognized Strep. pneumoniae separated lectin and nonlectin surface proteins in Western blot analysis and inhibited on average 80% of Strep. pneumoniae adhesion to epithelial cells in a concentration-dependent manner. However, sera longitudinally collected from healthy children attending day care centres from 18 months of age and over the course of the following 2 years revealed: (a) development of antibodies to previously unrecognized Strep. pneumoniae surface proteins with age; (b) a quantitative increase in antibody responses, measured by densitometry, towards separated Strep. pneumoniae surface proteins with age; and (c) inhibition of Strep. pneumoniae adhesion to epithelial cells, which was 50% on average at 18 months of age, increased significantly to an average level of 80% inhibition at 42 months of age equalling adult sera inhibitory values. The results obtained in the current study, from the longitudinally collected sera from healthy children with documented repeated Strep. pneumoniae colonization, show that repeated exposures are insufficient to elicit an immune response to Strep. pneumoniae proteins at 18 months of age. This inability to recognize Strep. pneumoniae surface proteins may stem from the inefficiency of T-cell-dependent B-cell responses at this age and/or from the low immunogenicity of the proteins.
Keywords: Streptococcus pneumoniae, surface proteins, immune response, bacterial adhesion, pathogenesis
INTRODUCTION
Streptococcus pneumoniae, a Gram-positive bacterium, is a major cause of morbidity and mortality worldwide [1,2]. The incidence and severity of disease is highest in children under 3 years of age, when immunoreactivity to existing polysaccharide-based vaccines is at its nadir [1,3,4]. In the elderly, the nonconjugate polysaccharide (PS) vaccine is only 60% effective in preventing invasive disease [5]. Promising new studies using Strep. pneumoniae PS conjugated to carrier proteins have yielded vaccines that are more immunogenic in children than soluble polysaccharides alone [6,7]. The high amount of conjugated protein required to elicit immunity to a single PS, however, limits the number of different conjugates that can be used.
Surface proteins of both Gram-negative and Gram-positive bacteria involved in early pathogen–host cell adhesion have been shown to be promising vaccine candidates. Some examples include the Escherichia coli FimH adhesins expressed by type 1 pili [8,9] and PapG [10,11], which are highly conserved proteins [12,13]. Among Gram-positive bacteria the I/II antigens found in Streptococcus mutans, Streptococcus sorbinus and in Streptococcus gordonii that bind salivary glycoproteins [14] have been shown to be protective in animal models [15].
Streptococcus pneumoniae immunogenic virulence proteins became logical targets for vaccine design [16] since children under 2 years of age are capable of producing antibodies against protein antigens [17]. Indeed, recent studies demonstrated that Strep. pneumoniae virulence proteins, among which are PspA [18] PsaA [19,20], pneumolysin [21], a combination of these [22], CbpA [23] and PpmA [24], can elicit protective immune responses and therefore prevent or delay mortality in a lethal-dose Strep. pneumoniae challenge model in mice. Recent studies in humans found that the natural immune responses to pneumolysin, PspA and PsaA are associated with pneumococcal exposure in children, either by carriage or infection [25].
Surface proteins that are involved in Strep. pneumoniae adhesion and invasion of the host are just beginning to be discovered [26]. In the initial stages of the Strep. pneumoniae–host interaction, Strep. pneumoniae binds avidly to cells of both the upper and lower respiratory tract [18,27,28] in a receptor-mediated fashion [29]. It is presumed that bacterial adhesins may act as ligands for host cell receptors. Adhesin characteristics have been attributed to several Strep. pneumoniae proteins [13].
Several putative host cell receptors involved in Strep. pneumoniae adhesion have recently been described [23,30,31]. The mammalian platelet activating factor receptor (PAF-R) contains N-acetyl glucosamine and promotes Strep. pneumoniae adhesion. PAF-R is expressed following inflammation of activated lung and endothelial cells [14]. Additional carbohydrates have been shown to interfere with Strep. pneumoniae adhesion to mammalian cells. For example, the carbohydrate Galβ1–4GlcNAc inhibited adhesion to conjunctival epithelial cells [19,32], GalNAcβ1–3Galβ1– 4GlcNaC inhibited adhesion to nasopharyngeal cells [33,34] and GalNAcβ1–4Gal inhibited adhesion to resting lung cells [33,34]. The cognate receptors for these carbohydrates, which are as yet unidentified, may provide additional portals of entry for Strep. pneumoniae and require further study.
Increases in antibody levels and an enhanced ability of the antibodies to interfere with the interaction of Strep. pneumoniae with its host target cells have been long considered surrogate markers for immunity. In the search for Strep. pneumoniae surface proteins that will be immunogenic and will elicit protection against Strep. pneumoniae infection, we have compared the antibody repertoire for Strep. pneumoniae surface lectin and nonlectin proteins in healthy adults previously exposed to Strep. pneumoniae, as judged by the presence of serotype specific antibodies to capsular PS, and in sera collected longitudinally from children beginning at 18 months of age. In addition, the ability of the immunoglobulins to interfere with Strep. pneumoniae adhesion to mammalian epithelial cells was analysed. The highest Strep. pneumoniae adhesion-blocking activity of the immunoglobulins was found in sera obtained from healthy adults. In children, the pattern of the qualitative and quantitative increased antibody recognition of Strep. pneumoniae surface proteins and their ability to interfere with Strep. pneumoniae adhesion to cultured cells correlated with the pattern of increased immunity to Strep. pneumoniae infection.
METHODS
Bacterial strains
The bacterial strains used in this study include: unencapsulated serotype 3 (DW3.8), 14 (DW14.8) Strep. pneumoniae mutants generated by Tn916 transposon mutagenesis of the glucotransferase gene of WU2 and a type 14 isolate, respectively, kindly provided by Dr David Watson, USA [35,36] and a clinical isolate from a patient at Soroka University Medical Centre, Beer-Sheva. Streptococcus pneumoniae bacteria were plated onto tryptic soy agar supplemented with 5% sheep erythrocytes and incubated for 17–18 h at 37°C in anaerobic conditions. Streptococcus pneumoniae were transferred to Todd-Hewitt broth supplemented with 0·5% yeast extract and grown to mid-late log phase. Bacteria were harvested and the pellets were stored at – 70°C.
Antibodies
A monoclonal antibody to phosphorylcholine TEPC 15 was purchased from Sigma Aldrich Co. (St Louis, MO, USA). Anti human IgG (Fc fragment) Horseradish peroxidase conjugate was purchased from Calbiochem (La Jolla, CA, USA).
Separation of cell wall (CW) and membrane (M) molecules
The method of fractionation of Strep. pneumoniae CW is a modification of a previously published protocol [37]. Briefly, bacterial pellets were resuspended in phosphate buffered saline (PBS), disrupted by sonication and centrifuged. Pellets were then treated with RNase and DNase, centrifuged and treated with lysozyme to release CW components. Supernatants containing the CW proteins were harvested. The membrane (M)-fraction pellet was solubilized with 0·5% Triton X-100. For the separation of Strep. pneumoniae lectin-like molecules from the nonlectin molecules, CW and diluted M protein (× 10) suspensions were separately adhered to the pan-lectin-binding highly glycosylated fetuin protein covalently bound to a sepharose column. The flow through from this affinity column contained the Strep. pneumoniae nonlectin-like (NL) molecules. The fetuin column adherent molecules, containing the lectin-like (L) fractions, were eluted with 50 mm ammonium acetate at pH 3·5.
Four fractions were thus obtained: CW-L, CW-NL, M-L and M-NL. These fractions were concentrated and analysed by 1D PAGE electrophoresis and either stained with Coomassie Blue for protein detection as previously described [38] or transferred onto nitrocellulose membrane for Western blot analysis. To verify the protein nature of the active components in the preparation, CW and M fractions were digested with proteinase K. To identify the location of teichoic acid and lipoteichoic acid, Western blots prepared from the CW and M fractions were probed with a monoclonal antibody to choline (TEPC-15) and with the human sera.
Sera and Western blots
Human sera were obtained from randomly chosen healthy adults and longitudinally collected at ages 18, 30 and 42 months from healthy children attending day care centres in the city of Beer-Sheva. The study was approved by the Soroka Medical Center Ethics Committee. Informed consent was obtained from the subject’s guardian. The presence of anti Strep. pneumoniae polysaccharide antibodies in the adults’ sera was confirmed as previously described [39,40]. Strep. pneumoniae CW and M extracts were probed with the human sera on Western blots as previously described [25]
Haemagglutination assays
Rabbit whole blood cells were washed three times with PBS. A 3% suspension of erythrocytes was prepared in PBS. Fifty μl of this suspension were mixed with diluted protein samples in V-shaped bottom, 96-well plates. Incubation of erythrocytes alone served as a negative control and Con-A (Sigma Aldrich Co, St Louis, MO, USA) treatment of erythrocytes served as a positive control.
Cells
The spontanously immortalized human keratinized cell line, HaCat [41] and primary nonkeratinized human mucosal epithelial cells were isolated and propagated according to Rheinwald et al. [42]. For the primary cultures, informed consent was obtained from the subjects donating epithelial cells. In short, cells were grown in DMEM supplemented with 10% heat-inactivated foetal calf serum (FCS; Biological Industries, Beith-HaEmek, Israel) penicillin and streptomycin (100 μg/ml each). The cells were removed from flasks by the addition of trypsin (0·25%), washed once in DMEM medium, plated and grown to confluence in 96-well flat-bottom plates (average of 6 × 104 cells/well). At least 16 h prior to the adhesion experiment the culture medium was changed to one without antibiotics. Cultures were then blocked with DMEM medium supplemented with 2·5% bovine serum albumin (BSA; Sigma Aldrich Co., St Louis, MO) and incubated at 37°C for 4 h.
Adhesion assay
DW3.8 Strep. pneumoniae bacteria were grown to mid-logarithmic phase determined by a growth curve (0·4 OD, 108 cfu/ml). Then 106 bacteria in DMEM supplemented with 2·5% BSA were added to the cultured epithelial cells. Bacterial colony forming units (cfu) were confirmed in each experiment. Following an additional 30-min incubation, facilitating bacterial-host cell adhesion, the cells were extensively washed with PBS-BSA (× 6) to remove nonadherent bacteria. Cells were liberated from the culture dish by incubation with either 1 mM EDTA or 0·25% trypsin-EDTA for 5 min at 37°C. This suspension and its 10-fold serial dilutions in DMEM were plated on sheep blood agar and the number of cfu determined.
For adhesion inhibition assays heat-inactivated pooled healthy adult human sera or heat-inactivated healthy childrens’ sera were incubated with 106 Strep. pneumoniae cells for 30 min prior to their addition to cultured epithelial cells at the denoted concentrations. In order to determine the extent of inhibition by Strep. pneumoniae surface proteins upon adhesion, fractionated CW and M proteins were added in conjunction with DW3.8 Strep. pneumoniae and incubated with HaCat cultured cells for 30 min and the Strep. pneumoniae cfu were assayed as described above. No effect on bacterial viability was observed under these conditions.
Statistical analysis
Statistical significance was analysed via 2-tailed Student’s t-test for two samples with equal variances. In addition, statistical analysis with the non-parametrical two sample Kolmogorov–Smirnov test for equality of distribution functions was performed as well.
In attempt to study both the time and dose effects of the inhibitory activity of childrens’ sera samples, a statistical analysis with two way ANOVA with repeated measurements was performed, as well.
RESULTS
Immune response to Strep. pneumoniae surface molecules
In order to identify different Strep. pneumoniae surface molecules we subfractionated Strep. pneumoniae cell wall (CW) and membrane (M) components as described in the Methods section. Using fetuin affinity chromatography and elution with 50 mM ammonium acetate, pH 3·5, the CW and M fractions were further separated into fetuin adherent, lectin-like (CW-L and M-L) and fetuin nonadherent, nonlectin (CW-NL and M-NL) fractions. While the fetuin-captured fractions agglutinated rabbit erythrocytes confirming the presence of lectins in these fractions, the flow-through proteins failed to agglutinate erythrocytes (data not shown). All four fractions were subjected to SDS PAGE analysis. Enrichment for lectin-like molecules could be achieved by using a clinical isolate or the DW3.8 unencapsulated WU2 mutant. However, in order to rule out capsular polysaccharide interference with Strep. pneumoniae surface molecules binding to the fetuin column, all the experiments described in this study involved work with the DW3.8 mutant. The protein nature of the bands was further substantiated, as no Coomassie blue stained bands could be detected on gels obtained from Proteinase K digested Strep. pneumoniae fractions (data not shown).
We have tested sera obtained from healthy adults for their ability to identify fractionated Strep. pneumoniae surface molecules. In healthy adults (n = 8), previous exposure to Strep. pneumoniae was assessed by verification of the presence of antibodies to Strep. pneumoniae capsular polysaccharide, albeit to a varying degree (data not shown). As expected, the sera obtained from the healthy adults demonstrated the existence of antibodies to many Strep. pneumoniae CW and M molecules by Western blot analysis (Fig. 1). Using pooled human sera, only the bands corresponding to teichoic acid and lipoteichoic acid (around 20 kD) remained after digestion with proteinase K of Strep. pneumoniae CW and M fractions (data not shown), in accordance with previously described results [43]. This was further verified by probing the blots with a monoclonal antibody to phosphorylcholine TEPC 15 (data not shown).
Fig. 1.
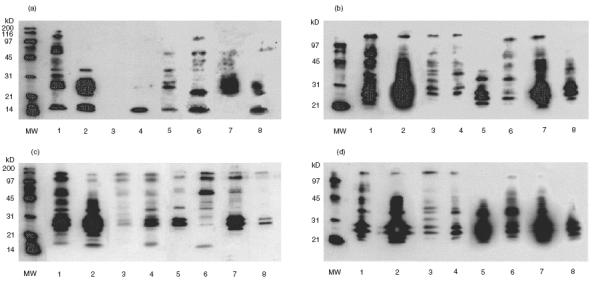
Antibodies to Strep. pneumoniae surface molecule in randomly chosen healthy adults. Sera from healthy adults were analysed by Western blot analysis for the presence of anti Strep. pneumoniae surface protein antibodies with Strep. pneumoniae fractionated surface molecules. (a) Cell wall (CW) lectin-like molecules. (b) CW proteins that did not adhere to the fetuin column. (c) Membrane (M) lectin-like molecules. (d) Fetuin nonadherent M molecules. MW: molecular weight markers. Lanes 1–8 represent probing with sera from eight different healthy adults. Only bands corresponding to teichoic acid and lipoteichoic acid (around 20 kD) could be identified on Western blots prepared from proteinase K digested CW and M fractions (data not shown).
To determine the extent of antibody responses in a Strep. pneumoniae susceptible population, sera from healthy children attending day care centres were tested. Eight healthy children with documented episodes of carriage of different Strep. pneumoniae serotypes were tested. These children demonstrated immunoreactivity to Strep. pneumoniae surface molecules isolated from DW3.8, as detected by Western blot analysis. A representative series revealing quantitative and qualitative enhancement of antibody responses to Strep. pneumoniae proteins over time is shown in Fig. 2. Both the number of proteins detected and the intensity of the response increased with childrens’ age. Summarizing the results obtained by Western blots probed with the childrens’ sera, we found that the following proteins were undetected at 18 months of age but elicited a response by 42 months of age: in the CW-L fractions: 48, 60, 70 and 83 kD; in CW-NL fraction: 50, 66, 73, 116 kD; in the M-L fraction: 50, 55, 62 and 83 kD; and in the M-NL fraction: 45, 60, 64, 70, 83 and 100 kD. To quantify the increase in the antibody response, the Western blots were analysed by TINA V2.10g densitometry software (Raytest Isotopenmegerifte GmbH) for assessment of densitometry. The intensity of the protein bands was determined per lane and the results are summarized in Fig. 3. In 90% of the cases, the childrens’ antibody responses were found to increase in the sera samples obtained at 30 and 42 months of age by 2 to 107 fold compared to the intensity found in the initial serum sample obtained at 18 months of age.
Fig. 2.
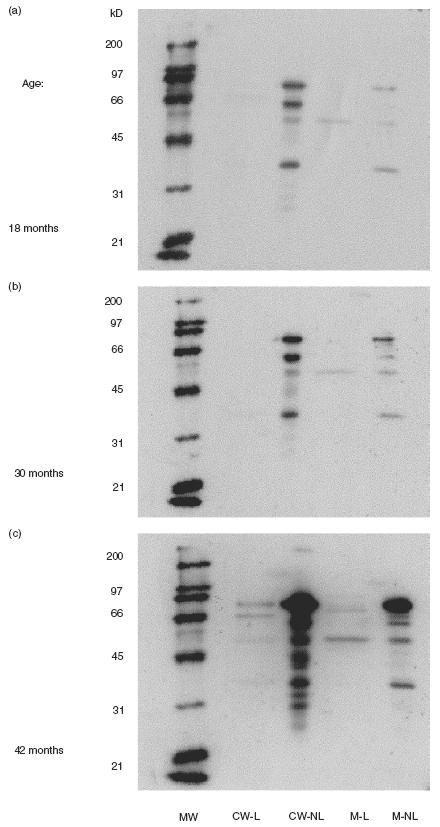
Qualitative development and de novo synthesis of antibodies to Strep. pneumoniae surface proteins in children. A representative series of Western blots prepared from Strep. pneumoniae surface molecules and probed with sera collected longitudinally at 18 (a), 30 (b) and 42 (c) months of age from a child attending a day care centre. Lane 1: MW markers; lane 2: cell wall lectin-like (CW-L) proteins; lane 3: cell wall nonlectin (CW-NL) proteins; lane 4: membrane lectin-like (M-L) proteins; lane 5: membrane nonlectin proteins (M-NL).
Fig. 3.
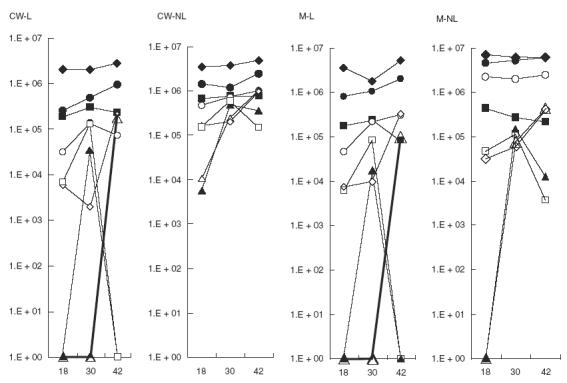
Quantitative increase of immune responses to Strep. pneumoniae surface proteins. Serial sera from eight healthy children attending a day care centre were tested for immune responses to Strep. pneumoniae surface proteins using Western blotting. To quantify the increase the blots were prepared from the same Strep. pneumoniae preparation and developed for 2 s (similar increases were obtained with longer exposure time but saturation prevented accurate quantification). TINA software was used for densitometry. An increase between 2 and 107 times was found in 90% of the cases in sera samples obtained at 30 and 42 months of age compared with sera obtained at 18 months of age. ✦ Child #1001; ▪ child #1007; ▴ child #1014; • child #1020; ▵ child #1021; ○ child #1029; ◊ child #1032; □ child #1048.
Inhibition of Strep. pneumoniae adhesion to epithelial cells
To determine whether the antibodies are important for adhesion of Strep. pneumoniae, we tested interference with adhesion to epithelial cells by the sera using the human HaCat epithelial cell line and human primary nonkeratinized oral mucosal cells. Heat-inactivated sera were incubated with Strep. pneumoniae prior to addition to cultured epithelial cells. Treated and untreated Strep. pneumoniae were then incubated with the epithelial cells in 96-well plates.
Heat-inactivated healthy adult pooled serum and its 40% ammonium sulphate precipitated immunoglobulin fraction interfered effectively with Strep. pneumoniae–epithelial cell adhesion, in a concentration-dependent manner, achieving 90% inhibition in both HaCat (Fig. 4a,b, respectively) and primary nonkeratinized mucosal epithelial cells (Fig. 4c). Serum components lacking immunoglobulins did not interfere with Strep. pneumoniae adhesion (data not shown). Streptococcus pneumoniae adhesion to HaCat epithelial cells could also be inhibited by the subfractionated CW and M proteins in a dose-dependent manner (Fig. 4d). It should be noted that at the highest concentration of the membrane protein preparation used, residual toxicity to the cultured cells was seen and this result was omitted from the graph. In control experiments a 90% reduction in Strep. pneumoniae adhesion was observed following 1 h treatment with trypsin, while bacterial viability was not affected (data not shown).
Fig. 4.
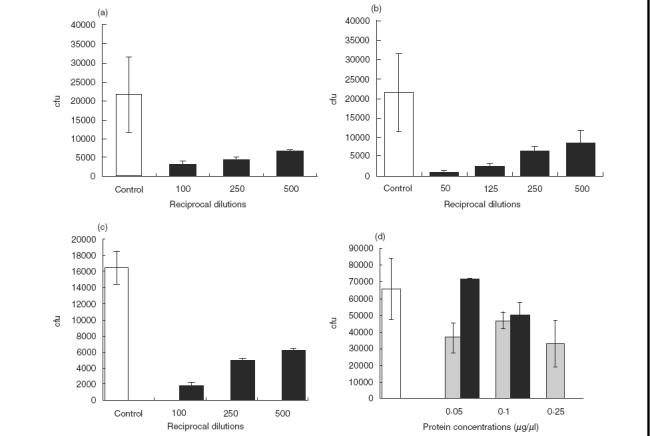
Inhibition of Strep. pneumoniae adhesion to epithelial cells. Inhibition of Strep. pneumoniae adhesion to HaCat epithelial cells was performed as described in the Methods section with: (a) pooled healthy adult sera; (b) immunoglobulin fraction from adult sera – it should be noted that the serum fraction devoided of immunoglobulins was also depleted of its inhibitory activity; (c) inhibition of adhesion to primary nonkeratinized oral mucosa cells; (d) dose-dependent inhibition of Strep. pneumoniae adhesion to HaCat cells by bacterial cell wall (CW) and membrane (M) proteins (□ BSA control; CW protein concentration; ▪ M protein concentration). These experiments were repeated on 3 separate experiments in triplicate each time, and a representative experiment is illustrated. cfu stands for cfu/well. The results are the mean of the triplicate wells ± standard error of the mean (s.e.m.).
Sera from all eight DCC children with documented Strep. pneumoniae carriage obtained longitudinally at 18, 30 and 42 months of age inhibited adhesion of Strep. pneumoniae to epithelial cells, using either HaCat or primary nonkeratinized oral mucosal cells. This inhibition increased from an average of 50% to 80% over the course of two years reaching adults values. Results of a representative experiment of Strep. pneumoniae adhesion to HaCat cells and its inhibition by the 1:250 dilution of the sera obtained from all the children in comparison to sera obtained from adults is shown in Fig. 5.
Fig. 5.
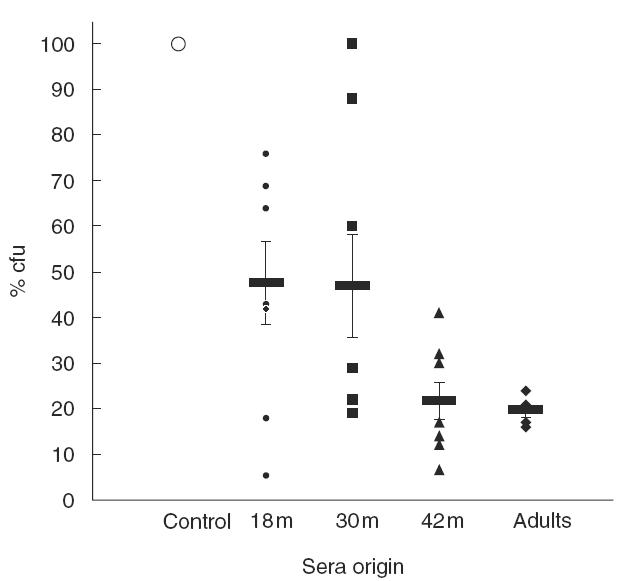
Development of inhibitory activity against Strep. pneumoniae adhesion to epithelial cells in children compared to adult. Sera were obtained longitudinally at 18, 30 and 42 months (m) of age (n = 8) from healthy children attending day care centres, with documented Strep. pneumoniae carriage. Individual adult’s sera (n = 5) were obtained from randomly chosen healthy adults. In a representative experiment HaCat cells were incubated with 106 Strep. pneumoniae cfu in the absence (control) or presence of these sera diluted 1:250. The number of bacteria bound to the cells was determined as decribed in the Methods section. Individual results are presented as % adhered bacteria. Marked are the calculated means and the standard errors of mean (s.e.m.).
Statistical analysis performed using two sample t-test with equal variance on five of the children revealed significant inhibition of Strep. pneumoniae adhesion to the HaCat cell-line in all sera assessed, as compared to untreated Strep. pneumoniae (two separate experiments each performed in triplicate, with three dilutions for each sample; P < 0·0001). Significant differences were also evident when comparing sera collected at 18 versus 42 months (P < 0·01) or 30 versus 42 months (P < 0·01). Similarly, statistical analysis of inhibition of adhesion to primary non- keratinized oral mucosal cells revealed significant differences between control and 18 month immune sera at either 1:100 (P < 0·0001) or 1:250 (P < 0·0005) dilutions and at all dilutions tested using 30 month serum (1:100 or 1:250, P < 0·0001; 1:500, P < 0·01) and 42 month serum (1:100 or 1:250 or 1:500, P < 0·0001). No significant differences could be found between children at 42 months of age and adults. Similar results of the statistical analysis were obtained by the non-parametrical two sample Kolmogorov–Smirnov test for equality of distribution functions.
In attempt to study both the time and dose effects of the inhibitory activity of childrens’ sera samples a statistical analysis with two way ANOVA with repeated measurements was performed. The experiments were statistically analysed in two ways: (1) for the significance of the inhibitory activity in all the childrens’ sera (drawn at 18, 30 and 42 months) in all dilutions (1:100; 1:250 and 1:500) over time; and (2) for the inhibitory activity in each serum dilution used over time. The statistical analysis revealed a significant effect of interaction between time and dilution in inhibiting Strep. pneumoniae adhesion to both HaCat and primary non-keratinized oral mucosa cells (P < 0·001). The analysis for each serum dilution further supported the significant inhibitory effect on Strep. pneumoniae adhesion to HaCat cells and its increase over time (at 1:100 dilution, P < 0·047; at 1:250 dilution, P < 0·012; at 1:500 dilution, P < 0·004). The inhibitory activity of the childrens’ sera on Strep. pneumoniae adhesion to primary mucosal cells was found to be significant using serum dilutions 1:250 (P < 0·06) and 1:500 (P < 0·004) but not significant at 1:100 dilution (P < 0·177).
DISCUSSION
In the current study we describe qualitative de novo synthesis and quantitative increases over time in previously existing antibody responses against Strep. pneumoniae surface proteins in children from 18 to 42 months of age. These increases coincided with an augmentation in the capacity of the childrens’ sera to inhibit adhesion of Strep. pneumoniae to primary and immortalised epithelial cells. In sera obtained from healthy adults, antibody recogni- tion of Strep. pneumoniae surface molecules was wide-ranging. The capacity to inhibit Strep. pneumoniae adhesion reached a maximum of 90% and resided in the immunoglobulin fraction. It is well established that newborn children have impaired T-cell-independent B-cell responses; this study suggests that the T-cell-dependent B-cell responses to Strep. pneumoniae surface proteins may also be inefficient in early childhood.
Several Strep. pneumoniae cell wall (CW) virulence proteins have been shown to be immunogenic and protective in animals. Among these, PspA has been shown to elicit protective immune responses and to reduce Strep. pneumoniae carriage in mouse models [44]. PspA, however, is serologically variable and the different serotypes are only partially cross-reactive [10]. A combination of pneumolysin, PspA and PsaA showed a synergistic effect [37,45]. Additional Strep. pneumoniae surface proteins are the choline binding proteins A (CbpA, or PspC). CbpA is a Strep. pneumoniae adhesin that binds to activated human cells. It is highly immunogenic and is a possible candidate for development of a future vaccine [15,23].
In the current study the serotype 3 un-encapsulated mutant of WU2 (DW3.8) [35,36] was used. It should be noted that type 3 WU2, the parental strain of the DW3.8 mutant, lacks PspC [46] and that the gpt unencapsulated DW3.8 mutant, used in this study, has also previously been reported to lack PspA [47]. Thus the inhibitory activity against Strep. pneumoniae adhesion in the childrens’ sera could not be assigned to antibodies to these proteins, but rather to additional adhesins. In addition, proteins undetected by sera obtained from children at 18 months of age but detected at 42 months of age were greater than 48 kD, excluding PsaA (37 kD protein; [19]).
The variations between different adults seen in Fig. 1 may stem from a different exposure to Strep. pneumoniae and from a different immunogenetic background among other possibilities. The importance of the immunogenetic background in response to bacteria is currently under investigation in our laboratory and in many laboratories worldwide.
Quantitatively and qualitatively the development of antibodies against the Strep. pneumoniae surface proteins coincides with the development of interference with Strep. pneumoniae adhesion to human cells (Figs 2, 3, 4 and 5). The ability of trypsin to eliminate Strep. pneumoniae adhesion suggests that the active components in the CW and M fractions that interfere with Strep. pneumoniae adhesion to the cultured epithelial cells (Fig. 4d) are of a proteineacious nature.
There are three possibilities that are not mutually exclusive and may be operational in parallel in determining the better response observed with age against Strep. pneumoniae proteins. (i) The immune response to the bacterial surface protein antigens is due to repeated exposures. These protein antigens may be less immunogenic than others and an adequate immune response, which may provide protection, may require multiple exposures over the years. (ii) The interstrain antigenic variability of surface-exposed bacterial antigens should not be excluded. Therefore, exposure to multiple different strains is needed before strong cross-reactivity to a specific antigenic species becomes apparent. (iii) Another possibility is that the T-cell-dependent B-cell responses to some bacterial proteins were inefficient in healthy children. This age-dependent antigenic preference could be a result of their T-cell repertoire being either limited or lacking in specificity at 18 months of age. The development of the thymus and the T-cell receptor repertoire with age has not been fully explored in healthy children [48]. The extent of the T-cell receptor repertoire in children is highly diverse but alteration in its expression occurs with increasing age [49].
The development of an appropriate T-cell immune response to Strep. pneumoniae surface proteins is currently under investigation in our laboratory using a mouse model. Moreover, we are currently characterizing the immunogenic Strep. pneumoniae proteins that may be instrumental in the development of more effective, protein-based vaccines.
Acknowledgments
We would like to thank Dr R. Teitelbaum for critical evaluation of this manuscript.
Financial support: YMN is a recipient of the Goisttela award. This study was partially supported by a grant from the Israeli MOH #4776 to YMB and by a grant from the Centre of Emerging Diseases #2506 to YMB.
References
- 1.Centre for Disease Control and Prevention. Pneumonia and influenza death rate – United States 1979–94. Morbid. Mortal. Weekly Report. 1995;44:535–7. [PubMed] [Google Scholar]
- 2.Austrian R. The enduring pneumococcus: Unfinished business and opportunities for the future. In: Tomasz A, editor. Mary Ann Liebert Inc. Streptococcus pneumoniae Larchmont; 1995. pp. 3–6. [DOI] [PubMed] [Google Scholar]
- 3.Robbins JB, Austrian R, Lee CJ, et al. Considerations for formulating the second-generation of pneumococal capsular polysaccharide vaccine with emphasis on the cross-reactive type within groups. J Infect Dis. 1983;148:1136–59. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 4.Cowan MJ, Ammann AJ, Wara DW, Howie VM, Schultz L, Doyle N, Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978;62:721–7. [PubMed] [Google Scholar]
- 5.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 6.Avery OT, Goebel WF. Chemo-immunologic studies on conjugate carbohydrate-proteins. II Immunological specificity synthetic sugar-protein antigens. J Exp Med. 1929;50:533–50. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayhty H, Eskola J. New vaccines for the prevention of pneumococcal infections. Emerg Infect Dis. 1996;2:289–98. doi: 10.3201/eid0204.960404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson MS, Brinton CC., Jr Identification and characterization of the Escherichia coli type 1 pilus tip adhesion protein. Nature. 1988;322:265–8. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- 9.Maurer L, Orndorff PE. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987;169:640–5. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bock K, Breimer ME, Brignole A, et al. Specificity of binding of a strain of uropathogenic Escherichia coli to Galα1–4Gal-containing glycosphingolipids. J Biol Chem. 1985;260:8545–51. [PubMed] [Google Scholar]
- 11.Stromberg N, Marklund BI, Lund B, Ilver D, Hamers A, Gaastra W, Karlsson KA, Normark S. Host specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Galα1–4Gal-containing isoreceptors. EMBO J. 1990;9:2001–10. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–11. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JA. Tropism in bacterial infections: urinary tract infections. J Urol. 1996;156:1552–9. [PubMed] [Google Scholar]
- 14.Demuth DR, Duan Y, Brooks W, Holmes AR, McNab R, Jenkinson HF. Tandem genes encode cell-surface polypeptide SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol Microbiol. 1996;20:403–13. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 15.Larsson C, Stalhammar-Carlemalm M, Lindahl G. Vaccination with highly purified cell surface proteins confers protection against experimental group B streptococcal infection. Adv Exp Med Biol. 1997;418:851–853. doi: 10.1007/978-1-4899-1825-3_202. [DOI] [PubMed] [Google Scholar]
- 16.Alonso de Velasco E, Verheul AF, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis and vaccine. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virolainen A, Jero J, Chattopadhyay P, Karrma P, Eskola J, Leinonen M. Comparison of serum antibodies to pneumolysin with those of pneumocccal capsular polysaccharides in children with acute otitis media. Pediatr Infect Dis. 1996;15:128–33. doi: 10.1097/00006454-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 18.McDaniel LS, Sheffield JS, Swiatlo E, Yother J, Crain MJ, Briles DE. Molecular localization of variable and conserve regions of PspA and identification of additional PspA homologous sequences in Streptococcus pneumoniae. Microb Pathog. 1992;13:261–9. doi: 10.1016/0882-4010(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 19.Sampson JS, O’Connor SP, Stinson AR, Tharpe JA, Russel H. Cloning and nucleotide sequence analysis of PsaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Immun. 1994;62:319–24. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades EW, Sampson JS, Briles DE. Mice challenged intranasally with Streptococcus pneumoniae are protected after intranasal immunization with pneumococcal recombinant PsaA (37 kDa) proteins. Presented at Pneumococcal Vaccines for the World Conference Washington DC Abstract 30.
- 21.Paton JC, Lock RA, Hansman DJ. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983;40:548–52. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briles DE, Ades E, Paton JC, et al. Intranasal immunization of mice with a mixture of pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68:796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. Contribution of novel choline-binding protein to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Molec Microbiol. 1997;25:819–29. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 24.Overweg K, Kerr A, Sluijter M, Jackson MH, Mitchell TJ, de Jong AP, de Groot R, Hermans PWM. The putative proteinase maturation protein A of Streptococcus pneumoniae is a conserved surface protein with potential to elicit protective immune response. Infect Immun. 2000;68:4180–8. doi: 10.1128/iai.68.7.4180-4188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapola S, Jantti V, Haikala R, et al. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in relation to pneumococcal carriage and acute otitis media. J Infect Dis. 2000;182:1146–52. doi: 10.1086/315822. [DOI] [PubMed] [Google Scholar]
- 26.Tuomanen E. Molecular and cellular biology of pneumococcal infection. Curr Opin Microbiol. 1999;2:35–9. doi: 10.1016/s1369-5274(99)80006-x. [DOI] [PubMed] [Google Scholar]
- 27.Barthelson R, Mobasseri A, Zopf D, Simon P. Adherence of Streptococcus pneumoniae to respiratory epithelial cells is inhibited by sialylated oligosaccharides. Infect Immun. 1998;66:1439–44. doi: 10.1128/iai.66.4.1439-1444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak R, Tuomanen E. Pathogenesis of pneumococcal pneumonia. Semin Respir Infect. 1999;14:209–17. [PubMed] [Google Scholar]
- 29.Cundell DR, Gerard N, Gerard C, Idanpaan-Heikkila I, Tuomanen E. Streptococcus pneumoniae anchores to activated eukaryotic cells by the receptor to platelets activating factor. Nature. 1995;377:435–8. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall component of innate immunity occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 31.Zhang JR, Tuomanen E. Molecular and cellular mechanisms for microbial entry into the CNS. J Neurovirol. 1999;5:591–603. doi: 10.3109/13550289909021288. [DOI] [PubMed] [Google Scholar]
- 32.Idanpaan-Heikkila I, Simon PM, Zopf D, Vullo T, Cahill P, Sokol K, Tuomanen E. Oligosaccharide interferes with the establishment and progression of experimental pneumococcal infection. J Infect Dis. 1997;176:704–12. doi: 10.1086/514094. [DOI] [PubMed] [Google Scholar]
- 33.Cundell DR, Tuomanen EI. Receptor specificity of adherence of Streptococcus pneumoniae to human type II pneumocytes and vascular endothelial cells in vitro. Microbiol Pathog. 1994;17:361–74. doi: 10.1006/mpat.1994.1082. [DOI] [PubMed] [Google Scholar]
- 34.Cundell DR, Pearce BJ, Sandros J, Naughton AM, Mazure HR. Peptide permeases from Streptococcus pneumoniae affect adherence to eukaryotic cells. Infect Immun. 1995;63:2493–8. doi: 10.1128/iai.63.7.2493-2498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson DA, Kapur V, Musher DM, Jacobson JW, Musser JM. Identification cloning and sequencing of DNA essential for encapsulation of Streptococcus pneumoniae. Curr Opin Microbiol. 1995;31:251–9. doi: 10.1007/BF00298383. [DOI] [PubMed] [Google Scholar]
- 36.Watson DA, Musher DM. Interruption of capsule production in Streptococcus pneumoniae serotype 3 by insertion of transposon Tn916. Infect Immun. 1990;58:3135–8. doi: 10.1128/iai.58.9.3135-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geelen S, Bhattacharyya C, Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993;61:1538–43. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasse J, Gallagter SR. Detection of protein. In: Coligan JE, Kruisbeek AM, Margulis DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. USA: Willey & Son; 1988. pp. 8.9.1–16. Chapter 8. [Google Scholar]
- 39.Plikaytis BD, Goldblatt D, Frasch CE, et al. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin Microbiol. 2000;38:2043–50. doi: 10.1128/jcm.38.6.2043-2050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massidda O, Anderluzzi D, Friedli L, Ferger G. Unconventional organization of the division and cell wall gene cluster of Streptococcus pneumoniae. Microbiology. 1998;144:3069–78. doi: 10.1099/00221287-144-11-3069. [DOI] [PubMed] [Google Scholar]
- 41.Boukamp P, Petrussevska RT, Breitkreutz D, Hormung J, Markham A, Fusenig NE. Normal keratinization in spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rheinwald JG. Serial cultivation of normal human epidermal keratinocytes. Methods Cell Biol. 1980;22:629–32. doi: 10.1016/s0091-679x(08)60769-4. [DOI] [PubMed] [Google Scholar]
- 43.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–77. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 44.McDaniel LS, Ralph BA, McDaniel DO, Briles DE. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192–260. Microb Pathogen. 1994;17:323–37. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 45.Ogunniyi AD, Folland RL, Briles DE, Hollingshead SK, Paton JC. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect Immun. 2000;68:3028–33. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks-Walter A, Briles DE, Hollingshead SK. The pspC gene of Streptococcus pneumoniae encodes polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun. 1999;67:6533–42. doi: 10.1128/iai.67.12.6533-6542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neeleman C, Geelen SP, Aerts PC, et al. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999;67:4517–24. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans JT, Okamoto Y, Douek DC, McFarland RD, Gatlin J, Koup RA, Garcia JV. Thymocyte differentiation from lentivirus marked CD34+ cells in infant and adult human thymus. J Immunol Methods. 2000;245:31–43. doi: 10.1016/s0022-1759(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 49.Wedderburn LR, Patel A, Varsani H, Woo P. The developing human immune system: T-cell receptor repertoire of children and young adults shows a wide discrepancy in the frequency of persistent oligoclonal T-cell expansion. Immunology. 2001;102:301–9. doi: 10.1046/j.1365-2567.2001.01194.x. 10.1046/j.1365-2567.2001.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


