Abstract
We conducted a phase I/II clinical trial of the safety and efficacy of intravesical administration of autologous IFN-γ-activated macrophages (MAK) in patients with superficial bladder cancer. Monocyte-derived MAK cells were prepared in vitro and patients received six instillations of 1·4 × 108 to 2·5 × 108 cells, once a week, for five consecutive weeks. Treatment was well tolerated, with seven grade 1 and five Grade 2 protocol-related adverse effects. Nine out of 17 included patients had no recurrences during the year following the first instillation of MAK. The aim of the present study was to search for immune parameters related to local immunostimulation induced by MAK. Monitoring of the patients showed that urinary IL-8, GM-CSF and, to a lesser extent, IL-18 were increased following MAK instillations, with inter-individual differences. The urinary IL-8 level was about 10-fold higher than that observed for other cytokines, and its biological activity was reflected by a concomitant increase of urinary elastase, indicating neutrophil activation and degranulation. We also showed that nine out of 12 patients investigated presented an increase of urinary neopterin, a marker of IFN-γ-activated macrophages, 7 days after MAK instillation, while serum neopterin levels were almost stable. These results are in line with persistence of activated macrophages in the bladder wall after infusions. Moreover, there was evidence of macrophages in urine smears 2 months after the sixth MAK instillation, and the score of macrophages correlated with the quantity of neutrophils in the urine. Overall, this study provides evidence of a local immunostimulation induced by this novel and safe immunotherapeutic approach of MAK instillations in patients with superficial bladder cancer.
Keywords: bladder neoplasms, macrophage, immunotherapy, urine, cytokines, interleukin-8
INTRODUCTION
Tumours of the urinary bladder are the fifth most common cancer in men, with an estimated incidence of 8000 new cases per year in France [1]. Seventy percent of bladder tumours are superficial at the time of presentation (CIS, Ta or T1 of the TNM classification) and are managed conservatively by endoscopic resection. These superficial tumours have a high potential of recurrence or progression [2], which has led to widespread use of adjuvant intravesical infusions of Bacillus Calmette-Guerin (BCG) preparation to eradicate disease that has not been controlled endoscopically, and to prevent recurrences [3]. The local immunostimulation exerted by BCG instillations is characterized by late induction of MHC class II antigens on urothelial cells, and a marked infiltration of CD4+ T lymphocytes with an increasing number of macrophages in the bladder wall [4,5]. In clinical practice, urinary Th1-associated cytokines IFN-γ, IL-2 [6], IL-12 [7] and IL-18 [8], with IL-8 [8] and GM-CSF [9], have been inconsistently observed in BCG responders. The clinical benefits of BCG must be weighed up against its systemic toxicity, unreliable efficacy and the unpredictable prognosis [9,10]. This prompted us to develop a novel immunotherapeutic approach, based on intravesical administration of IFN-γ-activated autologous macrophages (MAK = Macrophage Activated Killers cells), in patients with a superficial bladder tumour (Thiounn N et al. unpublished observation), as many studies have demonstrated that IFN-γ-activated macrophages are able to selectively kill tumour cells in vitro [11–13], or in experimental murine models of human tumours [14–16]. Clinical studies have also demonstrated the feasibility and potential value of macrophage instillations in the treatment of cancers [17–21]. Seventeen patients were included in this phase I/II open trial of intravesical administration of MAK. Monitoring of urine samples was performed during the course of MAK instillations. The aim of this study was to identify urine markers indicative of local immunostimulation induced by MAK.
PATIENTS
Seventeen patients with unifocal or multifocal superficial TaGIII, or recurrent TaGII bladder tumours with no associated carcinoma in situ (CIS), were included in this non-randomized, multi-centre phase I/II clinical trial. Each patient experienced from one to three resections for a uni- or multifocal superficial bladder tumour in the year before MAK treatment (total of 34 events) (Thiounn N et al. in preparation). Following transurethral tumour resection, apheresis was performed to collect mononuclear cell-enriched leucocytes. Mononuclear cells were then purified with a cell separator and cultured for 6 days in IMDM medium supplemented with 3–5% undecomplemented autologous serum, antibiotics and 500 IU/ml GM-CSF to allow differentiation into macrophages. Cells were activated with 250 U/ml IFN-γ on day 6 (MAK™). MAK cells were submitted to a microscopic examination and tested for CD14, CD64 and HLA-DR expression before storage. Patients received six intravesical administrations of 1·4 × 108 to 2·5 × 108 cells, once a week, for 5 consecutive weeks. Each MAK instillation was retained intravesically for 2 h before being spontaneously voided. Five patients received three additional instillations at months 3, 6 and 9, respectively. One patient received only three MAK instillations and was therefore not included in the biological monitoring. The patients were followed for 12 months after the day of the first instillation. Cystoscopy and urinary smears were performed at months 3, 6, 9 and 12. No severe adverse effects were observed during the course of the treatment. Nine patients reported adverse events. Most protocol-related adverse events had a CTC grade 1 severity, except for five adverse effects which had a CTC grade 2 severity (myalgia, urinary tract infection, prostatic disorders, headache and fatigue), each in one patient. Eight of the 17 patients included had a recurrence during the year following the first MAK instillation (one TaG1G2 at 3 months; five TaG2 at 3, 4, 9, 11 and 12 months; two TaG3 at 9 and 12 months). The study was approved by the Ethics Committee of Paris-Cochin Hospital, France and signed informed consent was obtained from all patients.
METHODS
Urine was collected before treatment, immediately following MAK (IDM, Paris, France) instillation number 1, 3 and 6, and 7 days after MAK instillation 2 and 5. Urine samples were immediately stored at 4°C and processed within 2 h. Samples were centrifuged at 3000 r.p.m. (3000 × g) for 10 min to remove cells and debris, aliquoted, and stored at –80°C until analysis. Blood samples (sera) were also collected 7 days after MAK instillation 2.
Urine cytokine determination
IL-8, GM-CSF, IL-18, IL-6, IL-1β, IL-12p70, TNF-α and IL-2 were determined with commercially-available, enzyme-linked immunosorbent assay kits (Quantikine ELISA assays, R & D Systems, Minneapolis, MN, USA). The assays typically provided sensitivities in the 10–20 pg/ml range. Each sample was analysed in duplicate and compared with the standard curve. To correct for hydration status, cytokine data were standardized to urinary creatinine. In four patients with normal hydration status, the mean urinary creatinine measured in the series was used, as data for urinary creatinine were not available for these patients. To investigate the stability of cytokines in urine, a known quantity of recombinant cytokine (50–100 pg/ml) was incubated for 5 min and 2, 4, 6 and 24 h at 4°C and 20°C. Cytokines were then measured by ELISA assay to estimate the percentage recovery.
Determination of urinary elastase
An enzyme-linked immunosorbent assay (Bender Medsystems, Vienna, Austria) was used for quantitative determination of human polymorphonuclear elastase levels in urine, according to the manufacturer’s instructions. Urine samples were analysed in duplicate and the results were standardized to urinary creatinine. The sensitivity of the test was determined to be 3 ng/ml.
Determination of serum and urine neopterin concentrations
Quantitative determination of serum neopterin was performed in duplicate with a competition enzyme immunoassay (Beckman Coulter, Fullerton, CA, USA), according to the manufacturer’s instructions (sensitivity: 0·2 ng/ml). For determination of urinary neopterin, samples was de-proteinized in 0·3 m HClO4 containing 2 mm ascorbic acid and centrifuged at 10 000 g for 5 min; the supernatant fluid was oxidized with acidic iodine and neutralized before injection into an HPLC column. The mobile phase was 0·1% acetic acid (pH 3·3):methanol, 99:1 (v/v), used with a reversed-phase column ODS Ultrasphere C-18 (Beckman Coulter); a fluorescence detector was used to detect neopterin (with excitation and emission wavelengths at 278 and 450 nm, respectively). Results were standardized to urinary creatinine. Values of a control group of healthy subjects ranged from 50 to 300 nmol/mmol creatinine.
Cytological evaluation of urine smears
Urine smears were performed in all centres of this open trial within 2 months following the sixth MAK instillation. The preparations of shed cells were collected and evaluated by a single pathologist (A. Vieillefond, Hôpital Cochin, Paris) for neutrophil and macrophage density. A semi-quantitative scoring system was performed as follows: 0 (absence); 1–4 (from weak to intense).
CD68 staining of bladder tumour recurrences
For three patients, sections from paraffin blocks of early bladder recurrences following treatment were evaluated for CD68 immunostaining. Briefly, after deparaffinization and rehydration, the 4 μm-thick tissue sections were quenched for endogenous peroxidase activity. Slides were incubated with a mouse IgG1 anti-human CD68 (1/200°) purchased from Dako (Copenhagen, Denmark) or the isotype match control at the same final concentration, for 1 h at room temperature. The biotinylated conjugate and streptavidin peroxidase were then applied, and DAB chromogen was used as the peroxidase substrate complex (all from LSAB + kit peroxidase, Dako). Tissue sections were counterstained with Harris’s haematoxylin.
Statistical analysis
Statistical analyses were performed using Statview software (Abacus Concept, Calabases, CA). Differences in cytokine secretion were evaluated using the sign test. Correlations between elastase and IL-8 concentrations, or between neutrophil and macrophage scores, were assessed by calculating the Kendall rank correlation coefficient (Kendall τ).
RESULTS
In vitro production of cytokines by MAK in urine
We first evaluated in vitro the capacity of MAK to produce cytokines. MAK were incubated for 2 h at 37°C in non-pH-adjusted urine, over the same concentration range as MAK administered intravesically. The viability of MAK was not altered by incubation in non-pH-adjusted urine (data not shown). Significant levels of IL-18, GM-CSF and IL-8 were detected by ELISA in immediately-processed samples. Similarly, MAK in urine incubated at 37°C for 2 h under non-adherent conditions (Teflon bag) produced the same cytokine pattern (data not shown). In the clinical study, urine voided after MAK instillation was stored at 4°C and centrifuged within 2 h to remove macrophages before storage. This could have induced a release of cytokines by MAK in vitro, leading to overestimation of the amounts of cytokines produced in the bladder. We therefore repeated the same experiment and kept the samples for 2 h at 20°C or 4°C before centrifugation and storage. The cytokine levels detected by ELISA were nearly identical to those observed in immediately-processed urine (less than 10% variation), arguing against ex vivo release of cytokines by MAK in the urine samples of this series. The mean and s.d. of urinary cytokine levels observed in the three experimental procedures were calculated and are shown in Fig. 1.
Fig. 1.
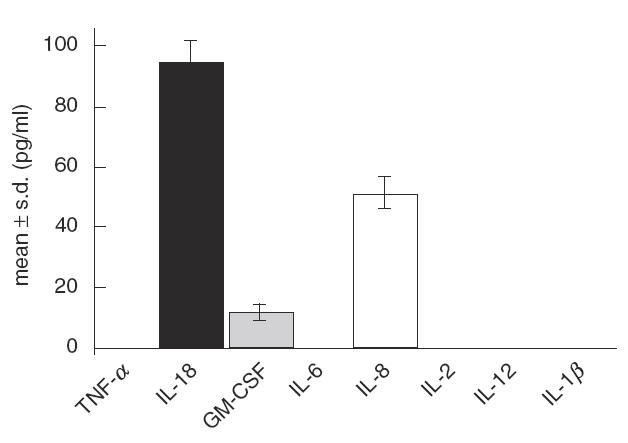
Cytokine production by MAK in vitro when incubated in urine for 2 h at 37°C. Urine samples with MAK were immediately centrifuged, or stored for 2 h at 4°C or 20°C before centrifugation and storage. Results are expressed for each cytokine as mean ± s.d. of the levels observed with the three sampling procedures. No cytokines were detected in the medium containing MAK before instillation, and the urine sample before MAK contained only a low level of IL-18 (>10 pg/ml).
We also investigated the stability of IL-18, GM-CSF and IL-8 in urine and found that GM-CSF and IL-18 were stable at 4°C and 20°C, whereas IL-8 was immediately unstable (52% recovery), even when the urine was adjusted to pH 7 (data not shown). For monitoring during the study, urine samples were therefore stored at 4°C and processed within 2 h, as immediate sampling and pH adjustment did not appear to be decisive.
Urinary cytokines in patients after MAK instillations
Samples of spontaneously-voided urine were collected before the first MAK instillation and immediately after MAK instillation numbers 1, 3 and 6. Urine samples were first evaluated in four patients of the series (two disease-free patients and two patients with recurrence) for IL-8, GM-CSF, IL-18, IL-6, IL-1β, IL-12p70, TNF-α and IL-2 levels by ELISA assays. TNF-α, IL-12 and IL-2 were always undetectable. Patients inconsistently exhibited low levels of IL-1β in their urine, whereas high levels of IL-8, IL-6, IL-18 and GM-CSF were detected in some patients following MAK instillations (data not shown). We then extended determination of urinary IL-8, IL-6, IL-18 and GM-CSF levels to all patients of the series (Fig. 2).
Fig. 2.
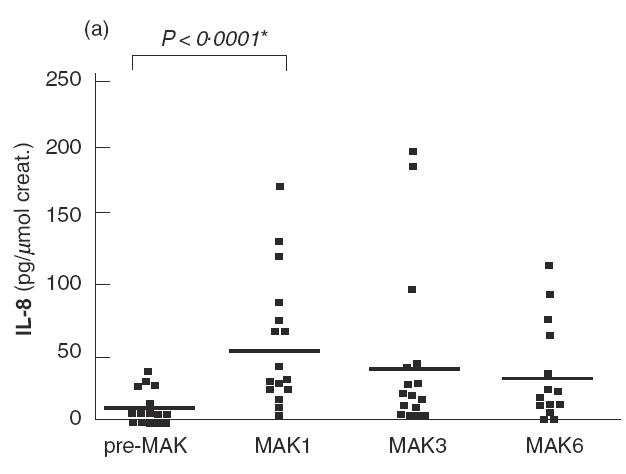
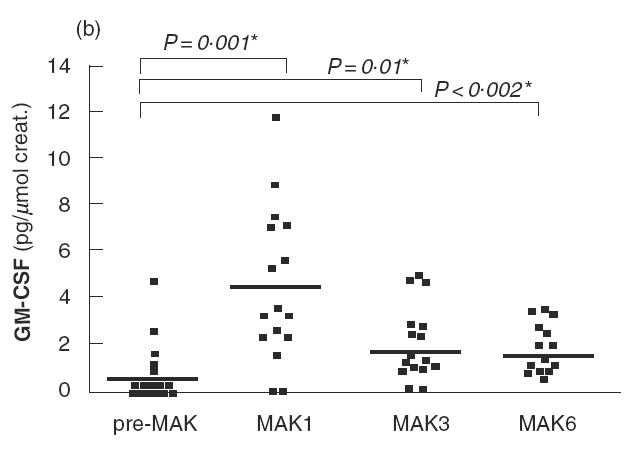
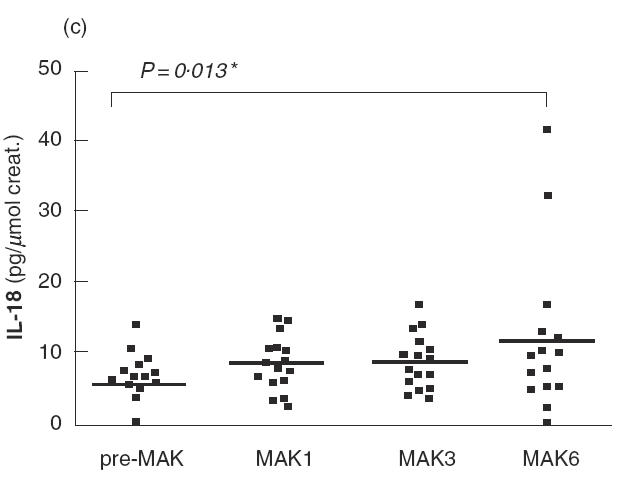
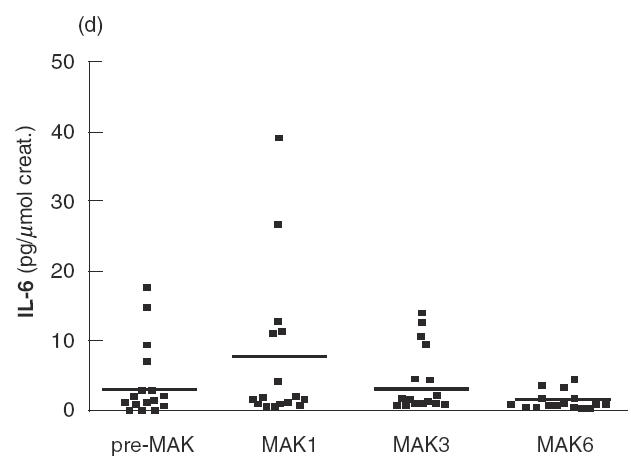
IL-8 (a), GM-CSF (b), IL-18 (c) and IL-6 (d) levels in urine are shown for patients of the series before the first instillation of MAK (pre-MAK), and in urine voided after MAK instillations number 1, 3 and 6. The horizontal line represents the mean values. * Analysed by sign test.
Before MAK instillations (pre-MAK), IL-8 was detected in the urine of five patients (mean 7·36; range 0–32·7 pg/μmol creatinine) (Fig. 2a). After MAK1, 15 out of 16 patients exhibited detectable urinary IL-8 levels, with a mean of 53 (range 0– 168 pg/μmol creatinine). This difference was significant (P < 0·0001 by sign test). A tendency towards decreasing IL-8 levels was observed with subsequent instillations (mean 43·5 and 29·4 for MAK3 and 6, respectively). Strikingly, the urinary IL-8 level detected following MAK instillations was about 10-fold higher than that observed for other cytokines studied. MAK instillations also induced or increased urinary GM-CSF levels during the course of instillations, with a more pronounced effect after MAK1 (Fig. 2b). GM-CSF was detected in 5/16 patients before MAK instillation (mean 0·6; range 0–4·6 pg/μmol creatinine), and in 14/16 after MAK1 (mean 4·5; range 0–11·7 pg/μmol creatinine). The increase in GM-CSF was statistically significant for MAK1, MAK3 and MAK6 (for MAK1; P = 0·001 by sign test). IL-18 levels increased slightly following the instillations, with a maximum observed after MAK6 (mean 12·4 versus 5·9 pg/μmol creatinine before MAK; P = 0·013 by sign test) (Fig. 2c). A transient and non-significant increase in IL-6 levels was observed after MAK1 (mean 7·7 versus 3·7 pg/μmol creatinine before MAK), which subsequently returned to normal (Fig. 2d).
Biological activity of IL-8 in the urine of patients
The observed instability of IL-8 in urine led us to investigate its biological activity in the patients’ urine following MAK instillations. We measured urinary levels of elastase, a molecule usually released by activated neutrophils in response to IL-8 stimulation. Among the 12 patients tested, significant levels of elastase were observed in six patients immediately before MAK3, and in nine patients in the urine voided 2 h after MAK3 (Table 1). The IL-8 level before MAK3 was correlated with urinary elastase secretion (Kendall rank correlation coefficient τ = 0·898, P < 0·0001). MAK instillation induced an increase in IL-8 secretion (mean 16·4 pg/μmol creatinine before MAK3 versus 52·2 pg/μmol creatinine after MAK3, P = 0·006), and a concomitant increase in urinary elastase (mean 213·3 ng/μmol creatinine before MAK3 versus 576·7 ng/μmol creatinine after MAK3, P = 0·02).
Table 1.
Comparison between urinary IL-8 and elastase, before and 2 h after MAK3
| Before MAK3 | After MAK3 | |||
|---|---|---|---|---|
| IL-8 | elastase * | IL-8† | elastase ‡ | |
| Patient no. | (pg/μmol creat.) | (ng/μmol creat.) | (pg/μmol creat.) | (ng/μmol creat.) |
| 1 | 0 | 0 | 8 | 179 |
| 2 | 82·4 | 1526·5 | 198·7 | 4207·1 |
| 3 | 0 | 0 | 14·6 | 0 |
| 5 | 42·3 | 445·8 | 96·8 | 1017·3 |
| 6 | 0 | 0 | 27·4 | 172·8 |
| 7 | 16·1 | 127·3 | 41·6 | 589·9 |
| 8 | 3·5 | 43·1 | 17·6 | 226·1 |
| 10 | 0 | 0 | 10·4 | 0 |
| 11 | 0 | 0 | 18·7 | 183·4 |
| 13 | 0 | 40·9 | 3·2 | 246·4 |
| 14 | 52·5 | 364·3 | 2·3 | 0 |
| 15 | 0 | 0 | 187·5 | 98·4 |
A correlation was observed between elastase and IL-8 (τ = 0·898; P < 0·0001).
Significant difference compared with the level before MAK3 (P = 0·006 sign test).
Significant difference compared with the level before MAK3 (P = 0·02 sign test).
Persistence of MAK after instillations
Table 1 shows that five out of 12 patients presented with significant levels of IL-8 in their urine 7 days after MAK2 (before MAK3). This does not formally indicate the presence of resident activated macrophages at distance from instillations. Therefore, we investigated urinary levels of neopterin, a marker of macrophage activation, 7 days after MAK2, and compared the values obtained with those observed before treatment. Nine of the 12 available samples presented with an increase in urinary neopterin, with a mean value of 375 (range 133–1019 nmol/mmol creatinine) versus 234 (range 0–703 nmol/mmol creatinine) before treatment (data not shown). We then investigated whether this increase was due to a modification of neopterin in peripheral blood. A neopterin ratio was calculated for each patient in urine and serum by dividing the neopterin levels observed 7 days after MAK2 by the value observed before treatment. Figure 3 clearly shows that the marked increase in urinary neopterin was associated with a minor modification of serum neopterin levels. In addition, 12 of the 16 patients presented macrophages on the urine smear performed within 2 months after the sixth instillation, and five of them showed a high macrophage score up to 1 (Fig. 4). A correlation (τ = 0·80, P < 0·0001) was observed between the macrophage score and the neutrophil score in urine smears (Fig. 4). Finally, the presence of numerous CD68-positive cells from the monocyte/macrophage lineage in disease-free urothelium and in bladder tumours was observed in the three patients with early recurrence following treatment (data not shown).
Fig. 3.
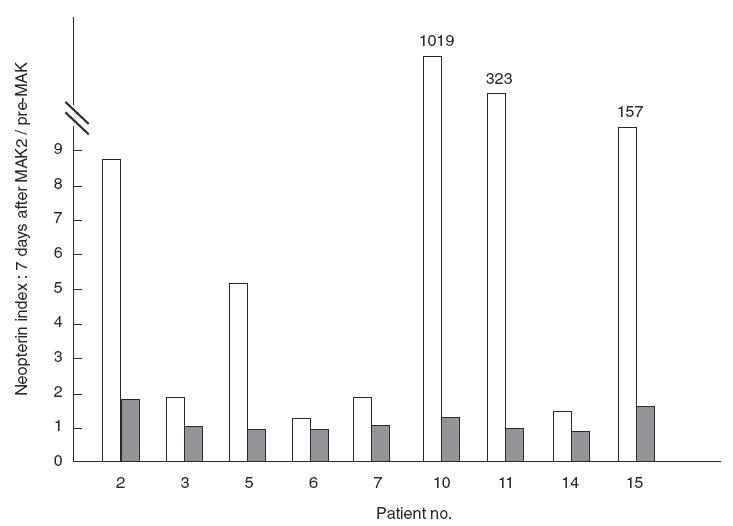
Neopterin was measured in urine and blood samples before treatment and 7 days after MAK2. A ratio was calculated by dividing the neopterin level observed 7 days after MAK2 by that detected before treatment (n-fold increase). The nine patients with an increase in urinary neopterin (□) 7 days after MAK2 are shown and compared with changes in serum neopterin levels.
Fig. 4.
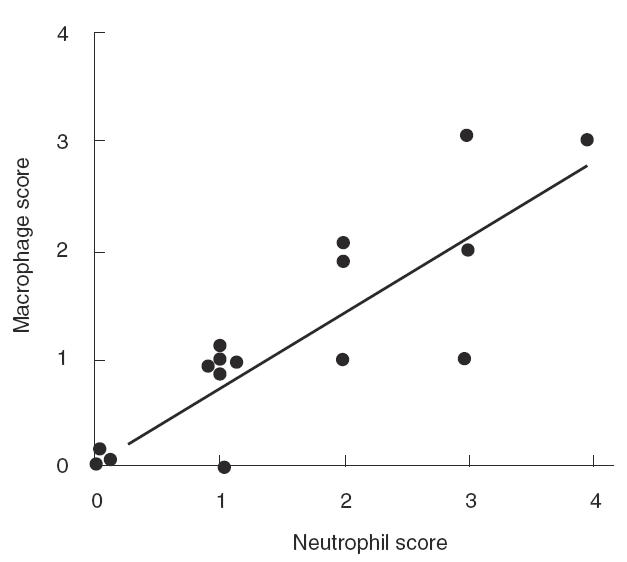
Cytological evaluation of urine smears performed 2 months after the sixth instillation in the 16 patients evaluated. A correlation is observed between the score of macrophages and the score of neutrophils (τ = 0·80 P < 0·0001).
DISCUSSION
We first evaluated in vitro the production of MAK in urine and observed a spontaneous release of IL-18, GM-CSF and IL-8. We excluded determination of urinary IFN-γ as this cytokine is extremely unstable in urine and requires immediate dialysis after collection [22]. Surprisingly, we did not detect any significant levels of IL-1β and TNF-α by ELISA assays. This feature was also observed in urinary samples during the course of MAK instillations. Soluble IL-1 receptor type II (sIL-1R type II), and soluble TNF receptor (sTNFR) types I and II, are detected in urine [23,24] and could interfere with IL-1β and TNF-α measurement by ELISA assays. This potential bias was excluded as we chose ELISA kits without interference with soluble cytokine receptors. Urine contains numerous immunomodulatory molecules, which could profoundly modify the nature of cytokines produced by macrophages and could account for the unexpected pattern of cytokine production.
In patients, there was clear evidence for a significant level of IL-8 and, to a lesser degree, IL-18 and GM-CSF in urine voided immediately after MAK instillations, in line with in vitro data. Surprisingly, IL-8 and GM-CSF tended to decrease with increasing number of instillations, while IL-18 increased with repeated instillations. The IL-8 produced in the bladder was probably greater than that detected by the ELISA assay, as we observed that about 50% of IL-8 was not detected by the ELISA assay when the cytokine was incubated in urine. Moreover, we observed that centrifugation of voided urine following MAK instillation at 3000 r.p.m. (3000 × g) for 10 min tended to result in lower IL-8 levels compared with deeply (1000 × g for lmin) centrifuged samples (data not shown), as previously reported for IL-1β [25].
IL-8 belongs to the CXC chemokine family. It acts as a potent chemoattractant and activator for neutrophils and a pro-inflammatory mediator [26,27]. Accumulated evidence suggests that IL-8 plays an important role in neutrophil migration across uroepithelial cell layers, as observed during urinary tract infection [28]. IL-8 activity is reinforced by GM-CSF, also induced by MAK, and IL-18 (another MAK-induced cytokine) has been shown to induce IL-8 in human peripheral blood mononuclear cells in the absence of co-stimuli [29]. The biological activity of IL-8 in urine can be impaired by its instability, formation of complexes with an inhibitor, or the concomitant presence of suppressor or antagonist cytokines. In this field, discrepancies between urinary ELISA for IL-1 and IL-2, and bioassays, have been observed in urine voided after BCG instillations [30]. We observed a correlation between urinary IL-8 and elastase which argues in favour of the biological activity of MAK-induced IL-8.
A subgroup of patients exhibited persistence of induced cytokines throughout the course of MAK infusions. Seven of 16 patients presented a significant increase in IL-8, IL-18 and/or GM-CSF levels 7 days after MAK2, and this effect persisted 7 days after MAK5 in four of these patients (data not shown). This result is compatible with prolonged local immunostimulation in some patients and suggests the presence of resident macrophages in the bladder wall. However, various cell types can produce IL-8 in the urinary tract, including epithelial, mesangial and endothelial cells, and tumour cells [31]. In previous immunohistochemical studies, we showed that normal urothelium stained positively for immature and/or mature IL-18 (unpublished data). Finally, GM-CSF has been reported to be produced by cell lines derived from bladder carcinoma [32]. The observed increase in urinary neopterin, the presence of macrophages in urine smears 2 months after MAK6, and the presence of CD68-positive cells in normal disease-free and tumour zones, strongly argues in favour of resident macrophages at a distance from instillations in some patients.
The comparison for each patient between levels of urinary IL-8 (or elastase) and neopterin 7 days after MAK2 showed that all patients with a significant level of urinary IL-8 presented with a high level of neopterin in urine. In contrast, a high level of neopterin was not always associated with an increase in urinary IL-8, particularly in patients with the highest levels of neopterin. The findings observed in this limited series may indicate that (i) urinary IL-8 is, in part, produced by activated macrophages, and (ii) the profile of cytokine production by MAK may differ from one patient to another. For example, two out of three patients with a high level of urinary neopterin and undetectable urinary IL-8 presented with increased urinary IL-18 (compared with pre-treatment values), and IL-6 was detected in the urine of the third patient.
It is of interest that urinary IL-8 and IL-18 levels detected in urine samples after MAK instillations were associated with a good prognosis in terms of disease-free survival in patients treated by BCG instillations for superficial bladder tumours [33,34]. We observed a strong decrease in the resection rate during the year after the first MAK instillation compared with that during the year before the first instillation (Thiounn N et al. in preparation). Altogether, the biological monitoring of this novel and well-tolerated immunotherapeutic approach provided evidence for a modulation of immune parameters, arguing for a local immunostimulation.
Acknowledgments
This work was financially supported by Immuno Designed Molecule (IDM, Paris, France). The authors would like to thank Cloé Despoix from IDM for help in collection of clinical data, Laurence Françoise and Pascal Bouchet for expert technical assistance, Dr Eric Tartour for advice concerning cytokine evaluation and helpful discussions, and Rachel Taylor from IDM for critically reviewing the manuscript.
References
- 1.Hill C, Doyon F, Sancho-Garnier H. Epidemiologie des cancers. In: Flammarion, editor. Médecine-Sciences. Paris: Epidemiologie descriptive; 1997. pp. 15–26. [Google Scholar]
- 2.Gilbert HA, Logan JL, Kagan AR, et al. The natural history of papillary transitional cell carcinoma of the bladder and its treatment in an unselected population on the basis of histologic grading. J Urol. 1978;119:488–92. doi: 10.1016/s0022-5347(17)57526-6. [DOI] [PubMed] [Google Scholar]
- 3.Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19:573–80. [PubMed] [Google Scholar]
- 4.Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette-Guerin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–8. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 5.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992;147:1636–42. doi: 10.1016/s0022-5347(17)37668-1. [DOI] [PubMed] [Google Scholar]
- 6.Patard JJ, Saint F, Velotti F, Abbou CC, Chopin DK. Immune response following intravesical bacillus Calmette-Guerin instillations in superficial bladder cancer: a review. Urol Res. 1998;26:155–9. doi: 10.1007/s002400050039. [DOI] [PubMed] [Google Scholar]
- 7.Poppas DP, Pavlovich CP, Folkman J, et al. Intravesical bacille Calmette-Guerin induces the antiangiogenic chemokine interferon-inducible protein 10. Urology. 1998;52:268–75. [PubMed] [Google Scholar]
- 8.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]
- 9.Jackson AM, Ivshina AV, Senko O, et al. Prognosis of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer by immunological urinary measurements: statistically weighted syndrome analysis. J Urol. 1998;159:1054–63. [PubMed] [Google Scholar]
- 10.Lamm DL. Complications of bacillus Calmette-Guerin immunotherapy. Urol Clin North Am. 1992;19:565–72. [PubMed] [Google Scholar]
- 11.Dumont S, Mabondzo A, Hartmann D, et al. Study of the dependence of human monocytes and macrophages antitumoral properties upon TNF-alpha expression or release. Anticancer Res. 1990;10:949–54. [PubMed] [Google Scholar]
- 12.Harwix S, Andreesen R, Ferber E, Schwamberger G. Human macrophages secrete a tumoricidal activity distinct from tumour necrosis factor-alpha and reactive nitrogen intermediates. Res Immunol. 1992;143:89–94. doi: 10.1016/0923-2494(92)80084-x. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Cheung NK. Phagocytosis of tumor cells by human monocytes cultured in recombinant macrophage colony-stimulating factor. J Exp Med. 1990;172:231–7. doi: 10.1084/jem.172.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartholeyns J, Lombard Y, Dumont S, et al. Immunotherapy of cancer: experimental approach with activated macrophages proliferating in culture. Cancer Detect Prev. 1988;12:413–20. [PubMed] [Google Scholar]
- 15.Chokri M, Freudenberg M, Galanos C, Poindron P, Bartholeyns J. Antitumoral effects of lipopolysaccharides, tumor necrosis factor, interferon and activated macrophages: synergism and tissue distribution. Anticancer Res. 1989;9:1185–90. [PubMed] [Google Scholar]
- 16.Chokri M, Lallot C, Ebert M, Poindron P, Bartholeyns J. Biodistribution of indium-labelled macrophages in mice bearing solid tumors. Int J Immunotherapy. 1990;6:79–84. [Google Scholar]
- 17.Chokri M, Lopez M, Oleron C, et al. Production of human macrophages with potent antitumor properties (MAK) by culture of monocytes in the presence of GM-CSF and 1,25-dihydroxy vitamin D3. Anticancer Res. 1992;12:2257–60. [PubMed] [Google Scholar]
- 18.Andreesen R, Scheibenbogen C, Brugger W, et al. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 1990;50:7450–6. [PubMed] [Google Scholar]
- 19.Faradji A, Bohbot A, Schmitt-Goguel M, et al. Phase I trial of intravenous infusion of ex-vivo-activated autologous blood-derived macrophages in patients with non-small-cell lung cancer: toxicity and immunomodulatory effects. Cancer Immunol Immunother. 1991;33:319–26. doi: 10.1007/BF01756597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartholeyns J, Lopez M, Andreesen R. Adoptive immunotherapy of solid tumors with activated macrophages: experimental and clinical results. Anticancer Res. 1991;11:1201–4. [PubMed] [Google Scholar]
- 21.Lopez M, Fechtenbaum J, David B, et al. Adoptive immunotherapy with activated macrophages grown in vitro from blood monocytes in cancer patients: a pilot study. J Immunother. 1992;11:209–17. doi: 10.1097/00002371-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Radio-immunoassay detection of interferon-gamma in urine after intravesical Evans BCG therapy. J Urol. 1990;144:1248–51. doi: 10.1016/s0022-5347(17)39713-6. [DOI] [PubMed] [Google Scholar]
- 23.Engelmann H, Aderka D, Rubinstein M, Rotman D, Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989;264:11974–80. [PubMed] [Google Scholar]
- 24.Olszyna DP, Prins JM, Buis B, van Deventer SJ, Speelman P, van der Poll T. Levels of inhibitors of tumor necrosis factor alpha and interleukin 1beta in urine and sera of patients with urosepsis. Infect Immun. 1998;66:3527–34. doi: 10.1128/iai.66.8.3527-3534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 26.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 27.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 28.Olszyna DP, Opal SM, Prins JM, et al. Chemotactic activity of CXC chemokines interleukin-8, growth-related oncogene-alpha, and epithelial cell-derived neutrophil-activating protein-78 in urine of patients with urosepsis. J Infect Dis. 2000;182:1731–7. doi: 10.1086/317603. [DOI] [PubMed] [Google Scholar]
- 29.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–21. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohle A, Nowc C, et al. Ulmer. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette-Guerin immunotherapy. J Urol. 1990;144:59–64. doi: 10.1016/s0022-5347(17)39366-7. [DOI] [PubMed] [Google Scholar]
- 31.Sander B, Damm O, Gustafsson B, Andersson U, Hakansson L. Localization of IL-1, IL-2, IL-4, IL-8 and TNF in superficial bladder tumors treated with intravesical bacillus Calmette-Guerin. J Urol. 1996;156:536–41. doi: 10.1097/00005392-199608000-00078. [DOI] [PubMed] [Google Scholar]
- 32.Steube KG, Meyer C, Drexler HG. Secretion of functional hematopoietic growth factors by human carcinoma cell lines. Int J Cancer. 1998;78:120–4. doi: 10.1002/(sici)1097-0215(19980925)78:1<120::aid-ijc19>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Thalmann GN, Dewald B, Baggiolini M, Studer UE. Interleukin-8 expression in the urine after bacillus Calmette-Guerin therapy: a potential prognostic factor of tumor recurrence and progression. J Urol. 1997;158:1340–4. [PubMed] [Google Scholar]
- 34.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]


