Abstract
Experimental autoimmune myasthenia gravis (EAMG) is an animal model for human myasthenia gravis (MG), characterized by an autoaggressive T-cell-dependent antibody-mediated immune response directed against the acetylcholine receptor (AChR) of the neuromuscular junction. Dendritic cells (DC) are unique antigen-presenting cells which control T- and B-cell functions and induce immunity or tolerance. Here, we demonstrate that DC exposed to TGF-β1 in vitro mediate protection against EAMG. Freshly prepared DC from spleen of healthy rats were exposed to TGF-β1 in vitro for 48 h, and administered subcutaneously to Lewis rats (2 × 106DC/rat) on day 5 post immunization with AChR in Freund’s complete adjuvant. Control EAMG rats were injected in parallel with untreated DC (naive DC) or PBS. Lewis rats receiving TGF-β1-exposed DC developed very mild symptoms of EAMG without loss of body weight compared with control EAMG rats receiving naive DC or PBS. This effect of TGF-β1-exposed DC was associated with augmented spontaneous and AChR-induced proliferation, IFN-γ and NO production, and decreased levels of anti-AChR antibody-secreting cells. Autologous DC exposed in vitro to TGF-β1 could represent a new opportunity for DC-based immunotherapy of antibody-mediated autoimmune diseases.
Keywords: experimental autoimmune myasthenia gravis, dendritic cells, TGF-β1
INTRODUCTION
In myasthenia gravis (MG) and its animal model, experimental autoimmune myasthenia gravis (EAMG), autoantibodies against the nicotinic acetylcholine receptor (AChR) at the postsynaptic membrane cause loss of functional AChR, resulting in disturbed neuromuscular transmission and muscle weakness [1]. The similarity of clinical symptoms and neurophysiological variables between human MG and EAMG make rat EAMG suitable for studying new strategies of immunomodulation and therapy.
Dendritic cells (DC) are specialized antigen-presenting cells (APCs) that capture antigen, migrate from the periphery to lymphoid organs and present the processed antigens to naive T cells. They not only activate lymphocytes, but can also tolerize T cells to self-antigens, thereby minimizing autoaggressive immune responses [2]. In mice, in vivo treatment with FLt3L, a growth factor that expands DC in vivo, enhanced the induction of oral tolerance [3]. Transfer of pancreas lymph node DC modulated autoimmunity and limited diabetes in NOD mice by the induction of regulatory cells [4]. Spontaneous autoimmune diabetes in NOD mice was also prevented by transferring human γ-globulin-pulsed DC [5]. In humans, DC pre-cultured with IL-10 induced a state of alloantigen-specific anergy in CD4+ T cells [6] and CD8+ T cells [7] by converting immunogenic DC into tolerogenic DC. We have observed that DC, upon exposure in vitro to encephalitogenic peptides, induced protection from experimental allergic encephalomyelitis [8]. The tolerogenic properties of DC are linked to their maturation state. Subcutaneous (s.c.) injection of immature DC can lead to peripheral tolerance by differentiation of regulatory T cells [9,10]. Thus, the concept of ‘tolerogenic’ DC reflects an additional property of these important APCs, which might be useful in the therapy of autoimmune diseases [11,12]. However, it is unclear whether DC can induce immune tolerance against antibody-mediated autoimmune disease.
TGF-β belongs to a well defined multi-potent cytokine family involved in many pathophysiological events [13]. Three isoforms (TGF-β1, β2 and β3) are expressed in mammals, among which TGF-β1 is a prototype and has most biological activities. We have reported that TGF-β1 ameliorates the development of EAE, accompanied by apoptosis of CD4+ T cells induced by DC-derived NO [14]. TGF-β1 promotes DC development in vitro [15] and suppresses DC maturation [16,17], and tolerogenicity of DC is enhanced by genetic modification with the introduction of vectors encoding TGF-β1 cDNA [18]. DC modulated by TGF-β adenoviral vectors suppressed T-cell alloreactivity [19]. In vitro manipulation of DC by exposure to a variety of factors (e.g. viral IL-4, CTLA4Ig) also confers tolerogenic properties [20].
Current therapy for MG is not specific and employs immunosuppression, which could partially block mechanisms of immune defense and cause complications. Here, we report that splenic DC, upon exposure in vitro to TGF-β1 (TGF-β1-DC) and followed by a single s.c. injection, ameliorate the development of EAMG in Lewis rats.
MATERIALS AND METHODS
Animals and reagents
Female Lewis rats, weighing 150–180 g, were obtained from Zentralinstitut fur Versuchstierzucht, Hannover, Germany. All animals were housed in pathogen-free conditions at the animal facilities. AChR was purified from electric organ of Torpedo californica (Pacific Biomarine, Venice, CA, USA) by affinity chromatography on a α-cobratoxin-agarose resin (Sigma, St. Louis, MO, USA) [21]. Purity was checked by SDS-PAGE. Recombinant human TGF-β1 (rh TGF-β1, 99% homology to rat TGF-β1) was from Genentech (San Fransisco, CA, USA).
Induction of EAMG
Lewis rats were immunized in both hind footpads with 200 μl inoculum containing 50 μg AChR, 2 mg Mycobacterium tuberculosis (strain H37RA, Difco, Detroit, MI, USA) in 100 μl saline and 100 μl Freund’s incomplete adjuvant (FIA, Difco). Animals were weighed and evaluated daily, in a blinded fashion by at least two investigators, for clinical signs. Clinical scores of EAMG were graded according to the following criteria: 0, asymptomatic; 1, mildly decreased activity, weak grip or cry, with fatigue; 2, markedly decreased activity and body weight, hunched posture at rest, head down and forelimb digits flexed, tremulous ambulations; and 3, severe generalized weakness, no cry, no grip [22].
DC preparation, modification and injection
Spleens were removed under aseptic conditions from healthy rats. Erythrocytes were osmotically lysed. DC were further enriched by differential adherence by incubating cells in 75 mm2 Falcon culture flasks (Becton Dickinson, Franklin Lakes, NJ, USA) in serum-free Dulbecco’s modification of Eagle’s medium (Gibco, Paisley, UK), containing 50 IU penicillin (GIBCO), 50 μg/ml streptomycin (GIBCO), 1% MEM amino acids (GIBCO) and 10 mM Hepes (Sigma), at 37°C in a 5% CO incubator. After 2 h, non-adherent cells were gently removed by swirling the flasks and aspirating the medium, and flasks were washed five times with serum-free medium to remove non-adherent cells. New medium containing 10% fetal calf serum (FCS) (GIBCO) was added to the flasks. After 18 h, re-floating cells were collected as a DC-enriched fraction, whereas the adherent cells mostly consisted of macrophages [23]. The DC fraction showed a purity of >85% as judged by flow cytometry using anti-rat CD3, CD11c, CD45RA, CD80, CD86, CD161, OX-42, OX-62, Mac-1 and MHC class II antibodies. In the DC-enriched fraction, contamination of T cells is about 2·3% (±s.d. 0·5), of B cells, 1·9% (±s.d. 0·6) and of NK cells, ≤1%.
DC were exposed to rh TGF-β1 (20 ng/ml, based on preliminary experiments). After 48 h, DC were harvested and washed with serum-free medium. In preliminary experiments, we tested different numbers of DC, ranging from 1 to 4 × 106/rat, but did not observe significant differences. In addition, we attempted to observe whether TGF-β1-exposed DC influence the first phase of EAMG from day 7 to 11 p.i. Thus, 2 × 106 DC/rat were s.c. injected into the backs of Lewis rats that had been immunized 5 days earlier with AChR + Freund’s complete adjuvant (FCA). Control EAMG rats were injected in parallel with untreated DC (naive DC) or PBS.
Preparation of lymph node and spleen mononuclear cells
Popliteal and inguinal lymph nodes and spleen were removed under aseptic conditions. Mononuclear cell (MNC) suspensions were obtained by grinding the organs through a stainless steel wire mesh in medium. The erythrocytes in spleen were lysed osmotically. Cells were then washed three times and re-suspended in medium at 2 × 106/ml. Because rat spleen MNC produce high levels of NO that inhibits T- and B-cell functions [24], lymph node MNC were used to investigate proliferation, cytokine- and anti-AChR IgG-secreting cells. To measure NO production, we used spleen MNC, since production of NO by lymph node MNC is very low [24].
Proliferation assay
MNC (2 × 106/ml) were cultured in the absence and presence of AChR (10 μg/ml). After 60 h of incubation at 37°C, the cells were labelled for an additional 12 h with 1μCi of 3H] methylthymidine (Amersham, Little Chalfont, UK). Cells were harvested and thymidine incorporation was measured. Cultures were run in triplicate and the results expressed as cpm.
Cytokine assay by ELISA
MNC (2 × 106/ml) were cultured in the absence and presence of AChR (10 μg/ml). After 48 h of incubation at 37°C, the supernatant fluids were collected and measured for IFN-γ and IL-10 by a sandwich ELISA kit (PharMingen, San Diego CA, USA) following the manufacturer’s instructions. Cytokine levels were quantified by reference to standard curves produced with recombinant rat IFN-γ and recombinant rat IL-10. Determinations were performed in duplicate and results expressed as pg/ml.
Nitrite assay
NO was assayed by measuring the end product nitrite, which was determined by a colorimeter assay based on the Griess reaction. MNC (2 × 106/ml) were cultured in the absence and presence of AChR (10 μg/ml). After 2 days of incubation at 37°C, supernatant fluids (100 μl) were mixed with 100 μl Griess reagent at Room Temperature for 10 min. Absorbance was measured at 540 nm in an automated plate reader. Concentration of nitrite was determined by reference to a standard curve of sodium nitrite (Sigma). Determinations were performed in triplicate and the results expressed as μM NO2−/ml.
Determination of anti-AChR antibody-producing cells by ELISPOT assay
Microtitre plates with nitrocellulose bottoms (Multiscreen-HA plates; Millipore, Mulsheim, France) were coated with AChR (10 μg/ml in PBS) overnight at 4°C. After washing with PBS twice, aliquots of 100 μl containing 2 × 105 MNC were added to individual wells in triplicate. After incubation for 24 h, the wells were emptied, followed by the addition of biotinylated rabbit anti-rat IgG (Dakopatts, Copenhagen, Denmark) and ABC (Vector, Burlingame, CA, USA). After peroxidase staining, the red-brown immunospots, which correspond to the cells that had secreted anti-AChR IgG antibodies, were counted in a blinded fashion by using a dissection microscope and standardized to number of spots per 105 MNC [25].
Statistics
Statistical analysis was performed using one-way ANOVA and Tukey-Kramer multiple comparisons test when ANOVA showed significant differences (P < 0·05).
RESULTS
TGF-β1-DC induce immune protection from EAMG
We evaluated the therapeutic potential of TGF-β1-DC in the Lewis rat EAMG model. Injection of TGF-β1-DC on day 5 post immunization (p.i.) reduced the severity of clinical symptoms demonstrable on day 39 p.i. (mean clinical score = 0·32) compared with rats receiving PBS (mean clinical score = 2·20, P ≤ 0·05) and naive DC (mean clinical score = 1·27) (Fig. 1a).
Fig. 1.


Effects of TGF-β1-DC on EAMG. (a) Clinical score; (b) body weight. Four female Lewis rats per group were first immunized with AChR and FCA. On day 5 p.i., rats were s.c. injected with either TGF-β1-DC, naïve DC (2 × 106DC/rat) or PBS (control EAMG). Arrows indicate day 5 p.i. when the injections were given s.c. All observations were performed in blinded fashion. Results are expressed as mean ± s.d. from four rats. Control EAMG; (▴) naive DC; (▪) TGF-β1-DC (✦). Data are representative of two independent experiments with similar results. *P < 0·05.
A marked and progressive reduction in body weight was observed in rats injected with PBS or naïve DC from day 26 to 39 p.i. (Fig. 1b) as the clinical muscle weakness became more severe (Fig. 1a). By contrast, there was no parallel loss of body weight in the rats injected with TGF-β1-DC (Fig. 1b).
Immune regulation induced by TGF-β1-DC in EAMG
We evaluated the ability of TGF-β1-DC to influence T- and B-cell responses in EAMG as reflected by AChR-induced T-cell proliferation, Th1/Th2 cytokines and levels of anti-AChR IgG antibody-secreting cells. Injection of TGF-β1-DC and naïve DC enhanced proliferation (P < 0·001 and 0·01, respectively) compared with DC from PBS-injected rats (Fig. 2). Proliferation was slightly lower in rats that had received naïve DC compared with rats injected with TGF-β1-DC. The augmented proliferation induced by DC injection seems to be antigen-independent (Fig. 2).
Fig. 2.

Spontaneous and AChR-induced proliferation (a) and anti-AChR-secreting cells (b). Lymph node MNC were obtained from rats receiving TGF-β1-DC, naive DC and PBS on day 39 p.i. Proliferation was measured by thymidine incorporation. Numbers of anti-AChR IgG antibody-secreting cells were detected by ELISPOT assays. Results are expressed as mean ± s.d. from four rats. (□) Control EAMG; ( ) naive DC; (▪) TGF-β1-DC. Data are representative of two independent experiments with similar results. **P < 0·01, ***P < 0·001.
) naive DC; (▪) TGF-β1-DC. Data are representative of two independent experiments with similar results. **P < 0·01, ***P < 0·001.
Both TGF-β1-DCs and naïve DC promoted increased spontaneous (P < 0·01) and AChR-induced IFN-γ levels (P < 0·001), detectable in supernatant fluids of lymph node MNC obtained day 39 p.i., compared with PBS-injected rats (Fig. 3a). For IL-10, no difference was observed between the three groups of rats (Fig. 3b).
Fig. 3.
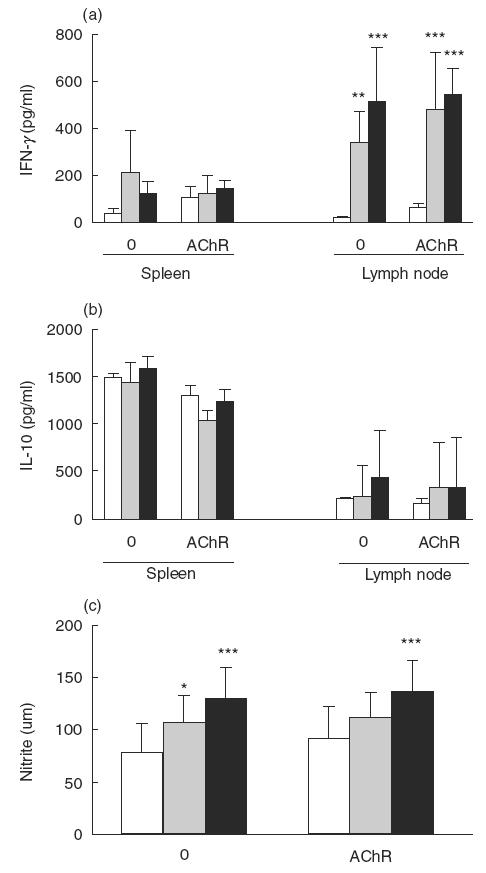
Spontaneous and AChR-driven IFN-γ (a), IL-10 (b) and nitrite (c). Spleen and lymph node MNC were obtained from rats receiving TGF-β1-DC, naïve DC or PBS on day 39 p.i. IFN-γ and IL-10 were detected by ELISA. Nitrite concentration was measured by Griess reaction. (□) Control EAMG; ( ) naïve DC; (▪) TGF-β1-DC. Results are expressed as mean ± s.d. from four rats. Data are representative of two independent experiments with similar results. *P < 0·05, **P < 0·01, ***P < 0·001.
) naïve DC; (▪) TGF-β1-DC. Results are expressed as mean ± s.d. from four rats. Data are representative of two independent experiments with similar results. *P < 0·05, **P < 0·01, ***P < 0·001.
Since IFN-γ promotes NOS up-regulation and NO production, we examined NO production by measuring nitrite concentration. Spontaneous and AChR-induced NO production by spleen MNC were significantly increased (P < 0·001 for both) in rats injected with TGF-β1-DC compared with PBS-injected animals (Fig. 3c). Levels of NO production by lymph node MNC were very low in the three groups of rats, with no detectable difference between the groups (data not shown).
As shown in Fig. 2b, TGF-β1-DC-treated rats had lower levels of anti-AChR IgG antibody-secreting cells among lymph node MNC compared with PBS- or naive DC-injected EAMG rats (P < 0·01, for both comparisons).
DISCUSSION
TGF-β is critical for differentiation and maturation of DC. Increasing evidence demonstrates that TGF-β can promote differentiation of DC and prevent final maturation [26,27]. Strobl and Knapp [28] reported that DC generated in cultures of CD34+ human haemopoietic progenitor cells stimulated with TGF-β1 are arrested in their maturation at an immature differentiation stage and lack specific phenotypic features of mature DC (CD83–). Yamaguchi et al. [16] observed that TGF-β1 addition to GM-CSF-supplemented cultures of murine bone marrow cells increases DC yields and suppresses DC maturation. These effects of TGF-β1 are reversed by addition of anti-TGF-β1 to the cultures. TGF-β also prevents final Langerhans cell maturation in response to TNF-α [26] and up-regulates expression of E-cadherin, which contributes to large homotypic cell clusters and maintenance of the immature state [29]. Immature DC can lead to peripheral tolerance by differentiation of regulatory T cells [9,10]. In the present study, we demonstrate that TGF-β1-DC, if administered s.c. on day 5 p.i. with AChR + FCA, protects Lewis rats from EAMG, accompanied by reduced levels of anti-AChR-secreting cells. The exact mechanism behind TGF-β1-DC-mediated immune protection from EAMG remains to be defined.
Induction of IFN-γ after therapeutic injection of TGF-β-DC is unexpected. However, IFN-γ is still a controversial factor in the pathogenesis of EAMG. B6 Mice with IFN-γ or IFN-γR deficiency are less susceptible to EAMG [25,30]. On the contrary, IFN-γ-deficient B6 mice developed EAMG with a frequency similar to wild-type mice [31]. IFN-γ neutralization strongly influenced the Th1/Th2 balance but did not affect the disease outcome, indicating that a Th1-dominated immune response is not necessarily associated with disease severity in EAMG [32]. Unexpected disease-ameliorating effects by IFN-γ have also been observed in EAE [33–36], lethal autoimmune myocarditis [37] and collagen-induced arthritis [38].
How does IFN-γ protect from EAMG? NO is considered to impair B-cell functions [39,40]. B-cell viability and antibody production were markedly reduced by NO, and were completely restored by the addition of NO inhibitor, suggesting that NO act as regulatory molecules in B-cell immune responses in three ways: cytostasis, reduction of cell growth and suppression of antibody synthesis [39]. In graft-versus-host disease, induction of the NO pathway is an important mechanism that mediates B-cell hyporesponsiveness to LPS [40]. Our results demonstrate that TGF-β1-DC treatment of EAMG results in lower levels of anti-AChR IgG antibody-secreting cells on day 39 p.i., accompanied by augmented NO production. We propose that DC-driven NO may induce apoptosis of activated T and/or B cells, resulting in low levels of anti-AChR antibodies, possibly through B-cell anergy and/or lack of T-cell help.
In conclusion, the present study shows that DC, upon exposure to TGF-β1 in vitro, when injected via the s.c. route to Lewis rats with incipient EAMG, inhibit the development of EAMG and the production of anti-AChR IgG antibodies. The exact mechanism(s) behind these phenomena remain to be investigated. The present data provide a new opportunity for DC-based immunotherapy of antibody-mediated autoimmune diseases.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council and Karolinska Institute Research Funds.
REFERENCES
- 1.Drachman DB, McIntosh KR, Yang B. Factors that determine the severity of experimental myasthenia gravis. Ann N Y Acad Sci. 1998;841:262–82. doi: 10.1111/j.1749-6632.1998.tb10935.x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinmann RM. Dendritic cells and control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Viney JL, Mowat AM, O’Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–25. [PubMed] [Google Scholar]
- 4.Clare-Salzler MJ, Brooks J, Chai A, van Herle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741–8. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaccio G, Nicoletti F, Pisanti FA, Bendtzen K, Galdieri M. Prevention of spontaneous autoimmune diabetes in NOD mice by transferring in vitro antigen-pulsed syngeneic dendritic cells. Endocrinology. 2000;141:1500–5. doi: 10.1210/endo.141.4.7437. [DOI] [PubMed] [Google Scholar]
- 6.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 7.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8 (+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 8.Huang YM, Yang JS, Xu LY, Link H, Xiao BG. Autoantigen-pulsed dendritic cells induce tolerance to experimental allergic encephalomyelitis in Lewis rats. Clin Exp Immunol. 2000;122:437–44. doi: 10.1046/j.1365-2249.2000.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link H, Huang YM, Xiao BG. Dendritic cells in experimental allergic encephalomyelitis and multiple sclerosis. J Neuroimmunol. 1999;100:102–10. doi: 10.1016/s0165-5728(99)00197-6. [DOI] [PubMed] [Google Scholar]
- 12.Link H, Huang YM, Thomas M, Xiao BG. Vaccination with autologous dendritic cells: from experimental autoimmune encephalomyelitis to multiple sclerosis. J Neuroimmunol. 2001;114:1–7. doi: 10.1016/s0165-5728(01)00247-8. [DOI] [PubMed] [Google Scholar]
- 13.Letterio JJ, Roberts AB. Regulation of immune response by TGF-β. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Jin YX, Xu LY, Guo H, Ishikawa M, Link H, Xiao BG. TGF-beta1 inhibits protracted-relapsing experimental autoimmune encephalomyelitis by activating dendritic cells. J Autoimmunity. 2000;14:213–20. doi: 10.1006/jaut.2000.0364. [DOI] [PubMed] [Google Scholar]
- 15.Riedl E, Strobl H, Majdic O, Knapp W. TGF-β1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol. 1997;158:1591–7. [PubMed] [Google Scholar]
- 16.Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–53. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Li W, Fu F, Chambers FG, Qian S, Fung JJ, Thomson AW. Blockade of the CD40–CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. 1997;64:1808–15. doi: 10.1097/00007890-199712270-00031. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 19.Dufter C, Watzlik A, Christ C, et al. Suppression of T-cell alloreactivity by gene-therapeutic modulation of human dendritic stimulator cells with TGF-beta adenoviral vectors. Transplant Proc. 2001;33:190–1. doi: 10.1016/s0041-1345(00)01971-0. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Lee WC, Takayama T, et al. Genetic engineering of dendritic cells to express immunosuppressive molecules (viral IL-10, TGF-beta, and CTLA4Ig) J Leukoc Biol. 1999;66:293–6. doi: 10.1002/jlb.66.2.293. [DOI] [PubMed] [Google Scholar]
- 21.Lindstrom J, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis: prevalence, clinical correlates and diagnostic value. Neurology. 1976;26:1054–9. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- 22.Lennon VA, Lambert EH, Leiby KR, Okarma TB, Talib S. Recombinant human acetylcholine receptor α-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol. 1991;146:2245–8. [PubMed] [Google Scholar]
- 23.Crowley M, Inaba K, Witmer-Pack M, Steinmann RM. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989;118:108–25. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- 24.Xu LY, Yang JS, Huang YM, et al. Limitation of nitric oxide production: cells from lymph node and spleen exhibit difference in nitric oxide production. Immunol Lett. 2000;71:177–84. doi: 10.1016/s0165-2478(00)00154-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang GX, Xiao BG, Bai XF, van der Meide PH, Orn A, link H. Mice with IFN-γ receptor deficiency are less susceptible to experimental autoimmune myasthenia gravis. J Immunol. 1999;162:3775–81. [PubMed] [Google Scholar]
- 26.Geissmann F, Revy P, Regnault A, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 27.Lee WC, Qiani S, Wan Y, et al. Contrasting effects of myeloid dendritic cells transduced with an adenoviral vector encoding interleukin-10 on organ allograft and tumour rejection. Immunology. 2000;101:233–41. doi: 10.1046/j.1365-2567.2000.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strobl H, Knapp W. TGF-β1 regulation of dendritic cells. Microb Infect. 1999;1:1283–90. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 29.Riedl E, Stöckl J, Majdic O, et al. Functional involvement of EC-adherin in TGF-β1-induced cell cluster formation of in vitro developing human Langerhans-type dendritic cells. J Immunol. 2000;165:1381–6. doi: 10.4049/jimmunol.165.3.1381. [DOI] [PubMed] [Google Scholar]
- 30.Balasa B, Deng C, Lee J, Bradleyu LM, Dalton DK, Christadoss P. Interferon gamma in necessary for genesis of actylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med. 1997;186:385–91. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karachunski PI, Ostlie NS, Monfardini C, Conti-Fine BM. Absence of IFN-γ or IL-12 has different effects on experimental myasthenia gravis in C57BL/6 mice. J Immunol. 2000;164:5236–44. doi: 10.4049/jimmunol.164.10.5236. [DOI] [PubMed] [Google Scholar]
- 32.Saoudi A, Bernard I, Hoedemaekers A, et al. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1- or Th2-type immune response in rats. J Immunol. 1999;162:7189–97. [PubMed] [Google Scholar]
- 33.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–86. [PubMed] [Google Scholar]
- 34.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–8. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weishaupt A, Jander S, Bruck W, et al. Molecular mechanisms of high-dose antigen therapy in experimental autoimmune encephalomyelitis: rapid induction of Th1-type cytokines and inducible nitric oxide synthase. J Immunol. 2000;165:7157–63. doi: 10.4049/jimmunol.165.12.7157. [DOI] [PubMed] [Google Scholar]
- 36.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–68. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 37.Eriksson U, Kurrer MO, Bingisser R, et al. Lethal autoimmune myocarditis in interferon-gamma receptor-deficient mice: enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation. 2001;103:18–21. doi: 10.1161/01.cir.103.1.18. [DOI] [PubMed] [Google Scholar]
- 38.Vermeire K, Thielemans L, Matthys P, Billiau A. The effects of NO synthase inhibitors on murine collagen-induced arthritis do not support a role of NO in the protective effect of IFN-gamma. J Leukoc Biol. 2000;68:119–24. [PubMed] [Google Scholar]
- 39.Takagi K, Nukaya I, Yasukawa K, Suteta Y. Inhibitory mechanisms of antibody production by nitrogen oxide released from activated macrophages during the immune response: relationship to energy consumption. Immunol Cell Biol. 1994;72:241–8. doi: 10.1038/icb.1994.36. [DOI] [PubMed] [Google Scholar]
- 40.Falzarano G, Krenger W, Snyder KM, Delmonte J, Jr, Karandikar M, Ferrara JL. Suppression of B-cell proliferation to lipopolysaccharide is mediated through induction of the nitric oxide pathway by tumor necrosis factor-alpha in mice with acute graft-versus-host disease. Blood. 1996;87:2853–60. [PubMed] [Google Scholar]


