Abstract
The prevalence of atopic diseases in children has increased during the last decades. Atopic symptoms usually appear early in life. This implies an early priming for atopic disease, possibly even at the fetal level. We therefore compared the presence and production of IgE in the local in utero environment during pregnancy in atopic and non-atopic women. Eighty-six women were included in the study. Fifty women were demonstrated to be atopics, based on clinical symptoms of atopic disease together with a positive Phadiatop and/or skin prick test. Placentas from these term pregnancies were obtained. Slices covering the full thickness of the placenta were cut clockwise around the umbilical cord and were analysed with immunohistochemistry. Surprisingly, numerous IgE+ cells, located primarily in the fetal villous stroma, were detected in a majority of the investigated placentas irrespective of the atopy of the mother or maternal or fetal total serum IgE levels. The placental IgE could not be demonstrated to be bound to IgE receptors, but was shown to be bound to fetal macrophages, possibly via FcγRI. No evidence was found for local fetal IgE production, although cells producing epsilon transcripts were occasionally detected in the decidua. We describe here the novel finding of numerous IgE+ cells in the human placenta, suggesting an hitherto unknown role for IgE in a successful pregnancy outcome, irrespective of whether or not the mother is atopic.
Keywords: allergy, Fc receptors, IgE, human placenta
INTRODUCTION
There has been a dramatic increase in the prevalence of allergic diseases during the last decades. Genetic influence is of importance in atopic diseases reviewed in 1], but the enhanced allergy prevalence has occurred over a very short period of time, which is indicative of a crucial role for environmental factors [2–4]. There is most probably an intricate interplay between genetic and environmental factors, which together play a role for atopy development and disease progression. Atopic symptoms usually appear very early in life. This implies that the neonate, or even the fetus, is subjected to maternal influence which could promote atopic disease. The impact of maternal influence on the fetus in utero was described recently in a mouse model [5], and several reports from human studies also support this. First, it has been reported that there is an increased risk of atopy development in children with atopic mothers compared to those of atopic fathers [6,7], a finding which could be explained by both genetic and environmental factors. Further, the detection of elevated IgE levels in cord blood could indicate in utero sensitization [8,9]. Finally, allergen-specific reactivity at birth to a range of common allergens, both dietary and inhalant allergens, has been observed [10–12].
The placenta acts as a continuous barrier between the mother and the fetus and is capable of inhibiting maternal immune responses against the fetus. The fetal part of the placenta consists principally of the two membranes, amnion and chorion, the umbilical cord and the chorionic villi with the covering trophoblast. The maternal part consists of the decidua and the intervillous space. The immune system is altered during pregnancy and a Th2 environment in utero has been suggested to be important for a successful pregnancy [13,14], although this is controversial [15,16]. In the placenta there is a local production of IL-4 and IL-10 [17,18]. Additionally, progesterone and prostaglandins, which are both detected at the placental level, contribute to the Th2 dominance [19,20]. Prostaglandin E2 has also been shown to synergize with IL-4 to induce IgE class switching [21,22]. IL-4 is known to induce germ-line (GL) IgE heavy chain transcription in both adult [23] and fetal [24] human B cells. The exact role of GL transcripts in the regulation of Ig class switching has not been elucidated but several findings suggest that GL mRNA is necessary for subsequent Ig class switch reviewed in 25].
As a Th2 dominance in utero also would provide a milieu that promotes atopic allergy [12], we were interested in investigating whether or not the local environment in the placenta differs between atopic and non-atopic mothers with respect to IgE expression. Here, we investigated the presence of IgE+ and IgE-receptor+ cells in the placenta as well as local IgE production.
MATERIALS AND METHODS
Subjects
Eighty-six pregnant women (median age 32; range 22–44 years) were recruited from the maternal ward or directly from the delivery unit in the Stockholm area. Maternal venous blood was obtained when mothers arrived at the delivery unit. Cord blood was collected immediately after birth. Sera were obtained by centrifugation and stored at –20°C until further analysis. The study was approved by the Ethics Committees of the Karolinska Hospital and Söder’s Hospital, Stockholm, Sweden. All mothers gave their informed consent to the study.
Analysis of IgE in serum
Maternal blood samples were analysed for total serum IgE levels (Pharmacia CAP System IgE FEIA; Pharmacia Diagnostics AB, Uppsala, Sweden). The detection limit was >2 kU/l, and values below this level were set to 2 kU/l for the evaluations and statistical analysis. Values above 122 kU/l are considered as elevated. Total IgE levels in cord blood serum were measured by an ultra-sensitive CAP-FEIA3 (detection limit 0·1 kU/l), modified by an extended standard curve but otherwise in accordance with the recommendations of the manufacturer (Pharmacia Diagnostics AB). Allergen-specific IgE against 11 common inhalant allergens was analysed in maternal and cord blood samples with Phadiatop (Pharmacia CAP System RAST FEIA; Pharmacia Diagnostics AB).
Skin prick test
Skin prick test (SPT) was performed on the mothers according to the manufacturer’s instructions using a panel of represen- tative inhalation allergens (cat, dog, horse, rabbit, birch, timothy grass, mugwort, Dermatophagoides pteronyssinus, D. farinae, Cladosporium herbarum and Alternaria). Histamine (10 mg/ml) served as a positive reference and the allergen diluent was used as the negative control. The allergen (Soluprick, 10 HEP, ALK, Copenhagen, Denmark) SPT was regarded as positive if the weal reaction was greater than or equal to 3 mm in diameter after 15 min.
Processing and immunohistochemical staining of placental tissue
Placentas from term pregnancies (18 elective caesarian sections and 68 normal vaginal deliveries) were collected. Placental tissue was taken immediately after delivery or kept refrigerated (at 4°C) until use (up to 48 h). The longer storage time did not influence placental histology or morphology (data not shown). Three to four placental slices were cut clockwise around and close to the umbilical cord in each placenta. Each one included the whole placental tissue, from the fetal membranes to the decidua. A segment of the umbilical cord was cut 15 cm from each placenta. Slices were fixed in formalin and paraffin embedded or frozen on dry ice and stored at –70°C until further analysis. Of the frozen material, acetone-fixed, 7 μm thick, cryostat sections were used for routine haematoxylin and eosin staining and for ABC-ELITE (Vector Laboratories Inc. Burlingame, CA, USA) immunohistochemical staining. To block endogenous peroxidase activity, sections were treated with 0·3% H2O2. Sections were then incubated with normal rabbit serum (dilution 1/10) followed by an avidin–biotin blocking step (Vector Laboratories Inc.). Thereafter the sections were incubated for 30 min with the following primary mouse IgG1 MoAbs diluted in 4% bovine serum albumin in PBS: 5 different anti-IgE MoAbs (clone B3102E8, Southern Biotechnology Associates Inc. Burlingame, CA, USA, dilution 1/50; clone CIA-E-7·12, Dako, Glostrup, Denmark, dilution 1/10; clone CGM9 (26), a kind gift from Dr CGM Magnusson, Unit of Clinical Immunology and Allergy, Department of Medicine, Karolinska Hospital, dilution 1/20; clones 16 and 35, kindly provided by Pharmacia Diagnostics AB, dilutions 1/16 and 1/13, respectively), anti-FcɛRIα (clone 3G6, Upstate Biotechnology, Lake Placid, NY, USA, dilution 1/1000), anti-CD23 (FcɛRII) (clone MHM6, Dako, dilution 1/5), anti-CD38 (clone AT13/5, Dako, dilution 1/40), anti-CD64 (FcγRI) (clone 10·1, Dako, dilution 1/80) followed by incubation with a biotin-labelled horse-antimouse secondary antibody (Vector Laboratories Inc.). The sections were then allowed to react with a preformed avidin–biotin–enzyme complex (ABC-ELITE reagent, Vector Laboratories Inc.) for an additional 30 min. The staining was developed by incubating the sections with three-amino-9-ethylcarbazole (AEC) substrate for 15 min. Sections were counterstained with Mayer’s haematoxylin. Staining was not observed when an irrelevant isotype-matched mouse IgG1 negative control (Dako) was used, nor when the primary antibody was omitted. As positive controls for the stainings, IgE-producing SKO cells (27) and biopsy specimens from lesional skin of atopic dermatitis patients were used.
Mast cells in the placenta were visualized on sections from formalin-fixed material with toluidine blue, which stains the granules of the mast cells red/violet-metachromatic.
Elution of tissue-bound IgE
To elute tissue-bound IgE, cryostat sections were treated with glycine hydrochloride buffer (0·05m glycine, 0·085M NaCl, 0·005M KCl, 0·01M ethylenediamine tetra-acetic acid, pH 2·5 [28]); or PBS for 30 min on ice and then washed twice. Sections were thereafter stained for IgE and FcɛRI as described above.
Evaluation of the immunohistochemical stainings
One section from two different areas of each placenta was stained for each antibody and examined under coded conditions. The evaluated sections spanned the whole placental tissue, from the fetal membranes to the decidual plate. A semiquantitative scale (+ = few positive cells, + + = moderate, and + + + = many positive cells) was used for the evaluation of the IgE staining.
Double immunofluorescence staining
Double immunofluorescence staining was performed on placental tissue from four women, two atopics and two non-atopics, selected on the basis of having extensive expression of IgE in the placenta. Frozen, acetone-fixed 7 μm-thick sections were blocked with normal goat serum (dilution 1/10) and incubated with mouse MoAbs anti-IgE (clone B3102E8, Southern Biotechnology Assoc., dilution 1/50) or anti-CD38 (clone AT13/5, Dako, dilution 1/40) for 30 min, followed by incubation with a rhodamine red-X conjugated donkey-antimouse secondary antibody (dilution 1/150, Jackson Immunoresearch Laboratories Inc., West Grove, PA, USA) for a further 30 min. The sections were then subjected to a secondary blocking step with normal mouse serum (dilution 1/100) followed by incubation with either FITC-conjugated mouse MoAb anti-HLA-DR (clone L243, Becton Dickinson, dilution 1/50) to visualize IgE+HLA-DR+ cells, or biotin-labelled mouse MoAb anti-IgE MoAb (clone B3102E8, Southern Biotechnology Assoc., dilution 1/100) followed by incubation with FITC-conjugated streptavidin (Dako, dilution 1/50) to detect CD38+IgE+ cells. The sections were mounted with glycerol in PBS containing p-phenylenediamine to reduce the fading of staining during microscopy. This double-staining technique enabled the identification of IgE+HLA-DR+ or CD38+IgE+ cells in the same section when the slides were analysed in a confocal laser scanning microscope (CLSM; Leica Instruments, Heidelberg, Germany) with separate settings for excitation and detection of the two fluorochromes. Staining was not observed in controls without the primary antibodies. The numbers of double positive cells were evaluated and compared with those observed in sections stained only for IgE, CD38 or HLA-DR. As a positive control for CD38+ IgE+ cells, the IgE producing SKO cell line was used.
In situ hybridization
Two different oligonucleotide probes were used as antisense probes. The probe detecting GL sterile epsilon transcripts was complementary to bases 650–663 (GenBank Accession no. X56797) of the IgE switch region and to bases 98–122 (GenBank Accession no. L00022 J00227 V00555) of the epsilon C-region. The probe detecting mature epsilon transcripts was complementary to bases 291–309 (GenBank Accession no. M99372) of the J segment and to bases 98–122 (GenBank Accession no. L00022 J00227 V00555) of the epsilon C-region. The sense probe (A3 adenosine receptor, GenBank Accession no. S65334) has been described elsewhere [29]. The probes were synthesized and desalted by Life Technologies (Sweden). They were labelled subsequently with 35S]-dATP (Amersham Pharmacia Biotech Uppsala, Sweden) at the 3′ end, using terminal deoxynucleotidyl transferase (Amersham Pharmacia Biotech) and purified on a NICK column (Amersham Pharmacia Biotech). Duplicates of five placental tissue specimens (three from atopic and two from non-atopic mothers) selected on the basis of having many IgE+ cells in the chorionic villi region were processed for in situ hybridization. Frozen tissue specimens were cut to 14 μm sections in a cryostat and stored at –20°C. As a positive control for sterile and mature epsilon transcript signals, IgE-producing SKO cells were used [25]. In situ hybridization was performed as described [30]. Dipping in Kodak NTB2 autoradiographic emulsion (Eastman Kodak Co., Rochester, NY, USA) was followed by a 5–6-week exposure (2-week exposure for SKO cells). The developed sections and cells were mounted with PBS/glycerine and evaluated with both light and dark field microscopy (Leica Instruments). The presence of an underlying cell was confirmed by a counterstain with haematoxylin.
Statistical analysis
The Mann–Whitney U-test was used to compare differences between the atopic and non-atopic groups. The difference was considered significant when P < 0·05. To evaluate the relationship between observed parameters, correlations were computed using the Spearman rank-order correlation coefficient (rs).
RESULTS
Analysis of total IgE levels in adult and cord blood serum
Fifty of the 86 women were diagnosed as atopics based on clinical symptoms of atopy together with positive Phadiatop (n = 40) and/or positive SPT (n = 45) (Table 1). There were no differences between the two groups regarding maternal age at delivery, mode of delivery, placental weight or birth weight of the baby. Children of non-atopic mothers had more siblings than children of atopic mothers (P < 0·0001) and the atopic mothers gave birth to more boys (56%) than non-atopic mothers (42%), as has been described previously [31] (Table 1). There was a statistically significant difference in total serum IgE levels between atopic and non-atopic mothers, P < 0·05 (Table 2). The children with atopic mothers did not show elevated cord blood IgE levels compared to the children with non-atopic mothers (Table 2), although maternal and cord blood IgE levels correlated, rs = 0·4, P < 0·001.
Table 1.
Demographic data of atopic and non-atopic mothers
| Group * | Cases (n) | Maternal age at delivery (years) | Mode of delivery (vaginal/caesarian) | Placental weight (g) | Birth weight (g) | Boy/girl (n) | Siblings (n) |
|---|---|---|---|---|---|---|---|
| Atopic | 50 | 31 (22–44)† | 42/8 | 597 (300–941)† | 3590 (2495–4800)2 | 28/22 | 1 (0–3) |
| Non-atopic | 36 | 33 (23–44) | 26/10 | 620 (468–1030) | 3587 (2750–4630) | 15/21 | 2 (1–4) |
The diagnosis of atopy was based on positive Phadiatop (Pharmacia CAP System RAST FEIA; Pharmacia Diagnostics AB) and/or positive skin prick test. All women in the atopic group had clinical manifestations of atopic disease (rhinoconjunctivitis, asthma or atopic eczema). None of the 37 women in the non-atopic group had symptoms of atopic disease.
Median (range).
Table 2.
IgE levels
| Group | |||
|---|---|---|---|
| Sample | Atopic | Non-atopic | P-value |
| Serum IgE * | 18 (2–530)† | 13 (2–94)† | |
| Mother | (n = 50) | (n = 36) | < 0·05 |
| Serum IgE * | 0·2 (0·1–1·8)† | 0·1 (0·1–1·8)† | |
| Cord blood | (n = 50) | (n = 36) | n.s.‡ |
Sera from 50 atopic and 36 non-atopic women and their neonates were analysed for total IgE as described in Materials and methods. Values are expressed as kU/l.
Median (range).
Non-significant.
IgE+ cells are present in the placenta and umbilical cord irrespective of maternal atopy
Cryostat sections from 86 placentas (50 from atopic and 36 from non-atopic mothers) and from 17 umbilical cords (randomly chosen, eight from the atopic and nine from the non-atopic group) were subjected to immunohistochemistry. The haematoxylin and eosin stained slides showed that the morphology was well preserved. The reproducibility between different slices of the same placenta was good.
Frequent IgE+ macrophage-like cells were seen all over the mesenchymal part of the chorionic villi, indicating a fetal origin of the IgE+ cells (Fig. 1a,b). Occasional IgE+ cells were also found in the decidua as well as in the intervillous space (not shown). Sparse, scattered IgE+ cells (Fig. 1c) were also observed in the vessel walls as well as in the mesenchyme of the cord. IgE+ cells were detected in all 86 placentas but differed in amount. Interestingly, no statistically significant difference (P = 0·7) between the atopic and non-atopic groups (Table 3) was found. There was no correlation between the amount of IgE+ cells in the placenta and any of the studied parameters in Table 1 or total serum IgE levels. The presence of IgE+ cells in the placenta was verified using five different MoAbs against IgE, all giving similar staining patterns, while no staining was observed with an isotype matched negative control (Fig. 1d).
Fig. 1.
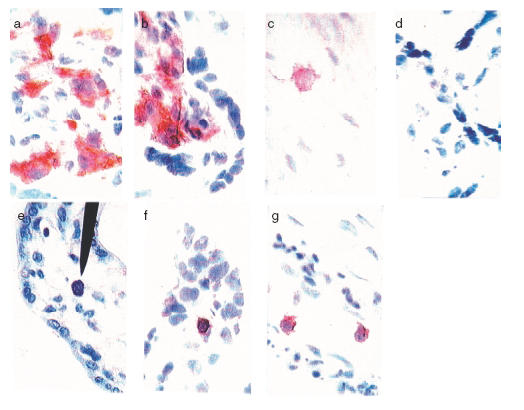
Detection of IgE+ cells, mast cells and CD38+ cells in the placenta. Acetone-fixed cryostat sections of frozen placental tissue (a–b, d, f–g) and umbilical cord (c) were stained with the ABC ELITE method, with anti-IgE MoAb (clone B3102E8, Southern Biotechnology Assoc.) (a–c) or anti-CD38 MoAb (clone AT13/5, Dako) (f-g) or an isotype matched control MoAb (Dako) (d). A counterstain was performed with Mayer’s haematoxylin. Positive cells are seen as brown/red. For the detection of mast cells (e), sections from formalin fixed biopsies were stained with toluidine blue. (a–b) IgE+ cells in the fetal part of the placenta in the chorionic villi mesenchyme. There was no difference in placental IgE expression between atopic (a) and non-atopic (b) mothers. (c) An IgE+ cell in a umbilical cord section from an atopic mother. (e) A single mast cell (indicated by arrow) present in the chorionic villi region. (f–g) CD38+ cells present in the villous part of the placenta, both as scattered cells (f) and present in fetal vessels (g). Magnification ×600.
Table 3.
IgE scores and number of mature ɛ transcript producing cells in placental biopsies
| IgE+ cells * | ||||
|---|---|---|---|---|
| Group | + | + + | + + + | ɛ transcript+ cells ‡ (n) |
| Atopic | 7† | 19† | 24† | 5§ |
| Non-atopic | 5† | 16† | 15† | 6§ |
A semiquantitative scale (+ = few positive cells, + + = moderate, and + + + = many positive cells) was used for the evaluation of the IgE staining with immunohistochemistry.
Number of placentae within each group.
Cells producing mature ɛ transcripts as analysed with in situ hybridization.
Values are expressed as a median number of positive cells detected in the maternal part of the placenta (duplicate sections from biopsies of 3 atopic and 2 non-atopic mothers, selected on the basis of having many IgE+ cells in the chorionic villi region) with the antisense probe. Positive cells detected with the sense probe are subtracted.
IgE is not expressed on mast cells but on placental macrophages
We then stained formalin-fixed placental tissue sections from 12 women (six atopic and six non-atopic) with toluidine blue, in order to see whether the IgE we detected in the placenta might be bound to mast cells. Scattered mast cells were identified in the villous stroma, identified by their red-to-violet granules (Fig. 1e). In contrast to the IgE+ cells, the mast cells were rather sparse. Accordingly, mast cell-bound IgE could not be responsible for a majority of the IgE detected in the placenta.
The morphology and location of the IgE+ cells present in the chorionic villi indicated that they were macrophages. To test this we performed double stainings for HLA-DR and IgE. The IgE+ cells detected were HLA-DR+ (Fig. 2a–c). In addition, the HLA-DR+ cells were also found to be CD14+ (data not shown), which further shows that the IgE+ cells were of macrophagic origin.
Fig. 2.
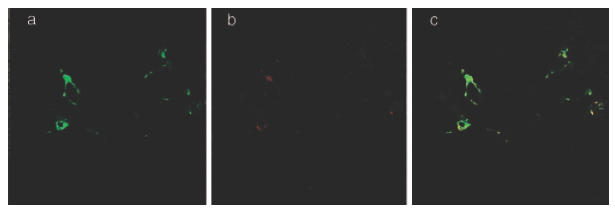
IgE+ cells in the placenta are HLA-DR+. Acetone-fixed cryostat sections of frozen placental biopsies were double-stained with a FITC conjugated anti-HLA-DR MoAb (clone L243, Becton Dickinson) and an unconjugated anti-IgE MoAb (clone B3102E8, Southern Biotechnology Assoc.) revealed by a rhodamine Red-X conjugated secondary antibody. The sections were mounted with glycerol in PBS containing p-phenylenediamine to reduce the fading of staining during microscopy and analysed with a CSLM (Leica Instruments). (a–c) The double-staining of a placental section from an atopic mother shows HLA-DR+ (a), IgE+ (b) and HLA-DR+ IgE+ (c) cells in the villous part of the placenta. Magnification ×500.
Role of IgE receptors in sequestering IgE in the placenta
To investigate whether the IgE+ cells in the placenta reflected IgE bound to IgE receptors on cells other than mast cells, we stained for the presence of the high and low affinity receptors, FcɛRIα and CD23 (FcɛRII). In order to detect the FcɛRI with our MoAb (clone 3G6), which recognizes only free receptors, we eluted tissue-bound IgE from the sections with glycine hydrochloride [28]. The sections were then subjected to immunohistochemistry as described in Materials and methods. The low pH treatment successfully eluted the IgE antibodies from the tissue sections (compare Fig. 3a,b). However, we could not detect any expression of FcɛRI+ cells, either before (Fig. 3c) or after (Fig. 3d) the treatment. To ascertain that the negative staining for FcɛRI in the placenta was not due to destruction of the receptor by the low pH treatment, we subjected skin biopsies from atopic dermatitis patients, positive for IgE and FcɛRI, to the same treatment (Fig. 3e–h). Here, strong staining with the FcɛRI antibody could be observed in the low pH treated sections (Fig. 3h), showing that this treatment did not make it impossible to stain for the receptor. This implies that the IgE detected in the placenta is not bound to FcɛRI.
Fig. 3.
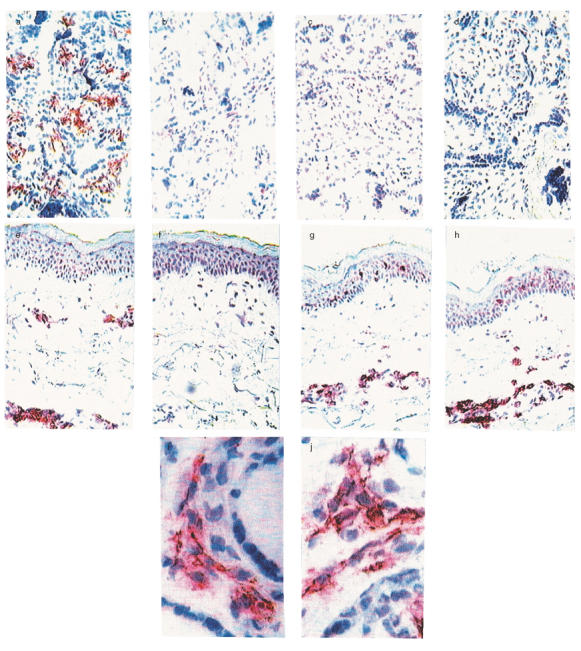
Expression of IgE and IgG receptors in the placenta. In order to detect FcɛRI in the placenta, tissue-bound IgE was eluted from the sections by low pH treatment. As control tissue, sections from a biopsy from lesional skin from an atopic dermatitis patient were used. Acetone-fixed cryostat sections were then stained for IgE (clone B3102E8, Southern Biotechnology Assoc.) and FcɛRI (clone 3G6, Upstate Biotechnology) before and after IgE elution, as described in Materials and methods. Staining of placental (a) and skin (e) sections for IgE+ cells on slides treated with PBS. The low pH treatment readily elutes IgE from both placenta (b) and skin (f). Staining for the FcɛRI before low pH treatment reveals staining of FcɛRI in skin (g) but not in placenta (c). Staining following IgE elution reveals no FcɛRI+ cells in placenta (d) but strong FcɛRI staining in skin (h), magnification ×150. To compare the expression of IgE and FcγRI, acetone-fixed cryostat sections of frozen placental tissue were stained either with anti-IgE (clone B3102E8, Southern Biotechnology Assoc.) or with anti-CD64 mAb (clone 10·1, Dako) with the ABC ELITE method as described in Materials and methods. (i–j) Consecutive sections of the same slice stained with anti-IgE (i) and anti-CD64 (j). Magnification ×600.
Scattered round cells positive for the low affinity IgE receptor, CD23, were detected in the maternal part of the placental sections (data not shown). However, the location and morphology of the CD23+ cells did not correlate with the IgE+ cells, indicating that this receptor does not bind IgE to a major extent in the placenta. In contrast, the distribution of FcγRI present on the placental macrophages correlated very clearly with that of the IgE+ cells (Fig. 3i–j).
Local IgE production in placenta
To trace the origin of the IgE detected in the placenta, sections from 12 women (six atopic and six non-atopic) were stained for plasma cells (CD38+). CD38+ cells were present in placenta, although at low frequency. The cells were scattered throughout the villous stroma and located in fetal vessels inside the villi (Fig. 1f,g) but also present in the maternal part of the placenta. This indicates that antibody production may occur locally in the placenta, making it possible that the IgE detected is produced by the fetus.
To determine whether the placental plasma cells were IgE+, double immunofluorescence stainings with anti-CD38 and anti-IgE antibodies were performed, and the results analysed with CLSM. No CD38 and IgE double-positive cells were detected (data not shown), indicating that the IgE detected in the placenta is produced elsewhere.
To further investigate the possibility of placental IgE production, we performed in situ hybridization in order to detect epsilon transcripts, as an indication of local IgE production. As shown in Table 3, placentas both from atopic and non-atopic women exhibited sparse scattered cells containing mature epsilon transcripts. However, these cells were located primarily in the decidua and in the intervillous space, outside the chorionic villi region, indicating a maternal origin of these cells. The same was true for GL epsilon transcripts (data not shown).
DISCUSSION
We present the novel finding of frequent IgE+ cells in the human placenta, irrespective of maternal atopy. IgE+ cells were distributed primarily in the villous stroma and in the umbilical cord mesenchyme. The presence of IgE+ cells was verified using five different MoAbs giving similar staining patterns. The placental IgE was expressed on macrophage-like, HLA-DR+ cells and had an identical staining pattern to FcγRI. In addition, the similar expression of CD14 and HLA-DR on these cells indicates further that the IgE+ cells are placental macrophages (our unpublished observations). The placenta-specific tissue macrophages express several known Fcγ-receptors, the neonatal Fc receptor (FcRn) and other proteins known to bind immunoglobulins reviewed in 32]. However, there is little information on their ability to bind IgE. FcɛRI or CD23 (FcɛRII) did not seem to play any major part in sequestering IgE in placenta. The observation that the staining pattern for FcγRI is the same as that for IgE indicates that IgE is bound to fetal cells indirectly via IgG antibodies. However, it has also been demonstrated that IgE can bind to FcγR II and III, although at low affinity [33].
An IgE binding capacity in the placenta has been reported previously [34], where mast cells were detected in part by anti-IgE antibodies. However, the numbers of mast cells in the placenta were not of predictive value for development of atopic disease in children [35]. Our study showed further that mast cell frequency and distribution does not correspond to that of the IgE+ cells. As infiltrating eosinophils are not present in placentas from normal pregnancies [36], IgE bound to eosinophils is not a probable explanation for the placental IgE.
The presence of IgE in the placenta raises the question as to whether it is of maternal or fetal origin. Several observations support a fetal origin of the placental IgE. The location of the IgE+ cells behind the trophoblast barrier is indicative of a fetal origin. It has also been reported that the human fetus has the potential to produce IgE as early as week 11 of pregnancy [37]. Moreover, it has been demonstrated recently that the human fetus contains B cells that are primed to undergo IgE class switching from the first trimester and onwards, and that these B cells can produce endogenous IgE by 20 weeks of gestation [38].
The placental barrier is widely believed to prevent all immunoglobulin isotypes except IgG from being transported from the mother to the fetus [39]. However, human IgE administered to pregnant animals has been reported to pass from mother to fetus at a fractional rate similar to that of albumin [40]. This indicates that IgE might possibly be transported across the placental barrier, although not at all with similar efficacy as IgG. Alternatively, IgE may be bound to placental cells via IgG–IgE immune complexes. In support of this theory, is the fact that we were able to detect the presence of IgE-IgG immune complexes in cord blood (unpublished observations). This could be a mechanism to protect the fetus from the potential hazards of allergen-specific IgE. Actually, high IgG anti-IgE serum levels at birth have been reported to be associated with a reduced allergy prevalence [41]. In addition, it has been shown that blocking IgG antibodies induced by specific allergy vaccination are able to prevent IgE-mediated allergen presentation, and thereby T cell proliferation and cytokine production at low allergen concentrations in birch-allergic patients [42]. A potential risk from postpartum leakage of IgE from mother to child is not considered as important, since we collected placental biopsies immediately following caesarian sections as well as up to 48 h after normal vaginal deliveries and could not correlate the amount of IgE+ cells in tissue biopsies with the time period between birth and freezing of the biopsies. However, it has been reported that the fetus is exposed to maternal IgE in the amniotic fluid [43]. The authors described a positive correlation between amniotic fluid IgE levels and maternal plasma concentrations. This could imply either that maternal IgE crosses the fetal membranes into the amniotic fluid during pregnancy, or that the IgE-stimulating factor(s) affecting maternal IgE levels also influence fetal IgE production. Fetal exposure to maternal IgE via the gastrointestinal tract, respiratory tract and the skin in the amniotic cavity could mediate allergen uptake via IgE receptors and prime the baby for atopy.
We found no evidence for IgE production in the fetal part of the placenta. Possible IgE-producing cells were, however, localized in the decidua and the intervillous space using in situ hybridization. Therefore, if the IgE is of fetal origin it is not produced locally in the placenta; instead, placental IgE probably originates from the fetal circulation. However, cord blood IgE levels were generally very low, but a discrepancy between IgE detected locally in tissue and serum has been described previously [44]. Cord blood IgE levels were not elevated in the group with atopic mothers compared to the group with non-atopic mothers, although we observed a correlation between maternal and cord blood IgE levels. It has been proposed that cord blood IgE levels are predictive of atopy development [8,9]. This is debatable, however, as other investigations contradict this predictive role for cord blood IgE [45,46]. We are following these children at present, to record their atopy development.
Several explanations for the presence of fetal IgE in the placenta can therefore be proposed. It could be merely a reflection of the Th2 dominance described for pregnancy and subsequently a marker of the baby’s response to Th2 type cytokine production [12]. It is, however, intriguing to speculate that the finding of such frequent IgE+ cells in the fetal part of the placenta in atopic as well as non-atopic women may indicate that the IgE is of some immunological importance to the fetus during its intrauterine life.
Acknowledgments
We are grateful to Ms A. Svensson and Ms M. Nordlund for excellent technical assistance with immunohistochemistry and ultra-sensitive CAP-FEIA of cord blood samples, Prof M. Troye Blomberg for providing antibodies for detection of immune complexes in ELISA, Dr C.G.M. Magnusson for providing the CGM9 anti-IgE antibody, Dr G. Jacobsson Ekman for assistance with confocal microsopy, Mr J. Kowalski, Statistical Consultant, Stockholm for help with statistical calculations and Drs K. Bremme and E. Östlund and the staff at the delivery units of Karolinska Hospital and Söder’s Hospital for help with collecting blood samples and placental material. This work was supported by grants from the Karolinska Institutet, the Swedish Asthma and Allergy Association, the Swedish Medical Research Council (grant no. 16X-7924), the Swedish Council for Work Life Research, the Swedish Foundation for Health Care Sciences and Allergy Research, the Hesselman’s Foundation, Magnus Bergwall’s Foundation, Konsul Th. C. Bergh’s Foundation and Tore Nilsson’s Foundation.
REFERENCES
- 1.Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999;402(Suppl. 6760):B5–11. doi: 10.1038/35037002. [DOI] [PubMed] [Google Scholar]
- 2.Asher MI, Weiland SK. The international study of asthma and allergies in childhood (ISAAC) ISAAC steering committee. Clin Exp Allergy. 1998;28(Suppl. 5):52–66. doi: 10.1046/j.1365-2222.1998.028s5052.x. [DOI] [PubMed] [Google Scholar]
- 3.Wold AE. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy. 1998;53:20–5. doi: 10.1111/j.1398-9995.1998.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 4.Alm JS, Swartz J, Lilja G, Scheynius A, Pershagen G. Atopy in children of families with an antroposophic lifestyle. Lancet. 1999;359:1485–8. doi: 10.1016/S0140-6736(98)09344-1. [DOI] [PubMed] [Google Scholar]
- 5.Herz U, Ahrens B, Schefold A, Joachim R, Radbruch A, Renz H. Impact of in utero Th2 immunity on T cell deviation and subsequent immediate-type hypersensitivity in the neonate. Eur J Immunol. 2000;30:714–8. doi: 10.1002/1521-4141(200002)30:2<714::AID-IMMU714>3.0.CO;2-P. 10.1002/(sici)1521-4141(200002)30:02<714::aid-immu714>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz RG, Kemeny DM, Price JF. Higher risk of infantile atopic dermatitis from maternal atopy than from paternal atopy. Clin Exp Allergy. 1993;22:762–6. doi: 10.1111/j.1365-2222.1992.tb02816.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuehr J, Karmaus W, Forster J, et al. Sensitization to four common inhalant allergens within 302 nuclear families. Clin Exp Allergy. 1993;23:600–5. doi: 10.1111/j.1365-2222.1993.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 8.Michel FB, Bousquet J, Greillier P, Robinet-Levy M, Coulomb Y. Comparison of cord blood immunoglobulin E concentrations and maternal allergy for the prediction of atopic diseases in infancy. J Allergy Clin Immunol. 1980;65:422–30. doi: 10.1016/0091-6749(80)90234-1. [DOI] [PubMed] [Google Scholar]
- 9.Magnusson CGM. Cord serum IgE in relation to family history and as predictor of atopic disease in early infancy. Allergy. 1988;43:241–51. doi: 10.1111/j.1398-9995.1988.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 10.Szepfalusi Z, Nentwich I, Gerstmayr M, et al. Prenatal allergen contact with milk proteins. Clin Exp Allergy. 1997;27:28–35. [PubMed] [Google Scholar]
- 11.Van Duren-Schmidt K, Pichler J, Ebner C, et al. Prenatal contact with inhalant allergens. Ped Res. 1997;41:128–31. doi: 10.1203/00006450-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Prescott SL, Macaubas C, Holt BJ, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–7. [PubMed] [Google Scholar]
- 13.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–82. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 14.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nature Med. 1998;4:1020–4. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 15.Vince GS, Johnson PM. Is there a Th2 bias in human pregnancy? J Reprod Immunol. 1996;32:101–4. doi: 10.1016/s0165-0378(96)00995-3. [DOI] [PubMed] [Google Scholar]
- 16.Svensson L, Arvola M, Sällström M-A, Holmdahl R, Mattson R. The Th2 cytokines IL-4 and IL-10 are not crucial for the completion of allogenic pregnancy in mice. J Reprod Immunol. 2001;51:3–7. doi: 10.1016/s0165-0378(01)00065-1. [DOI] [PubMed] [Google Scholar]
- 17.De Moraes-Pinto MI, Vince GS, Flanagan BF, Hart CA, Johnson PM. Localization of IL-4 and IL-4 receptors in the human term placenta, decidua and amniochorionic membranes. Immunology. 1997;90:87–94. doi: 10.1046/j.1365-2567.1997.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–48. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]
- 20.Van der Pouw Kraan TCTM, Boeije LCM, Smeenk RJT, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roper RL, Brown DM, Phipps RP. Prostaglandin E2 promotes B lymphocyte Ig isotype switching to IgE. J Immunol. 1995;154:162–70. [PubMed] [Google Scholar]
- 22.Kelly RW, Carr CG, Elliott CL, Tulppala M, Critchley HOD. Prostaglandin and cytokine release by trophoblastic villi. Human Reprod. 1995;10:3289–92. doi: 10.1093/oxfordjournals.humrep.a135904. [DOI] [PubMed] [Google Scholar]
- 23.Jabara HH, Schneider LC, Shapira SK, et al. Induction of germline and mature Cɛ transcripts in human B cells stimulated with rIL-4 and EBV. J Immunol. 1990;145:3468–73. [PubMed] [Google Scholar]
- 24.Punnonen J, Cocks BG, de Vries JE. IL-4 induce germ-line IgE heavy chain gene transcription in human fetal pre-B cells. Evidence for differential expression of functional IL-4 and IL-13 receptors during B cell ontogeny. J Immunol. 1995;155:4248–54. [PubMed] [Google Scholar]
- 25.Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson CGM, Aalberse RC, Johansson SGO. Monoclonal antibodies against human IgE. Int Archs Allergy Appl Immunol. 1986;80:329–32. [PubMed] [Google Scholar]
- 27.Nilsson K, Bennich H, Johansson SGO, Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970;7:477–89. [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizaka T, Ishizaka K. Mechanisms of passive sensitization. IV. Dissociation of IgE molecules from basophil receptors at acid pH. J Immunol. 1974;142:1078–84. [PubMed] [Google Scholar]
- 29.Walker BAM, Jacobson MA, Knight DA, et al. Adenosine A3 receptor expression and function in eosinophils. Am J Respir Cell Mol Biol. 1997;16:531–7. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- 30.Van der Ploeg I, Matuseviciene G, Fransson J, Wahlgren CF, Olsson T, Scheynius A. Localization of interleukin-13 gene-expressing cells in tuberculin reactions and lesional skin from patients with atopic dermatitis. Scand J Immunol. 1999;49:447–53. doi: 10.1046/j.1365-3083.1999.00513.x. 10.1046/j.1365-3083.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 31.Lilja G, Dannaeus A, Fälth-Magnusson K, et al. Immune response of the atopic woman and foetus: effects of high- and low-dose food allergen intake during late pregnancy. Clin Allergy. 1988;18:131–42. doi: 10.1111/j.1365-2222.1988.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 32.Simister NE, Story CM. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J Reprod Immunol. 1997;37:1–23. doi: 10.1016/s0165-0378(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 33.Takizawa F, Adamczewski M, Kinet JP. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as FcγRII and FcγRIII. J Exp Med. 1992;176:469–76. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purcell WM, Hanahoe THP. Histamine release from mast cells and basophils. A novel source of mast cells: the human placenta. Agents Action. 1991;33:8–12. doi: 10.1007/BF01993113. [DOI] [PubMed] [Google Scholar]
- 35.Mikkelsen AL, Felding C, Larsen LG, Andersen B, Sander P. Mast cells in the placenta. Is there a relation to the development of atopic disease in infants before 18 months of age. J Perinat Med. 1994;22:273–8. doi: 10.1515/jpme.1994.22.4.273. [DOI] [PubMed] [Google Scholar]
- 36.Maddox DE, Kephart GM, Coulam CB, Butterfield JH, Benirschke K, Gleich GJ. Localization of a molecule immunochemically similar to eosinophil major basic protein in human placenta. J Exp Med. 1984;60:29–41. doi: 10.1084/jem.160.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller DL, Hirvonen T, Gitlin D. Synthesis of IgE by the human conceptus. J Allergy Clin Immunol. 1973a;52:182–8. doi: 10.1016/0091-6749(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 38.Lima JO, Zhang L, Atkinson TP, Philips J, Dasanayake AP, Schroedrer HW., Jr Early expression of Iɛ, CD23 (FcɛRII), IL-4Rα, and IgE in the human fetus. J Allergy Clin Immunol. 2000;106:911–7. doi: 10.1067/mai.2000.110228. [DOI] [PubMed] [Google Scholar]
- 39.Avrech OM, Samra Z, Lazarovich Z, Caspi E, Jacobovicg A, Sompolinsky D. Efficacy of the placental barrier for immunoglobulins: correlations between maternal, paternal and fetal immunoglobulin levels. Int Arch Allergy Immunol. 1994;103:160–5. doi: 10.1159/000236622. [DOI] [PubMed] [Google Scholar]
- 40.Miller DL, Zapata R, Hutchinson DL, Gitlin D. Maternofetal passage of human IgE in the pregnant monkey, mouse, rat and guinea pig [Abstract] Fed Proc. 1973b;32:1013. [Google Scholar]
- 41.Vassella CC, Odelram H, Kjellman NIM, Borres MP, Vanto T, Björkstén B. High anti-IgE levels at birth are associated with a reduced allergy prevalence in infants at risk: a prospective study. Allergy. 1994;24:771–7. doi: 10.1111/j.1365-2222.1994.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Neerven RJJ, Wikborg T, Lund G, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- 43.Jones CA, Warner JA, Warner JO. Fetal swallowing of IgE. Lancet. 1998;351:1859. doi: 10.1016/S0140-6736(05)78805-X. [DOI] [PubMed] [Google Scholar]
- 44.Montan PG, Biberfeld PJ, Scheynius A. IgE, IgE receptors, and other immunocytochemical markers in atopic and non-atopic patients with vernal keratoconjunctivitis. Ophtalmology. 1995;102:725–32. doi: 10.1016/s0161-6420(95)30962-1. [DOI] [PubMed] [Google Scholar]
- 45.Bergmann RL, Edenharter G, Bergmann KE, et al. Predictability of early atopy by cord blood-IgE and parental history. Clin Exp Allergy. 1997;27:752–60. [PubMed] [Google Scholar]
- 46.Eiriksson TH, Sigurgeirsson B, Ardal B, Sigfusson A, Valdimarsson H. Cord blood IgE levels are influenced by gestational age but do not predict allergic manifestations in infants. Pediatr Allergy Immunol. 1994;5:5–10. doi: 10.1111/j.1399-3038.1994.tb00211.x. [DOI] [PubMed] [Google Scholar]


