INTRODUCTION
Graves’ disease (GD) is a common autoimmune condition [1] in which thyroid stimulating antibodies (TSAB) mimic the action of thyrotropin (TSH). Since both the growth and function of the thyroid are controlled by TSH [2], TSAB lead to hyperthyroidism and diffuse goitre. The target of the autoimmune response in GD [3] is the thyrotropin receptor (TSHR). The majority of patients with GD have some degree of ocular involvment that is self resolving, but 3–5% of patients develop thyroid eye disease (TED), an autoimmune condition that requires intervention [4].
The main clinical features of TED are proptosis, conjunctival injection, chemosis, diplopia, corneal ulceration and, in extreme cases, loss of sight due to compression of the optic nerve.These signs and symptoms can be explained by the increase in volume of the orbital contents by three mechanisms: (1) oedema; (2) production of hydrophilic glycosaminoglycans (GAG) and (3) hypertrophy of the adipose tissue by adipogenesis [5]. The first two induce expansion of the extra-ocular (EOM) muscles, which are greatly enlarged in TED, and have been the focus of investigations to identify the autoantigen in TED and the link with the thyroid gland.
AUTOANTIGENS IN TED
A variety of methods, including screening EOM expression libraries [6] and Western blotting [7], have identified a number of potential antigens, e.g. the flavoprotein subunit of mitochondrial succinate dehydrogenase [8] and extracellular matrix proteins [9]. A 64-kD protein ‘D1’ or tropomodulin, which is expressed in thyroid and EOM but not skeletal muscle, was isolated from a thyroid expression library [10]. Other investigators have studied the orbital connective tissue and adipose compartments and identified a 23-kD protein present in orbital fibroblasts as a potential TED autoantigen [11]. In fact fibroblasts are a prime suspect for the target of the autoimmune response, not only in TED but also in another extra-thyroidal manifestation, pretibial myxoedema (PM). In this context, it has been reported that immunoglobulins from GD patients stimulate the production of ICAM-1 from GD fibroblasts but not normal fibroblasts [12]. This highlights the presence of fibroblast antibodies in the circulation of GD patients and also a difference in the response to these antibodies between normal and GD fibroblasts. Finally the similarity between thyroglobulin (TG), an autoantigen in Hashimoto’s thyroiditis, and acetylcholinesterase (ACHE) led to the proposal that a TG/ACHE shared epitope could provide the link between the thyroid and the orbit [13]. TED sera were shown to bind to the neuromuscular junction [14] in vitro but, as in all of the preceeding examples, even though a proportion of TED patients’ sera were found to contain antibodies, their presence did not correlate with disease activity.
A plausible alternative is that the antigen in GD, the TSHR, also has a role in the pathogenesis of TED. This would require the presence of the TSHR, or a cross-reactive protein, in the orbit and evidence for autoreactivity to the receptor in all patients with TED. From the clinical standpoint, TED is usually found in GD patients having the highest titres of TSAB, although patients with euthyroid eye disease have also been described [15]. We have recently found antibodies, detectable by flow cytometry, which bind to the receptor but are not TSAB, in some patients with euthyroid TED unpublished observations].
Prior to the molecular cloning of the TSHR, TSH binding sites, TSH mediated adenylate cyclase activity and TSH induced lipolysis had been reported in orbital and other fat depots in rodents [16–19]. Results for human tissues were more controversial with some authors failing to show TSH binding to extra-thyroidal tissues [20] whilst others demonstrated low [21] or high-affinity binding [22] to human adipocyte membranes and TSH induced lipolysis in the neonate which is virtually extinguished by 10 years of age [23].
The application of molecular methods has led to the confirmation of many of the earlier functional studies. In rodents, adipose tissues from several locations have been shown to express TSHR transcripts [24] and a functional TSHR was cloned from rat fat cDNA [25]. Receptor expression was shown to be associated with differentiating preadipocytes [26] whilst the TSHR transcriptional control in these cells is different from that observed in the rat thyroid cell line, FRTL5 [27].
In man, recent Northern blotting data have revealed clear TSHR transcripts in infant abdominal fat but the levels are substantially reduced in the equivalent adult tissue [28]. In human disease, several methods, including RT-PCR [29], liquid hybridization analysis [30] and Northern blotting [31] have indicated that TSHR transcripts may be present in the orbit. Conclusions are sometimes conflicting, and arise because of methodological constraints/differences, e.g. analysing tissues following a period in culture vs. tissue ex vivo, the use of primers able to detect TSHR variant transcripts, and amplification of contaminating genomic DNA [32]. In this context, in earlier studies (reviewed in [32]) we found no evidence for full-length TSHR transcripts in orbital tissues but these did not include orbital fat. We have demonstrated TSHR transcripts in a single specimen of orbital fat, from a patient with TED, by Northern blotting [31] when signals in normal orbital and abdominal fat were at the limit of detection.
In recent studies, immunocytochemistry (ICC) using a monoclonal antibody to the membrane spanning region of the TSHR has demonstrated immunoreactivity for the receptor in orbital fibroblasts in culture and a small number of TED tissue specimens ex vivo [33]. A recent study from the same group suggests that functional receptor expression is induced by differentiation [34], although only orbital preadipocytes were investigated. Two more recent reports have demonstrated that the TSHR is induced during adipogenesis, irrespective of the depot [35,36] implicating either a further antigen to explain the orbital restriction or that the mechanisms operating in TED are systemic. The latter has been suggested previously following the measurement of urinary GAG secretion in TED patients, secretion that seemed excessive to be exclusively a product of the orbit [37]. However, no evidence for widespread fibroblast activation was found in GD forearm biposies assessed for mucin deposition when compared with samples from PM patients [38].
ANIMAL MODELS
From early in the twentieth century attempts were made to develop animal models that recapitulated the signs and symptoms of TED. The first work, in which exophthalmos was convincingly due to an increase in the volume of the orbital contents rather than to a nervous mechanism, is probably that of Smelser in 1936 [39], who administered pituitary extract to guinea pigs. All animals lost weight, had signs of thyroid hypertrophy and some had slight exophthalmos. When he repeated the experiment, but with the addition of thyroidectomy, the majority developed extreme exophthalmos and a 40% increase in the weight of the orbital contents was observed, when compared with noninjected thyroidectomized controls, predominantly in the orbital fat and lacrimal gland. The orbital tissues were examined histologically and found to be oedematous and infiltrated by lymphocytes and an eosin stainable mucopolysaccharide.
Some success in modelling GD and TED has been achieved by transferring TSHR primed T cells to naive syngeneic recipients. We have used unfractionated T cells and a CD4 + enriched population with the in vivo TSHR priming step performed using the extra-cellular domain of the receptor produced as a maltose binding protein fusion (ECD-MBP) in bacteria or genetic immunization (see below). In both cases in vivo priming was followed by an in vitro priming period using ECD-MBP. In BALBc and NOD recipients of syngeneic receptor primed T cells, both strains of mice displayed thyroiditis but with very different histological features [40]. In the BALBc mice, B cells and immunoreactivity for interleukin (IL)-4 and IL-10 were found but in the NOD mice there were very few B cells and immunoreactivity for interferon (INF)-γ, indicating the Th2 and Th1 nature of the induced disease, respectively.
In more recent experiments the mouse orbits have also been examined [41]. All of the NOD recipients of primed and nonprimed cells, displayed normal histology with intact well organized muscle fibre architecture. BALBc orbits of primed (but not nonprimed) T cells appeared strikingly different. The muscle fibres were disorganized and separated by periodic acid Schiff positive oedema. There was accumulation of adipose tissue and infiltration by immune cells, especially mast cells. These changes were observed in almost 70% of the BALBc recipients of receptor primed cells and did not correlate with thyrotropin binding inhibiting immunoglobulin (TBII) or thyroxine (T4) levels. However, orbital changes were observed only in mice having the most severe thyroiditis with 25–30% of the gland occupied by interstitium which also correlated with the most skewed Th2 response, B:T cell ratio 1·6–1·9 and IL-4:INF-γ ratio >2·5.
Similar results were obtained by genetic immunization of NMRI outbred mice [42]. 9/30 males displayed signs of hypothyroidism with reduced T4 and 5/29 females developed stable hyperthyroidism with circulating TSAB accompanied by increased thyroxine but undetectable TSH. In addition, Th2 thyroiditis and orbital changes, including infiltration by mast cells and macrophages, were induced. Analysis of the MHC haplotype of the mice revealed that they were predominantly H2q, irrespective of whether disease had been induced or not. This highlights the importance of non-MHC genes in the development of GD and also TED, and a number of further conclusions can be derived: the induction of a TED-like disease using TSHR cDNA or primed T cells is further support for this antigen being an important target in TED, as well as in GD, and demonstrates that a Th2 autoimmune response to the receptor can result in TED.
TH2 INVOLVEMENT
Perhaps the most convincing evidence for the Th2 nature of GD and TED is a human model happened on by chance. In patients with multiple sclerosis (MS) treated in vivo with a monoclonal antibody to CD52, >95% of their circulating T lymphocytes were eliminated and there was considerable amelioration of their disease. Eighteen months after this treatment, T cell numbers had returned to 35% and B cells to 180% of pretreatment values but 12/34 patients had developed GD with TSAB [43]. The deviation from Th1 to Th2, although beneficial for MS, was permissive for GD and stresses the importance of balance in maintaining appropriate immune responsiveness.
Additional support for the Th2 skew of TED comes from the positive correlation of levels of soluble CD30 (sCD30) with TBII activity and it has been suggested that sCD30 could be used as a marker to indicate when to complete antithyroid drug therapy [44]. Part of the TNF/nerve growth factor superfamily, sCD30 is preferentially expressed and secreted from Th2 cells. Cross-linking has expansion and effector functions particularly on B cells while blocking promotes Th1 phenotype cells developing [45]. In contrast, studies of T cell clones derived from TED patients indicate a predominance of Th1 type autoreactivity in early stages of the disease but a Th2 predominance as the condition develops [46,47]. The culture conditions could influence the clonal phenotype, and ex vivo analysis of cytokines in orbital tissues by RT-PCR has found a Th2 spectrum [48].
One feature of Th2 reactivity, the participation of mast cells, has been shown to induce prostaglandin synthesis and GAG production in human orbital fibroblasts, at least in vitro [49,50]. Mast cells have been reported in human TED biopsies [48] but their precise role warrants further investigation. Furthermore increases in circulating IgE, which could activate mast cells [51] and stem cell factor, a mast cell growth factor [52], have been reported in GD. We have been able to demonstrate IgE antibodies binding directly to the TSHR in a small number of GD patients with TED (unpublished observation) using flow cytometry. Interestingly IgE production by B cells requires T-cells, however, mast cells are capable of stimulating B-cell IgE production via their expression of CD154. This activates CD40 on B cells and is dependent on IL-4 [53]. IL-4 production by mast cells has also been demonstrated to augment fibroblast proliferation via cell-cell adhesion [54].
As will be discussed below, smoking is a major risk factor for TED and when cells from the mast cell lineage were exposed to cigarette smoke in vitro, the release of several cytokines including IL-4, IL-5, IL-10 and IL-13 and TNFα was induced. IL-4 and IL-13 induce class switching to IgE whilst TNFα is a major TED cytokine implicated in several aspects of pathogenesis [55].
CELLULAR MECHANISMS
The activity of cytokines marshals the recruitment of inflammatory cells to the orbit and their subsequent responses. A wide range of techniques has been used in the examination of the limited number of surgical specimens that are available for research investigation. The cellular protagonists as alluded to previously are lymphocytes, macrophages, mast cells and fibroblasts/preadipocytes. From an initial standpoint that IFNγ, TNFα and IL-1 are the primary effectors [56] have evolved the following mechanisms.
Facilitating entry of lymphocytes and monocytes to the orbit requires activation of adhesion molecules. In situ demonstration of ICAM-1, ELAM-1, VCAM-1 and LFA-3 on blood vessels and vascular endothelium from TED orbits has been linked with in vitro fibroblast production of ICAM-1 and LFA-1 stimulated by IFNγ, TNFα and IL-1 [57]. The expression of ICAM-1, ELAM-1, VCAM-1 and leucocyte integrins CD11a-c is greater in early disease in perimysial connective tissue and vascular endothelium in TED [58].
Chemoattractants implicated include IL-6, IL-8, IL-16, RANTES and monocyte chemotactic protein (MCP)-1. IL-16 is important for T-cell trafficking, acting as a ligand for CD4 + cells. IL-16 production is believed to follow RANTES production and both are responsible for T-cell trafficking in orbital and thyroid fibroblasts [59]. Such activity is induced by IL-1β simulating a pathological state. The IL-16 promolecule is cleaved by caspase 3 and is basally expressed by lymphocytes and stored preformed in mast cells. Similarly IL-6, IL-8 and MCP-1 chemokine production is induced by proinflammatory cytokines from orbital fibroblasts [60,61]. The CD40/CD154 costimulatory pathway has been demonstrated to have a potential role in IL-6 and IL-8 expression. T cells and mast cells express the CD154 ligand which activates the TNFα-related CD40 receptor that is up-regulated on orbital fibroblasts stimulated by INFγ [60]. CD154 triggers nuclear mobilization of NF-κB that in turn has binding sites within the promoter regions of IL-6 and IL-8.
Once in the orbit, specific antigen presentation to T cells is suggested to occur via the major histocompatibility complex (MHC) class II HLA-DR antigen [62]. IFNγ can induce orbital fibroblast expression of HLA-DR [60,63]. Antigen intracellular processing and presentation may also be facilitated by expression of heat shock proteins (HSP) present in TED orbital and pretibial fibroblasts [62,64].
As discussed previously, fibroblastic activation follows via one of two divergent routes (denoting a heterogeneous population). One engages in prostanoid synthesis and GAG production [65,66]; in the other, the preadipocyte undergoes an inflammatory mediated program of adipogenesis [67]. In the former both production of hyaluronan and prostaglandin E2 (PGE2) via prostaglandin endoperoxidase H synthase-2 (PGHS2) can be stimulated by CD40/154 activation [68] and as for IL-6 and IL-8 expression, these are linked to NF-κB translocation. PGE2 is a determinant for B cell maturation, mast cell activation and the phenotypic bias of Th0 lymphocytes to become Th2, and the interruption of CD40 activation may represent a specific site for therapeutic intervention [69].
SMOKING AND TED
When examining the environment for factors that exacerbate TED, smoking has turned out to be firmly associated and the risk appears to be immediate and direct given that current and not lifetime smoking is the most significant factor [70]. The effects of smoking on TED are summarized in Table 1. A cohort of newly diagnosed GD patients showed smokers had a 1·3 fold increase in symptomatic ophthalmopathy with objective measurements of proptosis and diplopia being 2·6 and 3·1 times more common, respectively. An assumed effect of smoking increasing tissue hypoxia was tested and it was shown in hypoxic conditions that TNFα, INFγ and IL-1 stimulated GAG production by orbital fibroblasts to a greater extent than in basal oxygen conditions [71]. The effect was greater in orbital than dermal fibroblasts. These effects are at a local tissue level as serum IL-1β, TNFα (and IL-6, IL-6R and IL-1RA) are not affected by smoking [72].
Table 1. Summary of the effects of smoking in TED.
| Current smoking habits have the greatest influence on proptosis, diplopia and symptomatic ophthalmopathy [70] |
| Smoking induced hypoxia promotes fibroblast production of glycosaminoglycans [71] |
| Nicotine increases orbital fibroblast expression of HLA-DR [76] |
| Mast cells production of IL-4,5,10,13 and TNFα is induced by cigarette smoke [55] |
| High levels of anti HSP antibodies are not linked to severity of ophthalmopathy [79] |
| Low levels of sIL-1RA in TED smokers is not a reproducible finding [73–75] |
Both TED patients and healthy smoking female controls have elevated levels of antibodies to HSP72 but the levels of antibody seem not to be linked with the severity of ophthalmopathy and are of doubtful significance other than as a marker of autoimmune susceptibility [73]. Initially it was demonstrated that TED smokers had lower levels of soluble IL-1 receptor antagonist (sIL-1RA) and were therefore less able to neutralize the inflammation generated by IL-1 which indicated a less favourable therapeutic response [74]. These findings have not been supported by follow up studies and therefore remain slightly ambiguous [75,76]. Another in vitro study has examined the role for the cigarette smoke constituents of tar and nicotine on orbital fibroblasts and found that HLA-DR expression occurs only in the presence of INFγ [77]. The enhanced potential for antigen presentation in smokers could increase their susceptibility.
CONCLUDING COMMENTS
We may be coming closer to a hypothesis that can ultimately be proved regarding the very earliest steps initiating orbital autoimmunity in TED. Whether or not the TSHR is the antigen of TED, TED is exquisitely sensitive to TSHR stimulation either from TSH or TSAB. This stimulation is mediated via a functional extrathyroidal TSHR present on small numbers of preadipocytes available for recruitment into adipogenesis or on those actively differentiating. A crucial trigger occurs locally within the orbit that sets in motion activation of mast cells and fibroblasts that initiates the adhesion molecule, chemokine, cytokine and prostaglandin cascade that results in fibrotic and adipogenic orbital remodelling that has been characterized as above. The trigger may be that TSAB or TSH drives an otherwise gentle turnover of orbital predipocyte differentiation to a point where products (such as glycosphingolipids [78] or via c-kit and CD40) are transferred to mast cells causing resident mast cell activation. The presence of antibodies to glycolipids has been demonstrated in Graves’ patients but has received little attention [79]. Alternatively it may be circulating IgE antibody mediated effects via mast cell Fc receptors that are the priming orbital trigger. Therefore our lines of investigation into TED are broadening with a view to understanding inflammatory mediated adipogenesis. The steps involved in the initiation of TED are summarized in Fig 1.
Fig. 1.
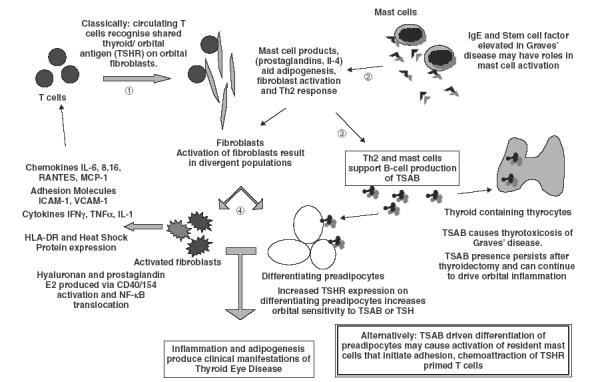
Summary of the steps involved in the initiation of TED. (1) T cells recognize TSHR on orbital fibroblasts. (2) Activated mast cells aid fibroblast activation, stimulate a Th2 response and (3) stimulate TSAB production. (4) Fibroblast activation results in the production of proinflammatory mediators and adhesion molecules with the preadipocyte fraction undergoing differentiation and increasing TSHR expression that heightens TSH/TSAB sensitivity. The resultant inflammation and adipogenesis produce the clinical manifestations of TED.
The clinical presentation of TED is varied but a significant proportion of patients have fat volume expansion without muscle enlargement. Whether this represents the most extreme end of a Th2 response and those with myopathy a more Th1 fibrotic effect remains unanswered.
REFERENCES
- 1.Weetman AP, McGregor AM. Autoimmune Thyroid Disease; Further Developments In Our Understanding. Endocr Revs. 1994;15:788–830. doi: 10.1210/edrv-15-6-788. [DOI] [PubMed] [Google Scholar]
- 2.Rees Smith B, McLachlan S, Furmaniak J. Autoantibodies to the Thyrotropin Receptor. Endocr Revs. 1998;9:106–20. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 3.Paschke R, Ludgate M. The Thyrotropin Receptor in Thyroid Disease. N Engl J Med. 1997;337:1675–81. doi: 10.1056/NEJM199712043372307. [DOI] [PubMed] [Google Scholar]
- 4.Perros P, Kendall-Taylor P. Thyroid-Associated Ophthalmopathy – Pathogenesis and clinical management. Baillieres Clin Endocrinol Metab. 1995;9:115–35. doi: 10.1016/s0950-351x(95)80867-1. [DOI] [PubMed] [Google Scholar]
- 5.Heufelder AE, Weetman AP, Ludgate M, Bahn RS. Pathogenesis of Graves’ ophthalmopathy. In: Prummel MF, Wiersinga WM, Mourits MP, editors. Recent Developments in Graves’ Ophthalmopathy. Dordrecht: Kluwer Academic Publishers; 2000. pp. 15–37. [Google Scholar]
- 6.Elisei R, Weightman D, Kendall-Taylor P, Vassart G, Ludgate M. Muscle Autoantigens In Thyroid-Associated Ophthalmopathy – The Limits of Molecular-Genetics. J Endocr Invest. 1993;16:533–40. doi: 10.1007/BF03348900. [DOI] [PubMed] [Google Scholar]
- 7.Ahmann A, Baker J, Weetman A, Wartofsky L, Nutman T, Burman K. Antibodies to porcine eye muscle in patients with Graves’ ophthalmopathy. identification of serum immunoglobulins directed against unique determinants by immunoblotting and enzyme linked absorbent assay. J Clin Endocrinol Metab. 1987;64:454–60. doi: 10.1210/jcem-64-3-454. [DOI] [PubMed] [Google Scholar]
- 8.Gunji K, Bellis AD, Kubota S, et al. Serum antibodies against the flavoprotein subunits of succinate dehydrogenase are sensitive markers of eye muscle autoimmunity in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1999;84:1255–62. doi: 10.1210/jcem.84.4.5640. [DOI] [PubMed] [Google Scholar]
- 9.Bernarczuk T, Stolarski C, Pawlik E, et al. Autoantibodies reactive with extracellular proteins in patients with thyroid associated ophthalmopathy. Thyroid. 1999;9:298–5. doi: 10.1089/thy.1999.9.289. [DOI] [PubMed] [Google Scholar]
- 10.Dong Q, Ludgate M, Vassart G. Cloning and Sequencing of A Novel 64-kDa Autoantigen Recognized By Patients With Autoimmune Thyroid-Disease. J Clin Endocrinol Metab. 1991;72:1375–81. doi: 10.1210/jcem-72-6-1375. [DOI] [PubMed] [Google Scholar]
- 11.Bahn RS, Gorman CA, Johnson CM, Smith TJ. Presence of antibodies in the sera of patients with Graves’ disease recognising a 23 kilodalton fibroblast protein. J Clin Endocrinol Metab. 1989;69:622–8. doi: 10.1210/jcem-69-3-622. [DOI] [PubMed] [Google Scholar]
- 12.Heufelder AE, Bahn RS. Graves’ immunoglobulins and cytokines stimulate expression of intercellular adhesion molecule-1 (ICAM-1) in cultured graves orbital fibroblasts. Eur J Clin Invest. 1992;8:529–37. doi: 10.1111/j.1365-2362.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 13.Ludgate M, Swillens S, Mercken L, Vassart G. Homology Between Thyroglobulin and Acetylcholinesterase – An Explanation For Pathogenesis of Graves Ophthalmopathy. Lancet. 1986;2:219–20. doi: 10.1016/s0140-6736(86)92515-8. [DOI] [PubMed] [Google Scholar]
- 14.Ludgate M, Dong Q, Dreyfus Pa, Zakut H, Taylor P, Vassart G, Soreq H. Definition, At The Molecular-Level, of A Thyroglobulin Acetylcholinesterase Shared Epitope – Study of Its Pathophysiological Significance In Patients With Graves Ophthalmopathy. Autoimmunity. 1989;3:167–76. doi: 10.3109/08916938909099014. [DOI] [PubMed] [Google Scholar]
- 15.Khoo DHC, Ho SC, Seah LL, et al. The combination of absent thyroid peroxidase antibodies and high thyroid stimulating immunoglobulins identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1175–80. doi: 10.1089/thy.1999.9.1175. [DOI] [PubMed] [Google Scholar]
- 16.Davies TF, Teng CS, McLachlan SM, Rees Smith B, Hall R. Thyrotropin receptors in adipose tissue, retro-orbital tissue and lymphocytes. Mol Cell Endo. 1978;9:303–10. doi: 10.1016/0303-7207(78)90072-2. [DOI] [PubMed] [Google Scholar]
- 17.Hart IR, McKenzie JM. Comparison of the effects of thyrotropin and the long acting thyroid stimulator on guinea pig adipose tissue. Endocrinology. 1971;88:26–32. doi: 10.1210/endo-88-1-26. [DOI] [PubMed] [Google Scholar]
- 18.Winand RJ, Kohn LD. Retrobulbar modifications in experimental exophthalmos. the effect of thyrotropin and exophthalmos-producing substance derived from thyrotropin on the 35SO4 incorporation and glycosaminoglycan content of Harderian glands. Endocrinology. 1973;93:670–7. doi: 10.1210/endo-93-3-670. [DOI] [PubMed] [Google Scholar]
- 19.Iida Y, Amir S, Ingbar S. Stabilization, partial purification and characterisation of thyrotropin receptors in solubilzed guinea pig fat cell membranes. Endocrinology. 1987;121:1627–36. doi: 10.1210/endo-121-5-1627. [DOI] [PubMed] [Google Scholar]
- 20.Perros P, Kendall-Taylor P. Demonstration of thyrotropin binding sites in orbital connective tissue: possible role in the pathogenesis of thyroid associated ophthalmopathy. J Endocr Invest. 1994;17:163–70. doi: 10.1007/BF03347708. [DOI] [PubMed] [Google Scholar]
- 21.Mullin BR, Lee G, Ledley F, Winand R, Kohn L. Thyrotropin interactions with human fat cell membrane preparations and the finding of a soluble thyrotropin binding component. Biochem Biophys Res Comm. 1976;69:55–62. doi: 10.1016/s0006-291x(76)80271-9. [DOI] [PubMed] [Google Scholar]
- 22.Marcus C, Erhen H, Bolme P, Armer P. Regulation of Lipolysis During The Neonatal Period Importance of Thyrotropin. J Clin Invest. 1988;82:1793–7. doi: 10.1172/JCI113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roselli-Rehfuss L, Robbins LS, Cone RD. Thyrotropin Receptor Messenger Ribonucleic Acid Is Expressed In Most Brown and White Adipose Tissue in the Guinea Pig. Endocrinology. 1992;130:1857–61. doi: 10.1210/endo.130.4.1547715. [DOI] [PubMed] [Google Scholar]
- 24.Endo T, Ohta K, Haraguchi K, Onaya T. Cloning and Functional Expression of A Thyrotropin Receptor cDNA From Rat Fat-Cells. J Biol Chem. 1995;270:10833–7. doi: 10.1074/jbc.270.18.10833. [DOI] [PubMed] [Google Scholar]
- 25.Haraguchi K, Shimura H, Lin L, Saito T, Endo T, Onaya T. Functional expression of thyrotropin receptor in differentiated 3T3-L1 cells: a possible model cell line of extrathyroidal expression of thyrotropin receptor. Biochem Biophys Res Comm. 1996;223:193–8. doi: 10.1006/bbrc.1996.0868. [DOI] [PubMed] [Google Scholar]
- 26.Haraguchi K, Shimura H, Lin L, Endo T, Onaya T. Differentiation of rat preadipocytes is accompanied by expression of thyrotropin receptors. Endocrinology. 1996;137:3200–5. doi: 10.1210/endo.137.8.8754740. [DOI] [PubMed] [Google Scholar]
- 27.Shimura H, Miyazaki A, Haraguchi K, Endo T, Onaya T. Analysis of differentiation induced expression mechanisms of thyrotropin receptor gene in adipocytes. Mol Endocrinol. 1998;12:1473–86. doi: 10.1210/mend.12.10.0175. [DOI] [PubMed] [Google Scholar]
- 28.Janson A, Rawet H, Perbeck L, Marcus C. Presence of thyrotropin receptor in infant adipocytes. Paediatric Res. 1998;43:555–8. doi: 10.1203/00006450-199804000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Feliciello A, Porcellini A, Ciullo L, Bonavolonta G, Avvedimento E, Fenzi G. Expression of thyrotropin receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342:337–8. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 30.Spitzweg C, Joba W, Hunt N, Heufelder AE. Analysis of Human Thyrotropin Receptor Gene Expression and Immunoreactivity In Human Orbital Tissue. Eur J Endocrinol. 1997;136:599–607. doi: 10.1530/eje.0.1360599. [DOI] [PubMed] [Google Scholar]
- 31.Crisp M, Lane C, Halliwell M, Wynford-Thomas D, Ludgate M. Thyrotropin Receptor Transcripts In Human Adult Adipose Tissue. J Clin Endocrinol Metab. 1997;82:2003–5. [PubMed] [Google Scholar]
- 32.Paschke R, Vassart G, Ludgate M. Current evidence for and against the TSH receptor being the common antigen in Graves-disease and thyroid-associated ophthalmopathy. Clin Endocrinol. 1995;42:565–9. doi: 10.1111/j.1365-2265.1995.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 33.Bahn R, Dutton C, Natt N, Joba W, Spitweg C, Heufelder A. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues; potential autoantigen in Graves’ Ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 34.Valyasevi R, Erickson D, Harteneck D, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–62. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 35.Crisp MS, Starkey KJ, Lane C, Ham J, Ludgate M. Adipogenesis in Thyroid Eye Disease. Invest Ophthal Vis Sci. 2000;41:3249–55. [PubMed] [Google Scholar]
- 36.Bell A, Gagnon L, Grunder S, Parekh SJ, Smith TJ, Sorisky A. Functional TS4 receptor in abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol. 2000;279:C335–40. doi: 10.1152/ajpcell.2000.279.2.C335. [DOI] [PubMed] [Google Scholar]
- 37.Hansen C, Fraiture B, Rouhi R, Otto E, Forster G. Kahaly GHPLC Glycosaminoglycan Analysis In Patients With Graves’ Disease. Clin Sci. 1997;92:511–7. doi: 10.1042/cs0920511. [DOI] [PubMed] [Google Scholar]
- 38.Peacey SR, Flemming L, Messenger A, Weetman AP. Is Graves’ dermopathy a generalised disorder? Thyroid. 1996;6:41–5. doi: 10.1089/thy.1996.6.41. [DOI] [PubMed] [Google Scholar]
- 39.Smelser GK. Experimental production of exophthalmos resembling that found in Graves’ disease. Proc Soc Exp Biol Medical. 1936;35:128–30. [Google Scholar]
- 40.Costagliola S, Many MC, StalmansFalys M, et al. Transfer of thyroiditis, with syngeneic spleen-cells sensitized with the human thyrotropin receptor, to naive BALB/c and nod mice. Endocrinology. 1996;137:4637–43. doi: 10.1210/endo.137.11.8895327. [DOI] [PubMed] [Google Scholar]
- 41.Many M-C, Costagliola S, Detrait M, Denef J-F, Vassart G, Ludgate M. Development of an animal model of autoimmune thyroid eye disease. J Immunol. 1999;162:4966–74. [PubMed] [Google Scholar]
- 42.Costagliola S, Many MC, Denef JF, Pohlenz J, Refetoff S, Vassart G. Genetic immunisation of outbred mice with thyrotropin receptor cDNA provides a model of Graves’ disease. J Clin Invest. 2000;105:803–11. doi: 10.1172/JCI7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coles AJ, Wing N, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354:1691–5. doi: 10.1016/S0140-6736(99)02429-0. [DOI] [PubMed] [Google Scholar]
- 44.Okumura M, Hidaka Y, Kuroka S, Takeoka K, Tada H, Akino N. Increased serum concentration of soluble CD30 in patients with Graves’ Disease and Hashimotos Thyroiditis. J Clin Endocrinol Metab. 1997;82:1757–60. doi: 10.1210/jcem.82.6.4000. [DOI] [PubMed] [Google Scholar]
- 45.Del Prete G, De Carli M, D’Elios M. CD30 mediated signalling promotes development of human T helper type 2-like T-cells. J Exp Med. 1995;182:1655–61. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2000;85:776–80. doi: 10.1210/jcem.85.2.6333. [DOI] [PubMed] [Google Scholar]
- 47.Yang D, Hiromatsu Y, Hoshino T, et al. Dominant infiltration of Th1-type CD4+ T cells at the retrobulbar space of patients with thyroid associated ophthalmopathy. Thyroid. 1999;9:305–9. doi: 10.1089/thy.1999.9.305. [DOI] [PubMed] [Google Scholar]
- 48.Hiromatsu Y, Yang D, Bednarczuk T, et al. Cytokine profiles in eye muscel tissue and orbital fat tissue from patients with thyroid associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85:1194–9. doi: 10.1210/jcem.85.3.6433. [DOI] [PubMed] [Google Scholar]
- 49.Smith TJ, Parikh SJ. HMC-1 mast cells activate human orbital fibroblasts in coculture. Evidence for up-regulation of prostaglandin E2 and Hyaluronan synthesis. Endocrinology. 1999;140:3518–25. doi: 10.1210/endo.140.8.6881. [DOI] [PubMed] [Google Scholar]
- 50.Hufnagel TJ, Hickey WF, Cobbs WH, et al. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves’ disease. Ophthalmology. 1984;91:1411–9. doi: 10.1016/s0161-6420(84)34152-5. [DOI] [PubMed] [Google Scholar]
- 51.Sato A, Takemura Y, Yamada T, et al. A possible role of immunoglobulin E in patients with hyperthyroid Graves’ disease. J Clin Endocrinol Metab. 1999;84:3602–5. doi: 10.1210/jcem.84.10.6038. [DOI] [PubMed] [Google Scholar]
- 52.Yamada T, Sato A, Aizawa T, et al. An elevation of stem cell factor in patients with hyperthyroid Graves’ disease. Thyroid. 1998;8:499–504. doi: 10.1089/thy.1998.8.499. [DOI] [PubMed] [Google Scholar]
- 53.Smyth LJC, Machado DC, Upton AP, Good S, Aufderheide M, Helm BA. Assessment of the molecular basis of the proallergic effects of cigarette smoke. Environ Sci Tecnol. 2000;34:1370–4. [Google Scholar]
- 54.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 55.Trautmann A, Krohne G, Brocker E-B, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1997;160:5053–7. [PubMed] [Google Scholar]
- 56.Heufelder A, Bahn RS. Detection and localisation of cytokine immunoreactivity in retro-ocular connective tissue in Graves’ ophthalmopathy. Eur J Clin Invest. 1993;23:10–7. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 57.Heufelder A, Bahn RS. Elevated expression in situ of selectin and immunoglobulin superfamily type adhesion molecules in retroocular connective tissue from patients with Graves’ ophthalmopathy. Clin Exp Immunol. 1993;91:381–9. doi: 10.1111/j.1365-2249.1993.tb05913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pappa A, Calder V, Fells P, Lightman S. Adhesion molecule expression in vivo on extraocular muscles in thyroid associated ophthalmopathy. Clin Exp Immunol. 1997;108:309–13. doi: 10.1046/j.1365-2249.1997.3621258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sciaky D, Brazer W, Lentre DM, Cruikshank WW, Smith TJ. Cultured human fibroblasts express constitutive IL-16 mRNA. cytokine induction of active IL-16 protein synthesis through a caspase 3 dependent mechanism. J Immunol. 2000;164:3806–14. doi: 10.4049/jimmunol.164.7.3806. [DOI] [PubMed] [Google Scholar]
- 60.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human Orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Amj Physiol. 1998;274:C707–14. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 61.Elner VM, Burnstine MA, Kunkel SL, Streiter RM, Elner SG. Interleukin-8 and monocyte chemotactic protein-1 gene expression and protein production by human orbital fibroblasts. Ophth Plas Reconstr Surg. 1998;14:119–25. doi: 10.1097/00002341-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Hiromatsu Y, Tanaka K, Ishisaka N, et al. Human histocompatibility leukocyte antigen-DR and heat shock protein-70 expression in eye muscle tissue in thyroid associated ophthalmopathy. J Clin Endocrinol Metab. 1995;80:685–91. doi: 10.1210/jcem.80.2.7531718. [DOI] [PubMed] [Google Scholar]
- 63.Heufelder AE, Smith TJ, Gorman CA, Bahn RS. Increased induction of HLA-DR by IFNγ in cultured fibroblasts derived from patients with Graves’ophthalmopathy and pretibial dermopathy. J Clin Endocrinol Metab. 1991;73:307–13. doi: 10.1210/jcem-73-2-307. [DOI] [PubMed] [Google Scholar]
- 64.Heufelder AE, Wenzel BE, Gorman C, Bahn RS. Detection, Cellular localisation and modulation of heat shock proteins in cultured fibroblasts from patients with extrathyroidal manifestations of Graves’ disease. J Clin Endocrinol Metab. 1991;73:739–45. doi: 10.1210/jcem-73-4-739. [DOI] [PubMed] [Google Scholar]
- 65.Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by INFγ in cultured retrocular fibroblasts. J Clin Endocrinol Metab. 1991;72:1169–71. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- 66.Wang HS, Cao HJ, Winn VD, et al. Leukoregulin induction of prostaglandin endoperoxidase H synthase-2 in human orbital fibroblasts. An in vitro model for connective tissue inflammation. J Biol Chem. 1996;271:22718–28. [PubMed] [Google Scholar]
- 67.Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence for adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81:3428–31. doi: 10.1210/jcem.81.9.8784110. [DOI] [PubMed] [Google Scholar]
- 68.Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in dramatic induction of hyaluronan synthesis and prostaglandin endoperoxidase H synthase-2 expression. J Biol Chem. 1998;273:29615–25. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 69.Smith TJ. Participation of orbital fibroblasts in the inflammation of Graves’ ophthalmopathy. In: Bahn RS, editor. Thyroid Eye Disease. Endocrine Updates 14. Vol. 200. Dordrecht: Kluwer Academic Publishers; pp. 83–98. [Google Scholar]
- 70.Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol. 1996;45:477–81. doi: 10.1046/j.1365-2265.1996.8220832.x. [DOI] [PubMed] [Google Scholar]
- 71.Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia, possible role in thyroid associated ophthalmopathy. Clin Endocrinol. 1994;40:67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 72.Salvi M, Pedrazonni M, Girasole G, Giuliani N, Minelli R, Wall J, Roti E. Serum concentrations of proinflammatory cytokines in Graves’ disease: effect of treatment, thyroid function, ophthalmopathy and cigarette smoking. Eur J Endocrinol. 2000;143:197–202. doi: 10.1530/eje.0.1430197. [DOI] [PubMed] [Google Scholar]
- 73.Prummel MF, Van Pareren Y, Bakker O, Wiersinga WM. Anti heat shock protein (hsp) 72 antibodies are present in patients with Graves’ disease (GD) and in smoking control subjects. Clin Exp Immunol. 1997;110:292–5. doi: 10.1111/j.1365-2249.1997.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofbauer LC, Muhlberg T, Konig A, Heufelder G, Schworm H-D, Heufelder AE. Soluble interleukin-1 receptor antagonist serum levels in smokers and nonsmokers with Graves’ ophthalmopathy undergoing orbital radiotherapy. J Clin Endocrinol Metab. 1997;82:2244–7. doi: 10.1210/jcem.82.7.4068. [DOI] [PubMed] [Google Scholar]
- 75.Bartelena L, Monetti L, Tanda ML, Dell’Unto E, Rouchi R, Barbesino G, Pinchera A, Marcocci C. Soluble interleukin-1 receptor antagonist concentration in patients with Graves’ ophthalmopathy is neither related to cigarette smoking nor predictive of subsequent response to glucocorticoids. Clin Endocrinol. 2000;52:647–51. doi: 10.1046/j.1365-2265.2000.00988.x. [DOI] [PubMed] [Google Scholar]
- 76.Wakelkamp IM, Gerding MW, Van der Mer JW, Prummel MF, Wiersinga WM. Both Th1 and Th2 cytokines are elevated in Graves’ ophthalmopathy. Clin Exp Immunol. 2000;121:453–7. doi: 10.1046/j.1365-2249.2000.01335.x. 10.1046/j.1365-2249.2000.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mack WP, Staisor GO, Cao HJ, Staisor OG, Smith TJ. The effect of cigarette smoking constituents on the expression of HLA-DR in orbital fibroblasts derived from patients with Graves’ ophthalmopathy. Ophth Plas Reconstr Surg. 1999;15:260–71. doi: 10.1097/00002341-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Katz HR, Dayton ET, Levi-Schaffer F, Benson AC, Austen KF, Stevens RL. Coculture of mouse IL-3 dependent mast cells with 3T3 fibroblasts stimulates synthesis of globopentaosylceramide (Forssman Glycolipid) by fibroblasts and surface expression on both populations. J Immunol. 1988;140:3090–7. [PubMed] [Google Scholar]
- 79.Ariga T, Yoshida T, Mimori T, Yu RK. Autoantibodies against Forssman glycolipids in Graves’ disease and Hashimoto’s thyroiditis. Clin Exp Immunol. 1991;86:483–8. doi: 10.1111/j.1365-2249.1991.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


