Abstract
Repeated challenge with antigen is involved in the pathogenesis of a variety of pulmonary diseases. Patients with cystic fibrosis (CF) experience recurrent pulmonary colonization with Pseudomonas aeruginosa before establishment of chronic lung infection. To mimic recurrent lung infections in CF patients, the lungs of susceptible BALB/c mice were re-infected with P. aeruginosa 14 days after the initial infection. Singly-infected BALB/c mice, as well as non-infected mice, were used as controls. Decreased mortality and milder lung inflammation in re-infected BALB/c mice, as well as a tendency for improved clearance of bacteria, was observed when compared with singly-infected mice. The improved outcome in re-infected mice correlated with changes in CD4 cell numbers. Surface expression of LFA-1 on pulmonary CD4 cells was increased in re-infected compared with singly-infected mice. Moreover, resistance to re-infection was paralleled by a shift towards a Th1-dominated response and increased IL-12 production. No significant increase in serum IgG was observed in the re-infected mice. In conclusion, these results indicate a protective role for a Th1-dominated response, independent of antibody production, in chronic P. aeruginosa lung infection in CF.
Keywords: Th1/Th2, infectious immunity-bacteria, cytokines, rodent, lung
INTRODUCTION
Cytokine responses are important controlling elements of the inflammation and immune reactions that occur during infection of an individual. Inappropriate inflammatory and immunological responses to chronic or repeated infections are major causes of pathology in several diseases [1–5]. In most patients with the inherited disease cystic fibrosis (CF), recurrent colonization with Pseudomonas aeruginosa is seen before chronic lung infection is established [6,7]. The inflammatory response in CF patients with chronic P. aeruginosa lung infection is characterized by numerous polymorphonuclear neutrophils (PMNs) surrounding the microcolonies of biofilm-growing P. aeruginosa [6,8]. The release of exoproteases from the PMNs results in degradation of the lung tissue, and the release of oxygen radicals results in a change to the mucoid phenotype of the P. aeruginosa [9]. Indeed, the conversion of the mucoid phenotype of P. aeruginosa is linked to the establishment of chronic lung infection in CF [6,10]. The spontaneous, non-treated course of chronic lung infection in CF has a dichotomized outcome. The immune response is dominated by a pronounced antibody response against P. aeruginosa antigens and a poor prognosis in most CF patients, whereas a few CF patients continue to have low antibody production and a better prognosis [11].
Since the original report that mouse CD4+ T-cell clones could be divided into Th1 or Th2 responders, the outcome of chronic infections has been ascribed to the type of T-helper (Th) cell response involved [1,12,13]. A Th1 response, characterized by IFN-γ production, low antibody response and activation of macrophages has been found in patients with the tuberculoid form of leprosy and in patients with curative leishmania infection [12,13]. In contrast, a Th2 response, characterized by IL-4 and IL-5 production and a high antibody response, has been observed in patients with lepromatous lepra and in patients with severe, visceral leishmaniasis [12,13].
Whereas humoral immunity to chronic P. aeruginosa lung infections in CF patients has been intensely studied, there has been limited focus on T-cell responses, although decreased T-cell proliferation to P. aeruginosa has been reported [14]. Recently, a Th2-dominated immune response in CF patients with chronic P. aeruginosa lung infection as compared with CF patients without chronic infection was observed [15]. Moreover, the chronically infected CF patients with the highest IFN-γ production also had the best lung function [15]. Another study reported significantly higher IFN-γ expression in bronchial biopsies from chronic stable compared with CF patients with acute excacerbations [16]. Thus, although P. aeruginosa is an extracellular bacterium to which the host would presumably benefit from a Th2 or mixed Th1/Th2 response, there is growing evidence that a Th1-dominated immune response might improve the prognosis of CF patients with chronic P. aeruginosa lung infection [15,17,18].
To investigate further the immunopathological mechanisms during chronic P. aeruginosa lung infection, animal models of chronic P. aeruginosa lung infection in BALB/c and C3H/HeN mice were established [19]. In these mouse models, a Th2- dominated pulmonary response in the susceptible BALB/c and a Th1-dominated pulmonary response in the resistant C3H/HeN mouse strain were reported [20]. The possible mechanisms in the early response responsible for development of the type of Th subset were described [20]. Since the Th-cell response tends to be more polarized upon long or repeated exposure to the same antigen, the aim of the present study was to investigate the outcome of pulmonary re-infection with alginate-embedded P. aeruginosa in relation to the immunological response in the susceptible BALB/c mice. Moreover, the aim was to establish an animal model which would mimic the situation in CF where patients experience recurrent lung infections with P. aeruginosa before they become chronically infected [6].
MATERIALS AND METHODS
Animals
Female 12 week-old BALB/c (n = 72) mice were purchased from M & B Laboratory Animals, Ry, Denmark. The mice were divided into two groups: (i) single infection group (n = 39 BALB/c) and (ii) re-infection group (n = 28 BALB/c). In the second group, five BALB/c mice died from the first infection, leaving only 23 BALB/c mice for re-infection; five mice were left uninfected as controls.
Immobilization of P. aeruginosa in seaweed alginate beads
Strain PAO 579 (IATS O: 2/5) of P. aeruginosa was used [21]. Immobilization of P. aeruginosa in seaweed alginate beads was performed as previously described [21]. The suspension was adjusted to 109 colony-forming units (cfu)/ml and confirmed by colony counts.
Challenge procedure
At the time of challenge, mice were anaesthetized subcutaneously with a 1:1 mixture of etomidate (Jannsen, Birkeroed, Denmark) and midazolam (Roche, Basel, Switzerland) (10 ml/kg body weight), and tracheostomized [19]. Intratracheal challenge with 0·04 ml (0·03 ml at first infection to decrease mortality) of P. aeruginosa embedded in alginate beads was performed with a bead-tipped needle. The inoculum was installed in the left lung 11 mm from the penetration site [19]. The re-infection group was challenged 14 days after the first infection when the bacteria had been cleared from the lung [19].
Pentobarbital (DAK, Copenhagen, Denmark), 2·0 ml/kg body weight, was used to sacrifice the animals [19]. Mice were sacrificed at day four after single or re-infection.
Bacteriology
Lungs from four singly-infected and six re-infected mice were prepared for bacteriological examination as previously described [19]. In brief, the lungs were removed aseptically and homogenized in 5 ml phosphate-buffered saline (PBS); serial dilutions of the homogenate were plated, incubated for 24 h and the numbers of cfu determined.
Histopathology
Lungs from 14 (six singly-infected and eight re-infected) were prepared for histopathological examination as previously described [19]. Briefly, the macroscopically affected lung parts were fixed in a 4% w/v formaldehyde solution (Bie & Berntsen, Copenhagen, Denmark) embedded in paraffin wax and cut into 5 μm thick sections, followed by haematoxylin and eosin staining. The entire lung slide was scanned at a low magnitude and, from an average evaluation of a minimum of five representative areas at higher magnitude (×400 and ×1000), the type of lung inflammation was estimated. The inflammatory responses were scored as acute (> 90% PMNs), chronic (> 90% mononuclear cells (MN)), both types present, neither dominating (PMN/MN), or no inflammation (NI) [19]. The histopathological evaluation was carried out blind.
Blood
Blood was obtained by cardiac puncture. Blood for flow cytometric investigations was isolated into a heparinized syringe and kept on ice. Red blood cells were removed by lysis in cold 0·87% NH4Cl buffer with EDTA, and the remaining cells were washed once in PBS before staining for flow cytometric analysis. Serum for antibody analysis was isolated after centrifugation of coagulated blood.
Broncho-alveolar lavage (BAL)
The lungs were lavaged with a total volume of 1·5 ml PBS by repeated flushing of the lungs. BAL failed in one mouse in the singly-infected group and in one non-infected mouse. BAL-fluid (BALF) was kept on ice and the cells separated by centrifugation. The number of nucleated cells was determined by counting after staining with crystal violet. BALF was kept at –70°C until determination of chemokine concentrations. The cells were kept on ice for flow cytometric analysis.
Lung cell suspensions
This method was modified from a previously described protocol [20,22]. Mice overdosed with pentobarbital were exsanguinated by cutting the vena cava. Blood vessels were washed by infusion of PBS to the right ventricle. Lungs were then removed aseptically and washed once in RPMI 1640 medium supplemented with 2% fetal calf serum (FCS; Biological Industries, Kibbutz Beit Haemek, Israel), 10 mm HEPES (local supply), 20 U/ml penicillin, 20 μg/ml streptomycin and 100 μg/ml gentamicin (GIBCO BRL, Life Technologies, Gaithersburg, USA), 58·4 mg/ml L-glutamin and 0·05 mM 2-mercaptoethanol. The lungs were sliced into pieces of 1–2 mm in 10 ml complete medium RPMI 1640 supplemented with 2 mg/ml collagenase (Sigma Chemical Co., St Louis, USA) and DNase 50 μg/ml (Boehringer Mannheim GmbH, Mannheim, Germany). The lung slices were incubated with shaking for 1·5 h at 37°C. Single cell suspensions were obtained by repeated pipetting, then passage through a 18G needle followed by a 23G needle. The cells were washed three times with complete RPMI 1640 medium and resuspended in 6 ml 10% FCS complete RPMI 1640 medium. The number of nucleated cells was determined by counting after staining with crystal violet, and the cell number was adjusted to 2 × 106 cells/ml.
Antigens and mitogens
Pseudomonas aeruginosa outer membrane protein (OMP) was prepared as previously described and used at a final concentration of 10 μg/ml [20,23,24]. The OMP antigen contains LPS and thus also stimulates macrophages. Monoclonal hamster anti-mouse CD3e without sodium azide (anti-CD3) (Pharmingen, San Diego, USA) for short stimulation of already activated T cells was used at a final concentration of 1μg/ml. OMP was incubated with the cells for 48 h and anti-CD3 was incubated with the cells for 6 h. Medium without antigen or anti-CD3 was used as control for 48 h or 6 h, respectively.
Cytokine production
Aliquots of 100 μl of the adjusted cell suspensions were added to each well in round-bottomed microtitre plates (Nunc, Roskilde, Denmark), and 100 μl medium with either OMP, anti-CD3 or medium alone were added to the cells in triplicate. Cells were incubated at 37°C in 5% CO2. Supernatant fluids were isolated and kept at –70°C until cytokine determination was performed.
IL-12 concentrations in the supernatant fluids were measured after 48 h of stimulation, and IL-4 and IFN-γ were measured after 6 h and 48 h. T lymphocytes have not been reported to produce IL-12 so IL-12 was only measured after OMP stimulation. Cytokine determination was achieved by enzyme-linked immunosorbent assay (ELISA) (Genzyme, Cambridge, UK or Pharmingen), following the manufacturer’s directions. The cytokine levels in supernatant fluids from cultures with medium alone were subtracted from the levels in anti-CD3- or OMP- stimulated cultures.
Immunofluorescence staining and flow cytometry
All antibodies were purchased from Pharmingen. The Th cells from BALF, lung cell suspensions and peripheral blood were stained by phycoerythrin (PE)-conjugated anti-CD4 (GK1·5), and activation of the CD4 cells was related to surface expression of the integrin molecule lymphocyte function-associated antigen-1 (LFA-1) stained by fluorescein isothiocyanate (FITC)- conjugated anti-CD11a (2D7). The cells were incubated on ice in the dark for 30 min and washed once in cold PBS. The samples were analysed using a FACSort (Becton Dickinson, San Jose, CA, USA) with a 15 mW argon laser tuned at 488 nm for excitation. Light scatter and logarithmically-amplified fluorescence parameters from 10 000 events were recorded in list mode after gating on forward light scatter to avoid debris, cell aggregates and bacteria. Using the CellQuest software (Becton Dickinson), the mean fluorescence intensity of all CD4+ cells was calculated.
IgG determination
IgG production against P. aeruginosa sonicate was determined by ELISA. Briefly, maxisorb microtitre plates (Nunc) were coated with P. aeruginosa sonicate (15 mg/ml diluted 1:1000 in PBS with 0·1% polyoxyethylenesorbitan monolaurate (Tween 20 from Sigma)) for 1 h at 37°C. The plates were washed in PBS with 0·1% Tween 20, blocked overnight with 0·1% goat serum in PBS with 0·1% Tween 20, washed again, and serum samples diluted 1:20 in PBS with 0·1% Tween 20 were added and incubated for 1h at 37°C. The plates were then washed and incubated for 1h at 37°C with peroxidase-conjugated rat anti-mouse (Nordic Immunology, Netherlands) diluted 1:10 000 in PBS with 0·1% Tween 20. After repeated washings, substrate solution (tetramethylbenzidine (TMB from Pharmingen)) was added and incubated until there was a clear colour reaction. The reaction was stopped with H2SO4 and the mean absorbance at 450 nm from double determinations was registered using an ELISA reader. The background read at 570 nm was subtracted. The results were presented as the relative increase above a standard obtained with serum from non-infected control mice of the relevant mice strains.
Statistical analysis
Statistical calculations were performed using Statview (Abacus Concepts, Berkeley, CA, USA). The chi-square test was used when comparing qualitative variables and the Mann–Whitney U-test was used when comparing quantitative variables. P ≤ 0·05 was considered statistically significant.
RESULTS
Improved outcome of re-infection in the susceptible BALB/c mouse strain
Significantly more singly-infected mice died compared with re-infected mice (24/39 singly-infected versus 3/23 re-infected; Fig. 1, P < 0·005).
Fig. 1.
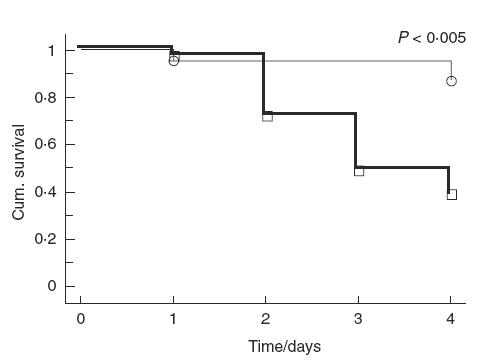
Cumulative survival of BALB/c mice after (—) single or (—) re-infection with alginate-embedded Pseudomonas aeruginosa in the lungs. Significantly more singly-infected BALB/c mice died than re-infected mice (P < 0·005). Event times (○) re-infected, (□) single.
The highest number of bacteria was isolated from the lungs of singly-infected mice (median 2·1 × 106 cfu/ml, range 0–5·8 × 106cfu/ml) compared with re-infected mice (median 4·8 × 102 cfu/ml, range 0–2·3 × 103cfu/ml), although this was not statistically significant.
Reduced lung inflammation in re-infected mice
All singly-infected mice had a PMN-dominated inflammation. Re-infected mice had reduced lung inflammation, since 6/8 re-infected mice had mixed inflammation or no signs of inflammation (P ≤ 0·005, Table 1).
Table 1.
Type of lung inflammation in the lungs of singly-infected or re-infected BALB/c mice. The inflammation was typed as acute (>90% PMNs), chronic (>90% MN), both types present, neither dominating (PMN/MN) or no inflammation (NI). Significantly more re-infected BALB/c mice had a milder inflammatory response compared with singly-infected BALB/c mice (P < 0·005)
| Singly-infected (n = 6) | Re-infected (n = 8) |
|---|---|
| PMN (n = 6) | PMN (n = 2)* |
| MN/PMN (n = 0) | MN/PMN (n = 5) |
| MN (n = 0) | MN (n = 0) |
| NI (n = 0) | NI (n = 1) |
Significantly fewer re-infected BALB/c mice with a PMN-dominated inflammation compared with singly-infected BALB/c mice (P ≤ 0·005).
Changes in numbers and proportions of cells after single or re-infection
The total number of cells in BALF from all mice was determined and was significantly higher in both singly- and re-infected mice compared with non-infected mice (P < 0·02; Fig. 2). The total number of cells in the lung tissue was determined after enzyme degradation but this did not differ significantly between the three groups (Fig. 2).
Fig. 2.
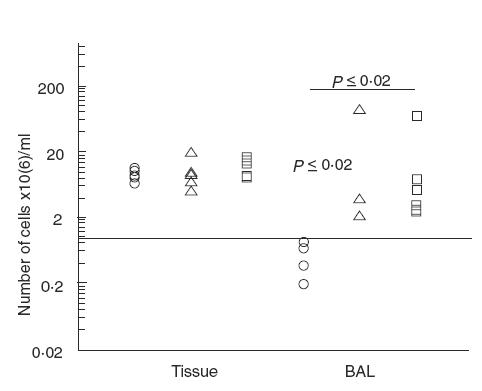
Total number of cells in the lungs. Numbers of nucleated cells were determined after staining with crystal violet. Significantly increased numbers of cells after infection with alginate-embedded Pseudomonas aeruginosa were observed in the BALF (P ≤ 0·02). Νο differences in cell numbers between the groups were observed in the lung tissue. (○) BALB/c non-infected (n = 5 (4 BAL)); (□) BALB/c re-infected (n = 6); (▵) BALB/c singly-infected (n = 5 (4 BAL)).
No significant differences were observed in the proportions of CD4 cells in BALF or lung cell suspensions (data not shown).
The number of CD4+ cells was obtained by multiplication of the cell number with the CD4 proportion. In both BALF and lung cell suspensions from re-infected mice, an increased number of CD4 cells was observed compared with non-infected mice (P < 0·02; Fig. 3). In addition, the number of CD4 cells was increased in BALF from singly- compared with non-infected mice (P < 0·05; Fig. 3).
Fig. 3.
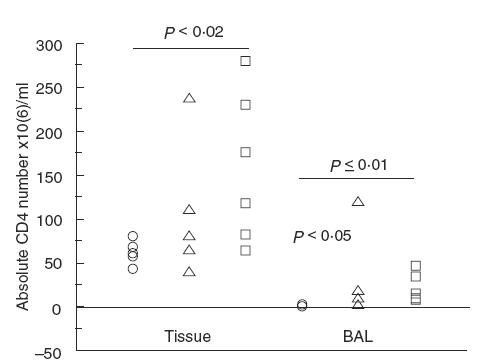
The number of CD4 cells in the lungs of singly-infected, re-infected or non-infected BALB/c mice. Re-infected mice had significantly of CD4 cells in the lungs compared with non-infected mice (tissue: P < 0·02 and BAL: P ≤ 0·01). In addition, singly-infected mice had increased numbers of CD4 cells in BALF (P < 0·05). No significant differences were observed between the singly- and re-infected groups. (○) BALB/c non-infected (n = 5 (4 BAL)); (□) BALB/c re-infected (n = 6); (▵) BALB/c singly-infected (n = 5 (4 BAL)).
Relationship between Th1 response and LFA-1 surface expression
The level of surface expression of LFA-1 on CD4 cells in BALF from infected mice was compared with the levels on blood CD4 cells from the same mice, since there were too few CD4 cells in the BALF from non-infected mice for comparison. The level of surface expression of LFA-1 on CD4 cells in BALF and lung tissue was increased in both singly- and re-infected mice compared with non-infected mice (P < 0·03; Fig. 4). Moreover, the surface expression of LFA-1 was increased on CD4 cells from the lung tissue in re-infected compared with singly-infected mice (P < 0·02; Fig. 4).
Fig. 4.
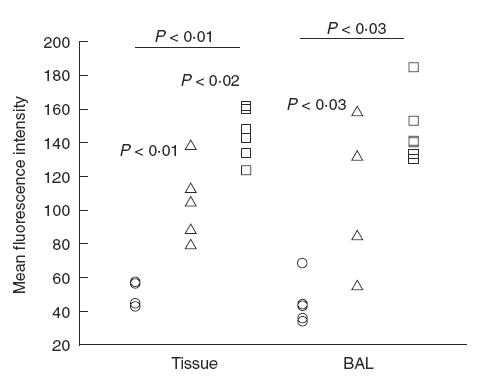
Surface expression of LFA-1 on CD4 cells in the lungs as analysed by FACS and presented as mean fluorescence intensity. An increase in LFA-1 surface expression on CD4+ cells from infected mice was observed both in tissue and BALF (P < 0·03). Moreover, an increase in LFA-1 surface expression on CD4+ cells from lung tissue was observed after re-infection as compared with single infection (P < 0·3). (○) BALB/c non-infected (n = 5 (4 BAL)); (□) BALB/c re-infected (n = 6); BALB/c singly-infected (n = 5 (4 BAL)).
Re-infection induces a Th1-dominated cytokine response in BALB/c mice
IL-4. Anti-CD3-induced (T-cell produced) IL-4 was significantly decreased in re-infected mice compared with non-infected or singly-infected mice (Table 2, P < 0·05). IL-4 concentrations in supernatant fluids were low or undetectable after OMP stimulation.
Table 2.
Release of IL-4 and IFN-γ from lung cells. Lungs were enzymatically-digested and cells stimulated for 6 h with anti-CD3. The concentration of cytokines was determined by ELISA. The IL-4 levels decreased significantly in re-infected BALB/c mice compared with non-infected BALB/c mice (P < 0·05). The IFN-γ levels were significantly increased in singly- and re-infected mice compared with non-infected mice(P < 0·01). Medians are presented with the range in parenthesis
| Non-infected median (range) | Singly-infected median (range) | Re-infected median (range) | |
|---|---|---|---|
| IL-4 | 42 (25–56 pg/ml) | 39 (25–88 pg/ml) | 23·5 (0–40 pg/ml)* |
| IFN-γ | 23 (0–90 pg/ml) | 350 (230–690 pg/ml)† | 465 (140–1110 pg/ml)† |
Significantly lower compared with non- or singly-infected mice (P < 0·05).
Significantly higher compared with non-infected mice (P < 0·01).
IFN-γ. Anti-CD3-induced IFN-γ production was significantly higher in both re-infected and singly-infected mice compared with non-infected mice (Table 2, P < 0·01). No significant differences were observed between singly- and re-infected mice.
IL-4/IFN-γ ratio. The ratio between anti-CD3-induced IL-4 and IFN-γ was significantly decreased in re-infected compared with singly-infected mice (P < 0·007; Fig. 5).
Fig. 5.
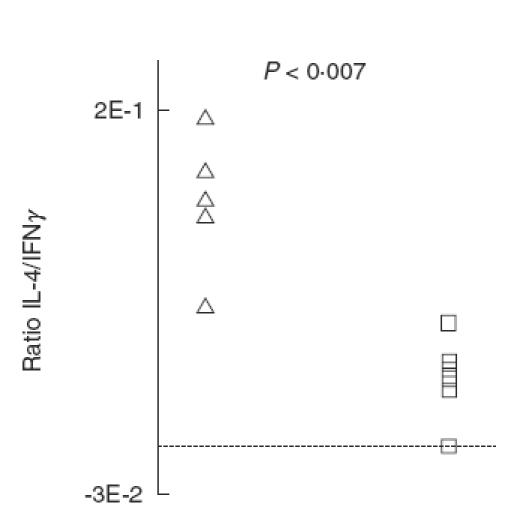
The ratio between pulmonary IL-4 and IFN-γ release from mice singly- or re-infected with Pseudomonas aeruginosa lung infection. Lungs were enzymatically-digested and the isolated cells were stimulated for 6 h with anti-CD3. The cytokine concentration was determined by ELISA. The ratio decreased significantly on re-infection of BALB/c mice (P < 0·007). (□) BALB/c re-infected (n = 6); BALB/c singly-infected (n = 5).
OMP-induced (antigen-specific) IFN-γ production was significantly increased in re-infected mice compared with non-infected and singly-infected mice (P < 0·05; Fig. 6a). In addition, IFN-γ production was significantly higher in singly-infected BALB/c mice compared with non-infected mice (P < 0·01; Fig. 6a).
Fig. 6.
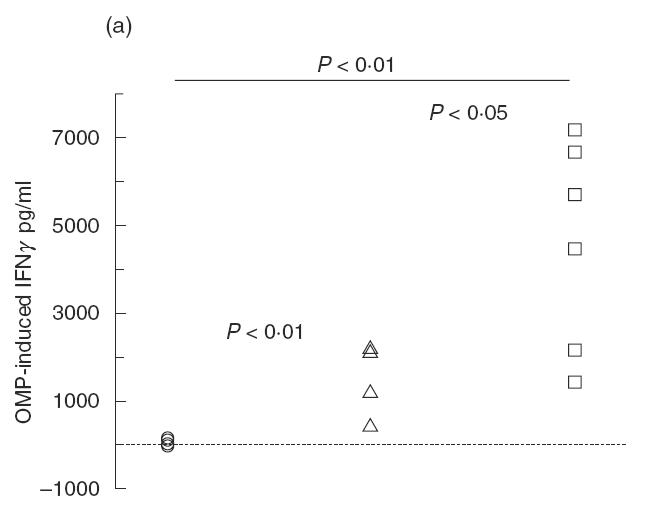
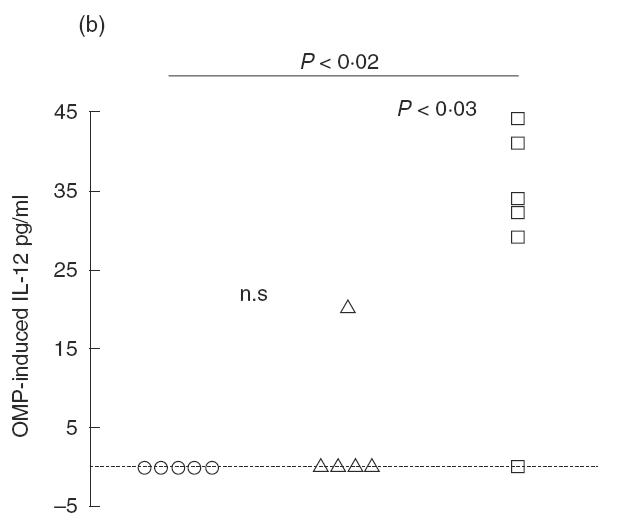
(a) Pulmonary IFN-γ release from mice singly- or re-infected with Pseudomonas aeruginosa in the lungs. Lungs were enzymatically-digested and the isolated cells were stimulated for 48h with P. aeruginosa OMP. The cytokine concentration was determined by ELISA. Both singly- and re-infected mice had significantly increased IFN-γ levels compared with non-infected mice (P < 0·01). However, re-infected mice had increased IFN-γ levels compared with singly-infected mice (P < 0·01). (b) Pulmonary IL-12 release from mice singly- or re-infected with P. aeruginosa in the lungs. Isolated cells from enzymatically-digested lungs were stimulated with P. aeruginosa OMP for 48 h. The cytokine concentration was determined by ELISA. Re-infected mice had significantly increased IL-12 levels compared with non-infected mice (P < 0·02) and singly-infected mice (P < 0·03). (○) BALB/c non-infected (n = 5); (□) BALB/c re-infected (n = 6); (▵) BALB/c singly-infected (n = 5).
IL-12. OMP-induced IL-12 production was low or undetectable in singly-infected mice (Fig. 6b). In contrast, IL-12 production was increased in BALB/c mice after re-infection compared with non-infected and singly-infected mice (P < 0·03; Fig. 6b).
No significant changes in the IgG response upon re-infection
No significant increases in total IgG production against P. aeruginosa were observed following repeat exposure, since the IgG response increased at comparable levels in singly-infected (median 1·61, range 1·38–2·08) and re-infected mice (median 1·85, range 1·48–2·38).
DISCUSSION
In contrast to mice models of leishmania infections where the micro-organism can survive for months, the limited duration of the chronic phase to only 2 weeks for the alginate or agar P. aeruginosa infection models prevents a significant discrimination between the contributions of the innate and the acquired immune response [19,25–27]. Therefore, this study was designed to amplify the influence of the adaptive immune response by re-infecting mice when the Th cells were highly activated.
Since the Th-cell response polarizes during maturation on re-infection or in the later stages of chronic infections, it was hypothesized that susceptible BALB/c mice would become more susceptible and present with a more pronounced Th2 response on re-infection with P. aeruginosa [13,28]. Surprisingly, increased resistance to the alginate-embedded P. aeruginosa lung infection was observed. More importantly, this improved outcome of infection was accompanied by changes in the Th-cell response, whereas no significant changes in IgG production were observed. Re-infection of BALB/c mice was accompanied by a shift to a more Th1-dominated response, as seen by the decreased IL-4/IFN-γ ratio and the increased OMP-induced IFN-γ production. In addition, the induced shift to a Th1 response in re-infected BALB/c mice was paralleled by reduced inflammation. In accordance, a shift from a pulmonary Th2 to a Th1 response and decreased lung inflammation after repeated challenge with sheep red blood cells (SRBC) in a mouse model of inappropriately-regulated pulmonary immune responses was recently reported [29]. Indeed, IL-12 and, especially, IL-18 increased after repeated challenge [29]. Moreover, a change in inflammation from being PMN-dominated to one dominated by mononuclear cells was induced by IFN-γ treatment of rats with chronic P. aeruginosa lung infection [30]. In accordance, CF patients without chronic lung infection revealed a Th1-dominated response compared with chronically infected CF patients [15]. In contrast, IL-4 treatment has been reported to improve the outcome of acute P. aeruginosa lung infection [31]. However, the IL-4 treatment was performed in an acute infection model with planktonic bacteria, and the outcome was evaluated within 24 h after challenge [31]. Thus, the findings are not comparable with the present results.
The surprising Th1 response in BALB/c mice might be due to the time chosen for re-infection. The previously reported Th2-dominated response in BALB/c mice was observed 7 days after a single infection, whereas the mice in the present study were re-infected on day 14. IL-4 is undetectable in supernatant fluids on day 14 after a single infection, whereas IFN-γ is produced at considerable levels (own observation). Day 14 was chosen for re-infection as the mortality due to the infection procedure is too high if the mice are re-infected earlier (own observation). Whether the cytokine response will differ if the mice are re-infected at another time point remains to be investigated.
Interestingly, the BALB/c mouse strain has been found to be resistant in another biofilm model of P. aeruginosa lung infection [26,27]. However, this model was established with a different bacterial strain and with the use of agar instead of alginate [26,27]. Furthermore, in the agar model, the BALB/c mouse strain is relatively resistant in comparison with the C57BL/6 mouse strain, which is relatively susceptible in the alginate model (own observation); there are no reports on the course of infection with the C3H/HeN mouse strain in the agar model.
The increase in OMP-induced IFN-γ production in re-infected BALB/c mice suggests that the antigen-specific clones were activated during the first infection, and subsequently selected and expanded upon re-infection [32]. Indeed, CD4 cells have been reported to be clonal, due to their antigen receptor, but heterogenous with respect to their cytokine production [32,33]. Change to a strongly polarized Th1 or Th2 response must be induced early to be effective, supporting the idea that the Th1 cells are generated during the first infection [34–36].
IL-4 levels in infected mice did not exceed the levels of non-infected mice; they were even found to be decreased in the re-infected BALB/c mice, whereas IFN-γ levels were significantly increased in all infected mice compared with non-infected mice. This was surprising since we have previously reported significantly increased IL-4 production in singly-infected mice compared with non-infected mice of both strains [20]. In the present study, IL-4 production was estimated on day 4, in contrast to the previous studies where IL-4 production was estimated on day 7. IL-4 production increases significantly from day 3 to day 7 (own observations). Furthermore, the previous studies on cytokine production were not preceded by BAL. Thus, by performing BAL, a selective flushing of Th-cell subsets may occur, favouring retention of Th1 cells in the endo-bronchial volume; Th1 cells might adhere better, since IFN-γ levels were increased compared with non-infected mice [37,38].
The importance of T cells in mice infected with P. aeruginosa has previously been reported [39–41]. Interestingly, a direct and strain-specific killing effect of T cells on the bacterium was reported, as well as a similar effect when using the supernatant fluid from cultured T cells from BALB/c mice infected intraperitoneally with P. aeruginosa [39]. Moreover, the killing was antibody independent [39]. Likewise, transfer of T cells from exposed or vaccinated mice or rats has been shown to generate immunity by an immunoglobulin-independent mechanism, as well as in granulocytopenic mice [40,42].
LFA-1 surface expression on CD4 cells increased simultaneously with the shift to a Th1 response in re-infected BALB/c mice. Reports on differential recruitment of Th1 and Th2 cells have been published [43]. P- and E-selectins primarily seem to recruit Th1 cells, and blocking LFA-1/ICAM-1 or LFA-1/ICAM-2 in transgenic mice leads to a 15–40-fold increase in the level of Th2 cytokines, probably by modifying the contact with the antigen-presenting cells [37,38].
Increased IL-12 production paralleled the shift in the Th-cell response in re-infected BALB/c mice. A pivotal role for endogenous IL-12 in improving the outcome of infectious diseases has been reported in several infection models, and exogenous IL-12 treatment of mice has yielded improved outcomes, especially for intracellular infections [44–46]. Whether IL-12 treatment of mice chronically infected with an extracellular micro-organism such as P. aeruginosa in the lungs could induce a Th1 response and thus, improve the resistance to infection, has to be investigated, although IL-12 treatment of mice with Klebsiella pneumoniae lung infection has been shown to improve the outcome [47].
In conclusion, the mouse model of alginate-embedded P. aeruginosa lung re-infection is useful for investigating the role of the Th-cell response in patients with CF. The development of an improved outcome after infection, paralleled by a Th1-dominated response with increased IL-12 production, is in accordance with our observations in CF patients, and indicates a beneficial role for a Th1-dominated response in CF patients with chronic P. aeruginosa lung infection also.
Acknowledgments
The skilful technical assistance of Lars Christophersen is greatly appreciated. This research was supported in part by the Novo Nordisk Foundation and the Danish Medical Association Research Fund.
REFERENCES
- 1.Kemp M, Theander TG, Kharazmi A. The contrasting roles of CD4+ cells in intracellular infections in humans: leishmaniasis as an example. Immunol Today. 1996;17:13–6. doi: 10.1016/0167-5699(96)80562-7. [DOI] [PubMed] [Google Scholar]
- 2.van Voorhis WC, Barret LK, Lolle DM, et al. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J Infect Dis. 1996;173:495–8. doi: 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- 3.Yammamura M, Uyemura K, Deans RJ, et al. Defining protective response to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 4.Surcel HM, Troye-Blomberg M, Paulie S, et al. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 5.van Dyke TE, Lester MA, Shapira L. The role of host response in periodontal disease progression: implications for future treatment strategies. J Periodontol. 1993;64:792–806. doi: 10.1902/jop.1993.64.8s.792. [DOI] [PubMed] [Google Scholar]
- 6.Koch C, Hoiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–9. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 7.Johansen JK, Hoiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax. 1992;47:109–11. doi: 10.1136/thx.47.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baltimore RS, Christie CDC, Smith GJW. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Am Rev Respir Dis. 1989;140:1650–61. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- 9.Mathee K, Ciofu O, Sternberg C, et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–57. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen SS. Lung infection with alginate-producing mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS. 1992;100(Suppl.):1–79. [PubMed] [Google Scholar]
- 11.Hoiby N, Flensborg EW, Beck B, et al. Pseudomonas aeruginosa infection in cystic fibrosis. Scand J Resp Dis. 1977;58:65–79. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. [PubMed] [Google Scholar]
- 12.Mosmann TR, Cherwinski H, Bond MW. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 13.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen RU, Stern RC, Polmar SH. Cellular immunity to bacteria: impairment of in vitro lymphocyte response to Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1977;18:735–40. doi: 10.1128/iai.18.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser C, Kjaergaard S, Pressler T, et al. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108:329–35. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 16.Wojnarowski C, Frischer T, Hofbauer E, et al. Cytokine expression in bronchial biopsies of cystic fibrosis patients with and without acute exacerbation. Eur Respir J. 1999;14:1136–44. doi: 10.1183/09031936.99.14511369. [DOI] [PubMed] [Google Scholar]
- 17.Lucey DR, Clerici M, Shearer GM. Type 1 and Type 2 cytokine dysregulation in human, neoplastic and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–62. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 19.Moser C, Johansen HK, Song Z, et al. Chronic Pseudomonas aeruginosa lung infection is more severe in Th2 responding BALB/c mice compared to Th1 responding C3H/HeN mice. APMIS. 1997;105:838–42. [PubMed] [Google Scholar]
- 20.Moser C, Hougen HP, Song Z, et al. Early immune response in susceptible and resistant mice strains with chronic Pseudomonas aeruginosa lung infection determines the type of T-helper cell response. APMIS. 1999;107:1093–100. doi: 10.1111/j.1699-0463.1999.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen SS, Shand GH, Hansen BL, et al. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS. 1990;98:203–11. [PubMed] [Google Scholar]
- 22.Stevenson MM, Kondratieva TK, Apt AS, et al. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shand GH, Anwar H, Kadurugamuwa HJ, et al. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985;48:35–9. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filip C, Fletcher G, Wulff JL, et al. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–22. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behin R, Mauel J, Sordat B. Leishmania tropica: Pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979;48:81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- 26.Gosselin D, DeSanctis J, Boulé M, et al. Role of Tumor Necosis Factor Alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–8. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morissette C, Francoeur C, Darmond-Zwaig C, et al. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect Immun. 1996;64:4984–92. doi: 10.1128/iai.64.12.4984-4992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 29.Todt J, Sonstein J, Polak T, et al. Repeated intratracheal challenge with particulate antigen modulates murine lung cytokines. J Immunol. 2000;164:4037–47. doi: 10.4049/jimmunol.164.8.4037. [DOI] [PubMed] [Google Scholar]
- 30.Johansen HK, Hougen HP, Rygaard J, et al. Interferon-gamma (IFN-γ) treatment decreases the inflammatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol. 1996;103:212–8. doi: 10.1046/j.1365-2249.1996.d01-618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain-Vora S, LeVine AM, Chroneos Z, et al. Interleukin-4 enhances pulmonary clearance of Pseudomonas aeruginosa. Infect Immun. 1998;66:4229–36. doi: 10.1128/iai.66.9.4229-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy E, Shibuya K, Hosken N, et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–13. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Openshaw P, Murphy EE, Hosken NA, et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatelain R, Varkila K, Coffman RF. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–7. [PubMed] [Google Scholar]
- 35.Sypek JP, Chung CL, Mayor SE, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belosevic M, Finbloom DS, Van Der Meide PH, et al. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–74. [PubMed] [Google Scholar]
- 37.Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–3. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 38.Salomon B, Bluestone JA. Cutting edge: LFA–1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–42. [PubMed] [Google Scholar]
- 39.Markham RB, Powderley WG. Exposure of mice to live Pseudomonas aeruginosa generates protective cell-mediated immunity in the absence of an antibody response. J Immunol. 1988;140:2039–45. [PubMed] [Google Scholar]
- 40.Powderley WG, Pier GB, Markham RB. T lymphocyte-mediated protection against Pseudomonas aeruginosa infection in granulocytopenic mice. J Clin Invest. 1986;78:375–80. doi: 10.1172/JCI112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markham RB, Pier GB, Powderley WG. Suppressor T cells regulating the cell-mediated immune response to Pseudomonas aeruginosa can be generated by immunization with anti-bacterial T cells. J Immunol. 1988;141:3975–9. [PubMed] [Google Scholar]
- 42.Dunkley ML, Clancey RL, Cripps AW. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83:362–9. [PMC free article] [PubMed] [Google Scholar]
- 43.D’Ambrosio D, Iellem A, Colantonio L, et al. Localization of Th-cell subsets in inflammation: differential thresholds for extravasation of Th1 and Th2 cells. Immunol Today. 2000;21:183–6. doi: 10.1016/s0167-5699(00)01590-5. [DOI] [PubMed] [Google Scholar]
- 44.Heintzel FP, Schoenhaut DS, Rerko RM, et al. Recombinant IL-12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flynn JL, Goldstein MM, Triebold KJ, et al. IL-12 increases resistance of BALB/c mice to Mycobacteria tuberculosis infection. J Immunol. 1995;155:2515–24. [PubMed] [Google Scholar]
- 46.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 47.Greenberger MJ, Kunkel SL, Strieter RM, et al. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–12. [PubMed] [Google Scholar]


