Abstract
Aspergillus fumigatus, a ubiquitous fungus, is implicated in the pathogenesis of a number of clinically different allergic diseases in man, including allergic broncopulmonary aspergillosis. Peptide-based immunotherapy may offer an alternative treatment strategy for the management of allergic disease. The objective of this study was to alter the allergen-specific immune response using dominant T cell epitopes of a major A. fumigatus allergen, Asp f2, expressed in yeast as virus-like particles (VLP). The T cell epitopes of Asp f2, recognized in mice with an H-2d background, were determined by producing T-cell hybridomas. Two dominant T cell epitopes, aa60–71 and aa235–249, were identified and expressed in a yeast VLP system. To induce tolerance VLP-peptides were injected subcutaneously into mice previously immunized with recombinant Asp f2. The T cell immune response was abrogated totally in 3 weeks following a single injection of VLP but was restored 2 months later following intranasal antigen exposure. T-cell depletion resulted in the reduction of 20–30% of all antigen-specific immunoglobulin classes. Thus, recombinant peptides expressed in the VLP system can be used successfully in the modulation of Asp f2-induced immune response in mice, although a single administration is not sufficient to maintain a state of tolerance for a long period of time.
Keywords: allergy, Aspergillus fumigatus, immunotherapy peptide-induced tolerance, virus-like particle
INTRODUCTION
Allergic diseases result from chronic activation of the immune system induced by proteins penetrating through epithelial and mucous membrane barriers. In many instances, innocuous proteins are presented by antigen-presenting cells (APC) to CD4+ T-lymphocytes, which induces a humoral immune response leading eventually to IgE production in predisposed individuals. Subsequent exposure of antigen to the immune system leads to the recognition of additional proteins from complex allergens, such as the ubiquitous fungus Aspergillus fumigatus. Disease develops upon antigen exposure and progresses subsequently to allergic asthma, hypersensitivity pneumonitis and other severe forms of disabling allergy, such as allergic bronchopulmonary aspergillosis (ABPA) [1,2].
Immunological alteration treatment modalities range from conventional specific immunotherapy (SIT) to the more modern antigenic peptide-based immunotherapy (PIT) [3–8]. These therapeutic measures are thought to induce down-regulation of a specific immune response resulting from peripheral tolerance induction in CD4+ T cells. SIT involves the administration of a series of subcutaneous injections of progressive doses of allergen until a safe maintenance dose is achieved that generates an effective result. The maintenance period needed to achieve tolerance may take as long as 3 years or more. The disadvantages of SIT are its questionable effectiveness, the long time required to decrease allergic responses and occasional adverse side-effects, including systemic anaphylaxis. Usually PIT is less harmful as the short linear peptides from allergens used for immunotherapy represent T cell epitopes and may not cause anaphylaxis. It has been shown that the peptides representing T cell epitopes often contain hydrophobic amino acids and form amphipathic helixes while B cell epitopes contain mainly hydrophilic amino acids [9,10]. PIT has been shown to be effective in controlling bee venom, birch pollen and cat dander allergy in humans [4–8].
Peripheral tolerance induction is caused by the interaction of APC displaying specific antigenic peptides on their surface with CD4+ T-cells in the absence of sufficient co-stimulatory signals [11,12]. The recognition of self peptides by the T cell receptor (TCR) in the absence of co-stimulatory molecules induces a state of anergy in T cells [12]. When peptides or proteins that have originated from allergens are injected subcutaneously (s.c.) or intraperitonally (i.p.) in a soluble form, and at low concentration, they induce anergy in allergen-specific T cells. The results from reported studies indicate that in allergic patients 3 or more years of SIT are needed to achieve successful therapy. In addition, this procedure may not yield successful results in all patients treated. Although the results of clinical trials with Fel dI and bee venom allergens are promising, the results indicate that PIT will not shorten this time significantly.
In the present study, we have investigated the effect of a new delivery vehicle for PIT using yeast virus-like particles (VLP). We used VLP containing T cell-dominant epitopes of Asp f2, a major allergen from A. fumigatus, the predominant fungus associated with a number of clinically different allergic diseases [1,2,13,14]. VLP are particles each formed by about 300 copies of the yeast p1 protein [15,16]. VLP have been shown to demonstrate adjuvant activity due to their size and have been used to express small proteins and peptides on the p1 protein at the C-terminal end [17–21]. It has been shown that VLP can be used as vehicles for peptide delivery to modulate the allergic immune response [21].
MATERIALS AND METHODS
Animals
Ten to 12-week-old inbred BALB/c (H-2d) mice purchased either from Charles River or bred at the Institute of Bioorganic Chemistry Animal Department, Moscow, were used in this study. The use of mice in the present investigation was approved by the Institutional Committee. As BALB/c mice have been reported to show both humoral and cellular immune responses in the ABPA model, this strain of mice was used in the present study [22,23].
Antigens
Recombinant Asp f2, a 37-kDa major antigen from A. fumigatus (ATCC 42202) was cloned and expressed in Escherichia coli using the pET vector [13,14]. The C-terminal histidine tag was used to purify the allergen by Ni-affinity chromatography. The crude antigen extract from A. fumigatus was prepared as described previously [1]. A mixture of culture filtrate and mycelial extracts of A. fumigatus was diluted to 5 mg/ml and kept frozen at –20°C.
Twelve T and B cell epitopes were predicted from the Asp f2 amino acid sequence using computer software [9,10]. These 11–22 mers were synthesized commercially using F-moc chemistry (Alpha Diagnostic Int., San Antonio, TX, USA). The sequences of these peptides are shown in Table 1.
Table 1.
Sequences of Asp f2 synthetic peptides
| Peptide | Position | Peptide sequence | Type of epitope |
|---|---|---|---|
| P1 | aa3–24 | GAVTSFPIHSSCNATQRRQIEA | |
| P2 | aa31–42 | ELARHAKAHILR | |
| P3 | aa48–58 | EIYRKYFGNRP | T epitope |
| P4 | aa60–71 | MEAVGAYDVIVN | T epitope |
| P5 | aa92–106 | GWGGHWRGANATSET | |
| P6 | aa113–127 | YTTRRWLVSMCSQGY | |
| P7 | aa140–158 | SDLMHRLYHVPAVGQ | B epitope |
| P8 | aa159–169 | GWVDHFADGYDEVIA | |
| P9 | aa172–186 | KSNGTESTHDSEAFE | |
| P10 | aa214–226 | GHDTGSASAPAST | |
| P11 | aa235–249 | SGSGATTTPTDSPSA* | T epitope |
| P12 | aa254–268 | PSNCHTHEGGQLHCT | B epitope |
Dominant T and B cell epitopes are shown in bold.
VLP fusion protein production
The yeast strain Saccharomyces cerevisiae YBS164 was used to produce recombinant VLP. Cells were cultured in a synthetic mineral medium as described previously [24]. Transformed cells were grown in YPDG medium at 30°C in a shaker incubator at a speed of 250 r.p.m. Recombinant DNA encoding the peptides was prepared and purified as described elsewhere [25,26]. DNA fragments encoding amino acids 1–380 of p1 protein from transposon Ty1 in S. cerevisiae [27] were obtained by PCR using the chromosomal DNA of the yeast (YBS164) as a template. The specific sense primer: 5′-aaaaccatggaatcccaacaattatct and antisense primer: 5′-gcaggatcctttgggtttggttgtattcg with NcoI and BamHI sites were used to obtain the desired DNA fragment by PCR subsequent to cloning in the expression vector. The NcoI/BamHI DNA fragment encoding the Ty A transposon gene YMLTy1–3 was cloned using the E. coli–S. cerevisiae shuttle vector PDX [25]. The resultant plasmid pPDX-TyA placed the Ty A gene under the control of the GAL1 promoter. Double-stranded DNA pieces, encoding either peptide 4 or 11 from Asp f2 were prepared by PCR using the following primer pairs:
P4-1: 5′-gatctatggaagcagttggtgcatatgatgtaatagtaaatg;
P4-2: 5′-gatccatttactattacatcatatgcaccaactgcttccata;
P11-1: 5′-gatcttctggttcaggtgctactactactccaactgattcaccatcag;
P11-2: 5′-gatcctgatggtgaatcagttggagtagtagtagcacctgaaccagaa.
Each DNA piece was then inserted in-frame at the BamH1 site within the Ty A gene in the plasmid pPDX-Ty A. This allowed expression of the original peptide fused to the carboxy-terminus of the viral particle protein p1. The resultant fusion proteins have been named VLP4 and VLP11. To obtain a particle with two T cell epitopes, fragments P11-1 and P11-2 were ligated into the BamHI site of the pPDX-TyA-CP4 plasmid coding for the VLP4 protein. The resulting construct encodes the fusion protein containing both VLP-4 and VLP11. All DNA constructs were sequenced and their integrity verified. VLP containing only p1 without inserts (VLP0) was used as a control. Extraction and purification of hybrid VLP was carried out as described previously [28].
T hybrid production
Mice were immunized by s.c. injection of 100 μg of Asp f2 in incomplete Freund’s adjuvant (IFA) in the footpad. Animals were sacrificed on day 14, and the draining popliteal lymph nodes (LN) were collected; 5 × 106 LN cells and 1 × 106 splenocytes (as additional APC) in RPMI-10 medium were incubated in 24-well plates (Costar, Cambridge, MA, USA) in air containing 5% CO2 at 37°C for 3 days in the presence of 10 μg/ml Asp f2. In vitro activated cells were separated by Ficoll-Hypaque gradient centrifugation and fused with the myeloma cell BW5147 at a ratio of 1:1 as described before [28]. The hybrids were screened for TCR expression after staining with an antimurine TCR antibody coupled with FITC (Pharmingen) and enumerated by flow cytometry (EPICS-ELITE, France).
T cell epitope analysis
T hybrids were used to map the T cell epitopes of Asp f2. Mapping was carried out as described before [28]. Briefly, 1 × 105 T hybrid cells and 5 × 105 of mitomycin C-treated splenocytes from naive BALB/c mice were cultured for 24 h in the presence of 5 μg/ml of synthetic peptides or recombinant Asp f2 protein in 96-well plates (Costar). Supernatants (SN) from the cultures were collected after 24 h and frozen until analysed. The frozen SN were thawed and studied for IL-2 production in a bioassay using an IL-2 dependent CTLL-2 cell line as described before [30].
Assay of IL-2
Briefly, 5 × 103 CTLL-2 cells/well in 50 μl of RPMI 10 and 50 μl of SN were cultured for 48 h in 96-well round-bottom plates (Costar). 3H]-thymidine was added for the last 6 h of incubation. Cells were transferred onto glass filters and 3H]-thymidine incorporation estimated by liquid scincillation counting. As some T hybrids produced low levels of IL-2 spontaneously, antigen-induced IL-2 production was estimated as an index of stimulation, calculated as the ratio of counts per minute (c.p.m.) in antigen-stimulated:unstimulated cultures.
Tolerance induction protocols
Four groups of five mice each (4 weeks old) were immunized with 30 μg of Asp f2 in IFA in the right hind footpad of each animal. Immunizations were carried out twice at monthly intervals, and following this the mice were allowed to rest for 2 months. The antibody response was checked before attempting to induce tolerance to ensure the persistence of Asp f2-specific memory cells. Tolerance was induced by s.c. injection of 500 μg of total protein per mouse of an equimolar mixture of VLP4, VLP11 and VLP-4–11 in PBS. This dose of VLP approximately corresponds to10 μg of peptides. Control mice received the same amount of VLP0. After 1 week of rest, mice were sensitized intranasally with a crude A. fumigatus extract as described previously [22]. Briefly, animals were lightly anaesthetized using Metofane aerosol (Methoxyflurane, Pitman-Moore, Mundelein, IL, USA) and 7 μg of A. fumigatus extract per injection in 20 μl of PBS was instilled in the nostrils using a pipetor tip. Injections were given five times a week for 3 weeks (total dose 100 μg per mouse). Five days after the last injection, mice from control and experimental groups were sacrificed and their blood, draining LN cells and spleens were collected. Splenocytes and LN cells were isolated and studied for their functional responses, including cytokine production. Serum separated from the blood was studied for total IgE and antigen-specific IgG antibody in the sera.
Two other groups of mice were used to determine the duration of tolerance induced from a single VLP-mix injection. After a rest of 1 month, mice were subjected to another series of allergen exposures i.n. as described above. Mice were sacrificed 5 days after the last exposure and their T and B cell responses were studied.
Spleen and lymph node cell cultures
Single cells from the spleens and LN were isolated as described previously [22,29]. Cells were washed and cultured at 3 × 105 cells per well in 0·2 ml RPMI 10 medium (Gibco-BRL) in 96-well flat-bottomed tissue culture plates (Linbro, UK). The cells were incubated in the presence of various concentrations of Asp f2 antigen in a CO2 incubator at 37°C. As LN contain low levels of APC, untreated peritoneal exudate cells obtained from the same animals were added at a concentration of 2 × 103/well. MHC class II restriction was analysed by blocking the IAd molecule with MK-D6 hybridoma (ATCC, HB-3) supernatant. Cell cultures were incubated for 24h and SN were collected to determine IL-2 production.
ELISA
Total IgE and antigen-specific antibody isotypes belonging to IgG1, IgG2a, IgG2b and IgG3 were studied using reagents from Pharmingen (San Diego, CA, USA) according to the manufacturer’s instructions (Pharmingen) [29]. In brief, 2 μg/ml of Asp f2 was coated onto the ELISA plates (Costar) in PBS. All incubations were performed in 1% BSA in PBS (BSA-PBS) at room temperature. Serum dilutions made in BSA-PBS were added to the antigen-coated wells and incubated for 2 h. The plates were then washed and biotinylated isotype-specific antimouse antibody was added and incubated. This was followed by washing and the addition of streptavidin-conjugated horseradish peroxidase (HRP) to the wells. After further incubation and washing, the colour was developed with orthophenylene diamine and the optical density determined using an ELISA reader (Multiscan MCC/340). The results are presented as net optical density values after subtracting the blanks. B cell epitopes of Asp f2 were determined by a peptide ELISA. Plates were coated overnight with 5 μg/ml of Asp f2 synthetic peptides (Table 1) dissolved in carbonate buffer, pH 9·0. All incubations were performed in PBS-Tween 20 for 1 h at room temperature as described above. Finally, HRP conjugated antimouse IgG antibody was added for 1 h and the colour developed after incubation and washing. The optical density was determined as described above using an ELISA reader.
RESULTS
T and B cell epitopes of Asp f2
Earlier we have shown that s.c. immunization of BALB/c mice with Asp f2 results in a mature IA-restricted Th2 immune response and antigen-specific IgG1 production [31]. In order to identify the dominant T cell epitopes of Asp f2 we produced T hybrids, some of which recognized the Asp f2 protein and synthetic peptides (Table 1). Peptides spanning 67% of Asp f2 sequence were selected as T or B cell epitopes based on computer algorithm prediction [9,10]. Three dominant T cell epitopes, P3, P4 and P11, were recognized by 22 antigen-specific T cell hybrids (Fig. 1). Splenocytes from individual mice usually recognized P11 and either P3 or P4. Some mice recognized all three dominant epitopes and various subdominant peptides (data not shown). The dynamics of the primary immune response of B cells was studied to compare the dominant T and B cell epitopes. In the present study, only two major B cell epitopes, P7 and P12, were recognized during the primary immune response (Fig. 1).
Fig. 1.
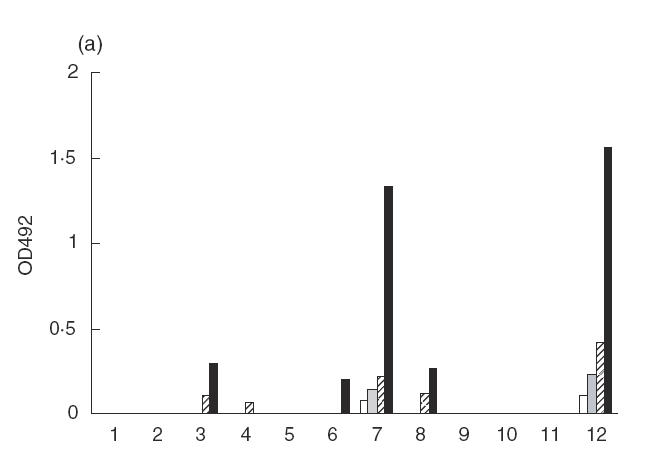
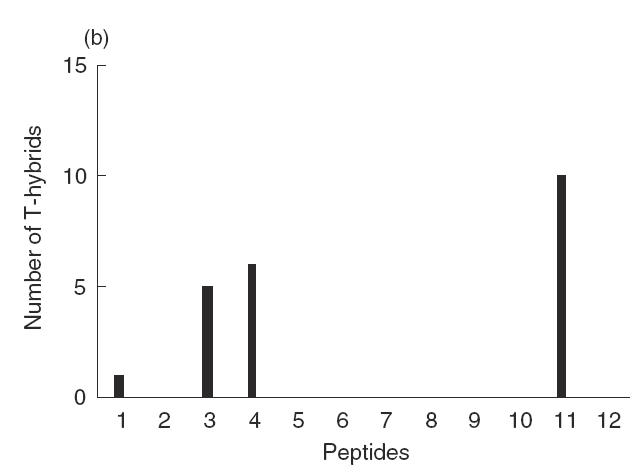
B and T cell epitopes of Asp f2. (a) Sera from mice immunized with Asp f2 were collected during the early immune response and linear B cell epitopes were analysed by ELISA using synthetic peptides as the coating agents. (b) T hybrids were produced by fusion of immune lymph node (LN) cells and TCR negative myeloma cells. The numbers of T hybrids recognizing different synthetic peptides are presented. □, 5 days;  , 10 days;
, 10 days;  , 15 days; ▪, 25 days.
, 15 days; ▪, 25 days.
Characterization of the mature T cell immune response to Asp f2
The memory immune response to Asp f2 was induced by two repeated s.c. immunizations at 1-month intervals. After 2 months of rest, the mice were subjected to 3 weeks of low dose exposure i.n. with A. fumigatus extract and, a week later, the T cell immune response was studied in vitro using cells from popliteal LN and pooled submaxillary LN and spleen cells. Asp f2 specific IL-2 production was observed only in spleen cells and was restricted mainly by the IA molecule (Fig. 2a). No immune response was observed in popliteal or submaxillary LN cells (data not shown). Dominant peptides were also recognized in vitro by splenocytes from mice immunized with the VLP mix (Fig. 2b). The peptide-induced response was found to be consistently IA restricted (Fig. 2b).
Fig. 2.
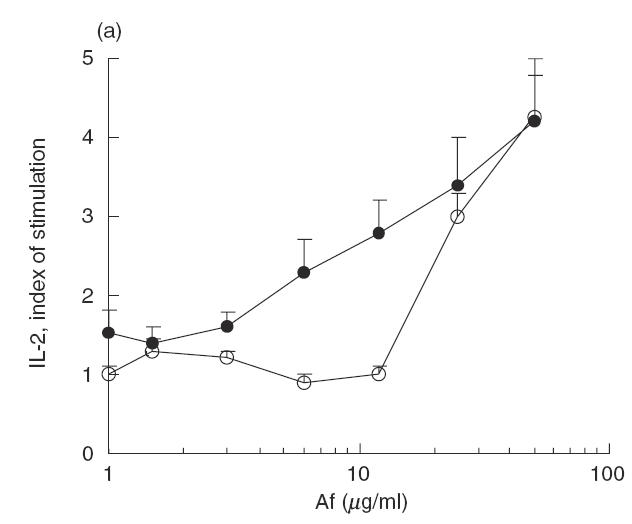
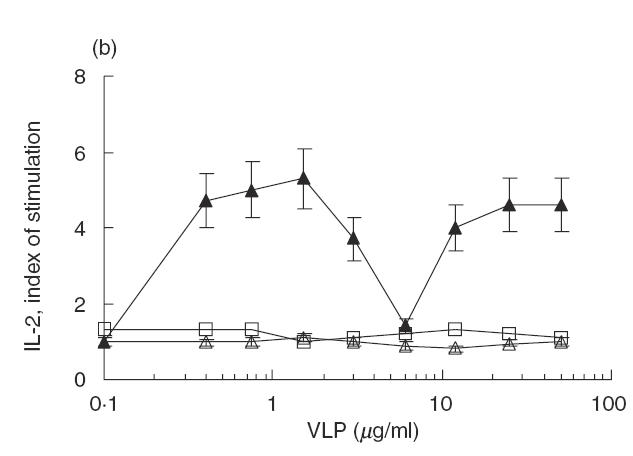
The immune response of T cells to Asp f2 and recombinant peptides. (a) MHC class II restriction of Asp f2-specific immune response was studied in vitro in the presence of anti-IAd antibodies. •, Asp f2; ○, Aspf2 + aIA. (b) LN cells from mice immunized with Asp f2 can recognize peptides representing the dominant T cell epitopes of Asp f2 expressed as a VLP mix but not the VLP vehicle alone. The immune response to the VLP mix containing Asp f2 peptides was IA restricted. ▴, VLP mix; ▵, VLP mix + aIA; □, VLP0.
Induction and duration of tolerance
We evaluated the effect of a single exposure to peptide epitopes expressed in VLP on the induction and duration of tolerance. The control group received VLP0 while the experimental group was injected with an equimolar VLP mix of peptides VLP4, VLP11 and VLP-4–11. After a week of rest, the mice were subjected to a series of immunizations i.n. with A. fumigatus extract (100 μg per series). The T and B cell immune responses were analysed 1 week after the last i.n. dose in short-term control and experimental groups of mice. Upon in vitro challenge with Asp f2, splenocytes from the control group of mice responded by producing IL-2 whereas splenocytes from the experimental group failed to respond in this way following challenge with the recall antigen (Fig. 3a).
Fig. 3.
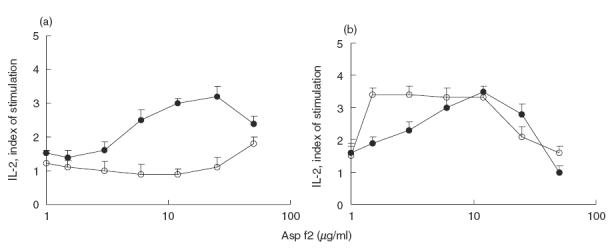
IL-2 production by T cells. Tolerance induction in T cells following a single injection of T cell dominant epitopes expressed in VLP. (a) VLP mix was injected s.c. to previously immunized mice and 1 week later the animals were challenged i.n. with A. fumigatus extract containing Asp f2. B. The long-term effect of the single injection of VLP-mix containing dominant T cell epitopes of Asp f2. Immune and VLP-treated mice were subjected to a second series of i.n. immunization 2 months after VLP treatment. The immune response was analysed a week after the last immunization and 3 months after the VLP mix injection. •, Asp f3 + VLP0; ○, Asp f2 + VLP mix.
A month later mice belonging to the long-term groups were challenged repeatedly with A. fumigatus extract as before. A week later the T and B cell immune responses were compared. There were no significant differences in the T cell immune responses observed between the peptide-treated and control groups at this time (Fig. 3b).
To analyse the effect of a single injection of VLP peptides on the B cell immune response, the production of antigen-specific immunoglobulins was studied. IgG2a, IgG3 and IgE production tested a month after injection showed a marked decrease in the experimental group, while IgG1 and IgG2b showed no significant reduction (Fig. 4).
Fig. 4.
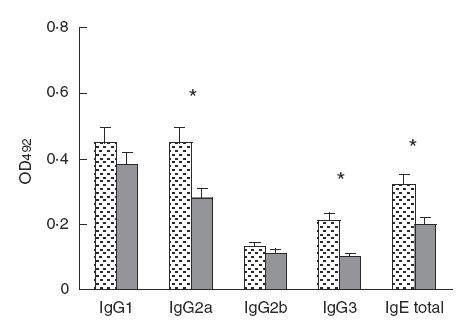
Effect of a single injection of Asp f2-dominant T cell peptides, expressed in VLP, on the B cell immune response. Antigen-specific IgG and total IgE content were determined by ELISA in sera from VLP0 and VLP mix-treated animals 1 month after VLP injection. A P-value of < 0·05 is considered as statistically significant and is indicated by an asterisk.  , Asp f2 VLP0;
, Asp f2 VLP0;  , Asp f2 + VLP mix.
, Asp f2 + VLP mix.
The dynamics of antigen-specific IgG production are shown in Fig. 5. Antibody levels slowly decreased a month after peptide treatment in comparison with the control group. New cycles of exposure i.n. to A. fumigatus enhanced antigen-specific IgG in both groups, although to a much lesser extent in the peptide-treated group. The antibody response in control mice treated with VLP0 also showed a slight decrease in comparison with the untreated group (data not shown).
Fig. 5.
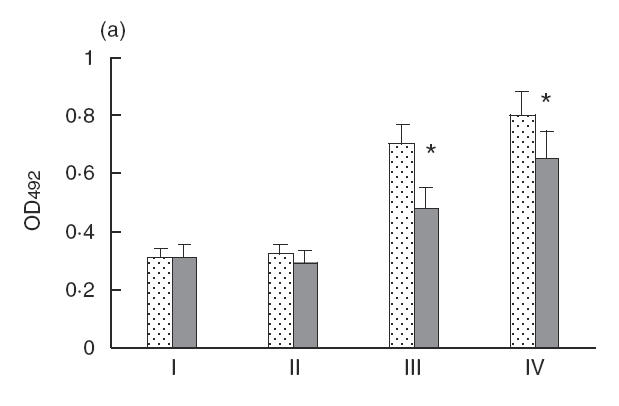
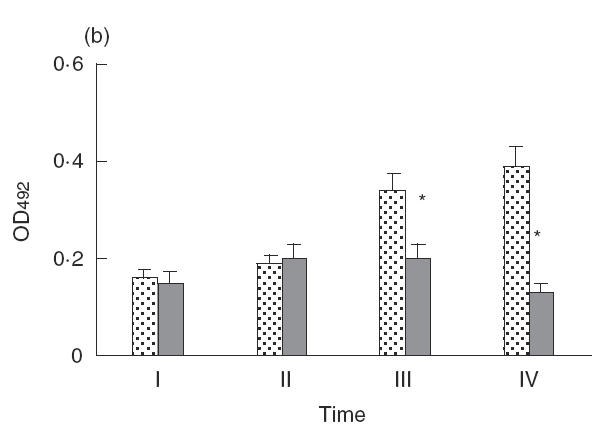
The dynamics of antigen-specific IgG (a) and total IgE (b) production determined by ELISA. Sera were collected at the moment of VLP injection (point I), 2 weeks after injection (point II), 1 month after the VLP injection and 1 week after i.n. challenge (point III), and 3 months after the VLP injection and after the second series of i.n. challenge (point IV). A P-value of <0·05 is considered as statistically significant and is indicated by an asterisk.  , Asp f2 VLP0;
, Asp f2 VLP0;  , Asp f2 + VLP mix.
, Asp f2 + VLP mix.
DISCUSSION
The allergic immune response is a result of the failure of the immune system to induce either tolerance or a self-limited immune response to allergens. Although several potential approaches have been investigated to modulate the Th2/Th1 response, the most successful result by far in the treatment of allergy has been with SIT [3–8, 23]. The doses of allergen used for SIT in humans are usually very low compared to those used in animal experiments and range from 1 to 100 μg per 100 kg of weight, with an average dose of 10 μg [32]. When this dose is translated to mice, only 2–10 ng of protein should be needed to induce tolerance. The use of low doses of allergen extracts represents a compromise between avoiding possible adverse side effects and inducing tolerance in T cells; higher doses of allergens have been shown to be more effective for tolerance induction, but the possibility of adverse reaction increases with these doses. A balance can be accomplished by the use of either short synthetic peptides or polypeptides containing T cell epitopes of the allergens [4–8].
It has become clear that short synthetic peptides have to be used in high doses due possibly to their significant loss in the body without any effect on the immune system. In order to increase the length of time therapeutic peptides remain in circulation, new vehicles with relevant capabilities need to be developed. In the present study we have demonstrated that yeast VLP expressing T cell-dominant epitopes of Asp f2 can be used effectively to modulate the immune response to Asp f2. To eliminate the possible side-effects resulting from IgE antibody-induced anaphylaxis, epitopes with T-cell binding activity expressed in VLP system were selected (Fig. 1). Two different approaches are usually used to identify T cell epitopes in a protein: by preparing overlapping synthetic peptides from sequences of the protein or by epitope prediction based on computer algorithm. The latter results in correct prediction in 70% of cases [9,10]. In the present study, we intended to obtain only a few selected epitopes, but not all dominant epitopes, hence, we used computer prediction to identify epitope. The recognition of recombinant peptides on the surface of VLP was verified in vitro by IL-2 production which was comparable to the level induced by recombinant Asp f2 (Fig. 2).
Molecular characterization of the immunodominant allergens and antigens of A. fumigatus has identified these to be complex proteins, polysaccharides, heat-shock proteins and antigens with enzyme activities such as elastase, protease, catalase, dismutase and ribonuclease [33]. It is unlikely that all these proteins are involved in A. fumigatus-induced allergy. Hence, we tried to use only a limited number of dominant peptides from the major allergen from A. fumigatus to modulate the immune response induced by the i.n. exposure of mice to Aspergillus antigens. This approach permitted us to reverse the allergic response by decreasing the total IgE production.
The concentration of recombinant peptides injected into mice for the induction of tolerance was high (10 μg per mouse), in line with the high doses of A. fumigatus allergens used in many of the reported experimental models [33]. We used a single high dose of peptide to estimate its effect on the dynamics of immune response. As predicted, a single injection of the recombinant peptide representing a dominant T cell epitope of Asp f2 decreased antigen-specific T and B cell immune responses (Figs 3a, 4 and 5). However, the effect of a single injection on T cells was not long-lasting (Fig. 3b). The production of antibodies of different IgG subclasses and IgE to Aspergillus decreased markedly in the treated group, although this was significant only for IgG2a, IgG3 and IgE antibodies (Fig. 4). The kinetics of the antibody response demonstrated that significant effects were present even 3 months after peptide–VLP injection (Fig. 5).
However, the activation of antigen-specific T cells, which orchestrates all the events in allergy induction, is the most important event resulting from sensitization to A. fumigatus proteins. When new T cell precursors generated in the thymus bearing specificity for a specific antigen encounter that antigen, they initiate a primary immune response even if the memory cells are non-responsive due to the induction of tolerance. Based on the results of SIT, it can be speculated that stable remission can be achieved only after a prolonged exposure to low doses of allergens or moderate doses of their T cell dominant peptides. This may be attributed to the emergence of new suppressor cells to modulate the immune response [34].
Acknowledgments
The research described in this publication was made possible in part by Award RBI-2020 of the US Civilian Research and Development Foundation for the Independent States of the Former Soviet Union (CRDF), by the grants 00-04-49524 and 01-04-06049 from Russian Foundation for Basic Research and by the Veteran Affairs Medical Research, USA.
REFERENCES
- 1.Kurup VP, Banerjee B, Hemmann S, Greenberger PA, Blaser K, Crameri R. Selected recombinant Aspergillus fumigatus allergens bind specifically to IgE in ABPA. Clin Exp Allergy. 2000;30:988–93. doi: 10.1046/j.1365-2222.2000.00837.x. [DOI] [PubMed] [Google Scholar]
- 2.Purkayastha S, Madan T, Shah A, Krishnamurthy HG, Sarma PU. Multifunctional antigens of A. fumigatus and specific antibodies. Appl Biochem Biotechnol. 2000;83:271–83. doi: 10.1385/abab:83:1-3:271. discussion 283–6, 297–313. [DOI] [PubMed] [Google Scholar]
- 3.Rolland J, O’Hehir R. Immunotherapy of allergy: anergy, deletion, and immune deviation. Curr Opin Immunol. 1998;10:640–5. doi: 10.1016/s0952-7915(98)80082-4. [DOI] [PubMed] [Google Scholar]
- 4.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–45. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 5.Pene J, Desroches A, Paradis L, et al. Immunotherapy with Fel d1 peptides decreases IL-4 release by peripheral blood T cells of patients allergic to cats. J Allergy Clin Immunol. 1998;102:571–8. doi: 10.1016/s0091-6749(98)70294-5. [DOI] [PubMed] [Google Scholar]
- 6.Muller UR, Akdis CA, Fricker M, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–54. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 7.Vrtala S, Akdis CA, Budak F, et al. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v1, induce blocking antibodies. J Immunol. 2000;165:6653–9. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- 8.Vrtala S, Hirtenlehner K, Vangelista L, et al. Conversion of the major birch pollen allergen, Bet v1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–81. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker J, Guo D, Hodges R. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data. correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986;25:5425–32. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- 10.Menndez-Arias L, Rodriguez R. A basic microcomputer program for prediction of B and T cell epitopes in proteins. Comput Appl Biosci. 1990;6:101–5. doi: 10.1093/bioinformatics/6.2.101. [DOI] [PubMed] [Google Scholar]
- 11.Gao JX, Madrenas J, Zeng W, et al. CD40-deficient dendritic cells producing interleukin-10, but not interleukin-12, induce T-cell hyporesponsiveness in vitro and prevent acute allograft rejection. Immunology. 1999;98:159–70. doi: 10.1046/j.1365-2567.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudmundsdottir H, Turka LA. T cell co-stimulatory blockade: new therapies for transplant rejection. J Am Soc Nephrol. 1999;10:1356–65. doi: 10.1681/ASN.V1061356. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee B, Kurup VP, Phadnis S, Greenberger PA, Fink JN. Molecular cloning and expression of a recombinant Aspergillus fumigatus protein Asp fII with significant immunoglobulin E reactivity in allergic bronchopulmonary aspergillosis. J Lab Clin Med. 1996;127:253–62. doi: 10.1016/s0022-2143(96)90093-1. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee B, Greenberger PA, Fink JN, Kurup VP. Immunological characterization of Asp f2, a major allergen from Aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis. Infect Immun. 1998;66:5175–82. doi: 10.1128/iai.66.11.5175-5182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth JF. The yeast Ty virus-like particles. Yeast. 2000;16:785–95. doi: 10.1002/1097-0061(20000630)16:9<785::AID-YEA550>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Hauber J, Nelböck-Hochstetter P, Feldmann H. Nucleotide sequence and characteristics of a Ty element from yeast. Nucl Acids Res. 1985;13:2745–58. doi: 10.1093/nar/13.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira-Ferreira J, Miyahira Y, Layton GT, et al. Immunogenicity of Ty-VLP bearing a CD8 (+) T cell epitope of the CS protein of P. yoelii: enhanced memory response by boosting with recombinant vaccinia virus. Vaccine. 2000;18:1863–9. doi: 10.1016/s0264-410x(99)00344-8. 10.1016/s0264-410x(99)00344-8. [DOI] [PubMed] [Google Scholar]
- 18.Buonaguro L, Buonaguro FM, Tornesello ML, et al. High efficient production of Pr55 (gag) virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Res 2001; 49:35–47. [DOI] [PubMed]
- 19.Goldmann C, Stolte N, Nisslein T, Hunsmann G, Luke W, Petry H. Packaging of small molecules into VP1-virus-like particles of the human polyomavirus JC virus. J Virol Meth. 2000;90:85–90. doi: 10.1016/s0166-0934(00)00226-3. [DOI] [PubMed] [Google Scholar]
- 20.Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93:284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 21.Hirschberg S, Layton GT, Harris SJ, Savage N, Dallman MJ, Lamb JR. CD4 (+) T cells induced by virus-like particles expressing a major T cell epitope down-regulate IL-5 production in an ongoing immune response to Der p1 independently of IFN-gamma production. Int Immunol. 1999;11:1927–34. doi: 10.1093/intimm/11.12.1927. [DOI] [PubMed] [Google Scholar]
- 22.Kurup VP, Xia JQ, Crameri R, et al. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin Immunol. 2001;98:327–36. doi: 10.1006/clim.2000.4993. [DOI] [PubMed] [Google Scholar]
- 23.Bauer L, Bohle B, Jahn-Schmid B, et al. Modulation of the allergic immune response in BALB/C mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v1. Clin Exp Immunol. 1997;107:536–41. doi: 10.1046/j.1365-2249.1997.d01-953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman F, Fink GR, Hicks JB. Cold Spring Harbor Laboratory Press. NY: Cold Spring Harbor; 1983. Methods in yeast genetics. A laboratory manual. [Google Scholar]
- 25.Maniatis T, Fritsch EF, Sambrook J. Cold Spring Harbor Laboratory Press. NY: Cold Spring Harbor; 1982. Molecular cloning. A laboratory manual. [Google Scholar]
- 26.Cryer DR, Eccleshall R, Marmur J. Isolation of yeast DNA. DM Presckott Meth Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- 27.Kim JM, Vanguri S, Boeke JD, Gabriel A, Voytas DF. Transposable elements and genome organization: a comprehensive survey of retrotransposons revealed by the complete Saccharomyces cerevisiae genome sequence. Genome Res. 1998;8:464–78. doi: 10.1101/gr.8.5.464. [DOI] [PubMed] [Google Scholar]
- 28.Burms NR, Gilmour JE, Kingsman SM, Kingsman AJ, Adams SE. Production and purification of hybrid Ty-VLPs. Mol Biotechnol. 1994;1:137–45. doi: 10.1007/BF02921554. [DOI] [PubMed] [Google Scholar]
- 29.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. John Wiley & Sons, Inc. NY: Brooklyn; 1998. Current protocols in immunology. [Google Scholar]
- 30.Gillis S, Ferm W, Ou W, Smith KT. T cell growth factor, parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–32. [PubMed] [Google Scholar]
- 31.Svirshchevskaya EV, Alekseeva LG, Andronova TM, Kurup VP. Do T helper 1 and 2 recognize different class II MHC molecules? J Clin Immunol. 2001;100:349–54. doi: 10.1006/clim.2001.5067. Humoral and cellular immune responses to soluble allergen from Aspergillus fumigatus Asp f2. [DOI] [PubMed] [Google Scholar]
- 32.Nelson HS. The use of standardized extracts in allergen immunotherapy. J Allergy Clin Immunol. 2000;106:41–5. doi: 10.1067/mai.2000.107197. [DOI] [PubMed] [Google Scholar]
- 33.Kurup VP, Crameri R. Aspergillus allergens, 2001. Aspergillus website: http //www.aspergillus.man.ac.uk/index.htm.
- 34.Levings MK, Roncarolo MG. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J Allergy Clin Immunol. 2000;106:S109–12. doi: 10.1067/mai.2000.106635. [DOI] [PubMed] [Google Scholar]


