Abstract
Treatment with the immunosuppressive drugs cyclosporin and tacrolimus, the mainstays of anti-graft rejection and autoimmune disease therapy, is limited by their hepato-and nephrotoxicity. The metabolic conversion of these compounds to more easily excretable products is catalysed mainly by hepatic cytochrome P4503A4 (CYP3A4) but also involves extrahepatic CYP3A5 and other P450 forms. We set out to study whether or not exposure to cyclosporin and FK506 in children undergoing organ transplantation leads to formation of autoantibodies against P450s. Immunoblotting analysis revealed anti-CYP reactivity in 16% of children on CyA for anti-graft rejection or treatment of nephrosis (n = 67), 31% of kidney transplant patients switched from CyA to FK506 (n = 16), and 21% of kidney and or liver transplant patients on FK506 (n = 14). In contrast, the frequency of reactive immunoblots was only 8·5% among the normal paediatric controls (n = 25) and 7% among adult kidney transplant patients on CyA or FK506 (n = 30). The CYP2C9+ sera were able to immunoprecipitate in vitro translated CYP2C9 and the immunoblot reactivity showed striking correlation to peaks in the age at onset of drug exposure. Sera were isoform selective as evidenced from Western blotting using human liver microsomes and heterologously expressed human P450s. These findings suggest that anti-cytochrome P450 autoantibodies, identified on the basis of their specific binding in immunoblots, are significantly increased among children on immunosuppressive drugs and in some cases are associated with drug toxicity and organ rejection.
Keywords: FK506, cyclosporin A, autoantibody, CYP450, GST fusion proteins
INTRODUCTION
Cytochrome P450s (CYPs) are thio-haemoproteins classified into families and subfamilies according to similarities in their amino acid sequences, yet each member exhibits a distinct substrate specificity, catalytic activity and immunological identity. Certain CYPs may be irreversibly modified during catalysis because of the binding of reactive metabolites, produced during drug or chemical biotransformation, to the protein or haeme moiety [1,2]. Usually this leads to the loss of CYP enzymatic activity. Subsequent catabolism of the modified CYP may lead to the presentation by the MHC of modified and native peptides that could lead to the breakdown of tolerance.
Neoepitopes generated by covalent modification of peptides during drug metabolism, although likely to initiate an autoimmune response, are not necessarily the direct target of self-reactive effector T and B cells. Autoantibodies from only a subset of patients actually recognize specific adducts derived from drug exposures to tienilic acid (anti-CYP2C9) [3]. CYP autoantibodies that show no detectable binding to adducts often bind to native protein sequences, as in the case with dihydralazine-associated hepatitis (anti-CYP1A2) [4], hypersensitivity reactions to aromatic anti-convulsants (anti-CYP3A) [5], and dislufiram toxicity (anti-CYP1A2) [6]. Autoantibodies to native CYP epitopes in CYP2D6 are found in type II chronic autoimmune hepatitis and in 5% of patients with virus C hepatitis [7], and to CYP1A2 are found in autoimmune polyendocrine syndrome type I [8] CYP21 in Addison’s disease [9,10].
It has become evident that hepatic CYP3A4 and extrahepatic CYP3A activities in addition to the MDR P-glycoprotein are important for intestinal drug absorption and metabolism and are the major determinants of the highly variable bioavailability and toxicity of CyA and FK506 observed between and within individuals [11].
Prompted by our recent finding of IgG autoantibodies against native CYP3A4 sequences among alcoholics [12], we set out to investigate if patients dependent on extensive treatments with immunosuppressants known to be CYP3A substrates had antibodies directed against the enzyme.
Cyclosporin and FK506 have serious nephrotoxic, hepatotoxic and neurotoxic side-effects [13,14] that have been correlated with CYP3A4 activity [15,16]. We reasoned that it would be advantageous to study formation of CYP autoantibodies in paediatric patients because careful monitoring of both dosage and blood trough values for individual children is required to avoid the risk of organ rejection due to under-dosage and toxic organ damage due to over-dosage and accumulation of metabolites [17,18].
In the present study, sera of paediatric patients undergoing treatment with the immunosuppressants cyclosporin A (CyA) or FK506 were examined for autoantibodies against the major drug-metabolizing human CYPs using ELISA, immunoprecipitation and immunoblot techniques. We demonstrate an increased frequency of anti-CYP antibodies among children on drug treatment over control groups that correlates with age at onset of drug exposure. The antibodies exhibit a consistent binding pattern to specific CYP enzymes and recognize epitopes within the C-terminal domain of CYP3A4.
MATERIALS AND METHODS
Patients and control subject recruitment
Sera of three distinct groups of paediatric patients were used. The first group consisted of 68 kidney transplanted children (31 boys and 35 girls; age range 0·5–17 years; mean age 7·5 years); 48 were treated with CyA, 16 were treated with both CyA and FK506 and four were treated with FK506. Patients were admitted, primarily for renal failure due to hereditary or congenital malformations, to the Department of Paediatrics, Huddinge Hospital and treated with prednisone (5–10 mg), azathioprine (10–50 mg) and either twice daily neoral CyA (Sandoz) mean dosage 5 mg/kg per day, range 2–7 mg/kg per day or FK506 (Prograf, Fujisawa) mean dosage 0·14 mg/kg per day, range 0·05–0·2 mg/kg per day.
In a second group of 14 nephrotics (nine boys and five girls; age range 2–13 years, mean age 6 years), nine were treated with CyA mean dosage 5 mg/kg per day, dosage range 2·5–8 mg/kg per day, three with prednisone alone 10–25 mg/day and two with no drug treatment. The third group of 20 liver transplanted children (13 boys and seven girls; age range 0·3–23 years, mean age 9 years), diagnosed as α1-trypsin deficiency (n = 2), extrahepatic biliary atresia (n = 5), chronic cholestasis (n = 1), acute fulminant hepatitis (n = 2), glycogenosis (n = 3), Budd–Chiari syndrome with hepatic vein occlusion (n = 1) and other congenital or inherited hepatic dysfunction (n = 6), were given postoperative treatments of either CyA, mean dosage 5 mg/kg per day, dosage range 3–9 mg/kg per day, or FK506, mean dosage 0·13mg/kg per day, dosage range 0·05–0·23 mg/kg per day, to maintain 12h trough values in the range of 100–300 ng/ml and 4–9 ng/ml, respectively. The sera of the 24 control paediatric subjects had no drug treatments and were normal donors (13 boys and 11 girls; age range 0·5–3 years, mean age 2 years).
The sera of 30 kidney transplant recipients (24 males and eight females), age range 15–62 years (mean 38 years), on postoperative CyA, mean dose 7 mg/kg per day (range 2·7–16 mg/kg per day), to maintain mean trough values of 187 ng/ml (range 42–316 ng/ml) or FK506 mean 0·12 mg/kg per day (range 0·03–0·2 mg/day) and mean trough values of 12 ng/ml were kindly provided by Professor M. I. Lorber (Section of Organ Transplantation and Immunology, Department of Surgery, Yale University School of Medicine, New Haven, CT). Sera of eight adult primary biliary cirrhosis (PBC) patients without immunosuppressive treatments were kindly provided by Dr U. Broome (Department of Medicine, Huddinge Hospital, Karolinska Institutet) and Dr E. Eliasson (Division of Molecular Toxicology, Karolinska Institutet).
This study was carried out with consent of the human subjects and approval by the Swedish Ethics Committee.
Human proteins
The human recombinant CYP2E1, CYP3A4, CYP1A2 and CYP2C9 were produced from over-expressing plasmid in Escherichia coli at Panvera Ltd (Madison, WI). The N-terminal modifications were as previously described [19,20]. Microsomes derived from AHH-1 TK+/− human lymphoblastoid B cell line co-expressed full-length cDNA of human CYPs: CYP2E1, CYP3A4, CYP1A2, CYP2C9 or CYP2D6 and the human cytochrome P450 reductase. Microsomes derived from baculovirus-infected insect cell system co-expressed cDNA of CYP3A4 or CYP3A5 with cytochrome P450 reductase or the cytochrome P450 reductase alone. All microsome preparations were produced from GENTEST Corp. (Woburn, MA). The human recombinant FK-binding protein 12 (FKBP12) was expressed in E. coli (Sigma Chemical Co., St Louis, MO).
Antigens
Rat CYP3A1 and NADPH reductase-cytochrome P450 was purified from microsomal fractions of rat liver, and human liver microsomes were from frozen stocks prepared as described previously [21].
Functional assays
Studies of drug bioactivation were performed by additions of CyA in DMSO or FK506 in ethanol at final concentrations of 100 μm drug and 0·5% v/v solvent to baculovirus microsomes containing 50 pmol of either CYP3A4 or CYP3A5 in 50 mm potassium phosphate buffer and 50 mm KCl pH 7·4 as described previously [22]. The above reaction mixtures were incubated for 1 h at 37°C in the presence of NADPH generating system; 1 mm NADP+, 50 mm glucose-6-phosphate and 0·5 U/ml glucose-6-phosphate dehydrogenase. CYP3A-dependent 6 β hydroxylation of 14C testosterone was monitored as described previously [21]. The activity of NADPH-cytochrome P450 reductase was measured spectrophotometrically using cytochrome c as an electron receptor [23].
ELISA
ELISA was performed in polystyrene microwell plates (Sigma) as described previously [12].
In vitro coupled transcription-translation of human CYP3A4his6
Full-length human 35S-CYP2C9 his6, 35S-CYP3A4 his6 and N-terminal truncated 35S-7Asp123 CYP3A4his6 were produced in vitro from their respective cDNAs in pGEM-4Z using a TnT T7-Quick coupled reticulocyte lysate system (Promega, Madison, WI). Plasmid DNA (1·5 μg was incubated for 90 min at 30°C in 40 μl rabbit reticulocyte lysate, 2 μl 10 mCi/ml 35S-methionine and nuclease-free water added to final volume of 50 μl reaction mixture. The reaction mix was stored at –70 μCi until needed and the percent incorporation of 35S-methionine in the reaction mixture was determined according to standard procedure (Promega Corporation Technical Manual no. 045, revised edition 9/99).
Immunoprecipitation of 35S-methionine-labelled cytochrome P450s
For each immunoprecipitation assay a 5-μl aliquot of in vitro translation reaction mixture (equivalent to 400 000–780 000 ct/min of TCA precipitable material) was suspended in 100 μl immunoprecipitation buffer containing 20% glycerol, 0·5% NP40 in NaH2PO4 0·3 m NaCl, 10 mm imidazole, 4 mm PMSF, 2 μg/ml leupeptin (pH = 7·9). Preclearing of the reaction mix was done by addition of 50 μl Protein A Sepharose (Pharmacia Biotech, Uppsala, Sweden) prepared according to the manufacturer, and incubated for 2 h under gentle shaking at 4°C. After 2000 g × 3 min centrifugation the supernatant was transferred to clean Eppendorff tubes. Human serum was then added to the precleared reaction mix at a final dilution of 1:100. After 4 h incubation with shaking at 4°C, 50μl of Protein A Sepharose were added as described previously and incubated at 4°C for 12 h with shaking. The protein A Sepharose–antibody complexes were then collected by centrifugation and washed five times in 1·5-ml volumes of immunoprecipitation buffer at 4°C. Immunoprecipitated radioactivity was dissolved in 1 ml of Hydrofluor scintillation fluid and evaluated in a liquid scintillation analyser. For analysis by SDS–PAGE, the protein A Sepharose antibody complexes were resuspended in 50 μl SDS sample buffer, boiled and centrifuged, and 30 μl of the recovered supernatant were loaded for electrophoresis.
SDS–PAGE electrophoresis and immunoblotting
SDS–PAGE was performed using 4% stacking gel and 8·7% separating gels [24]. Human liver microsomal preparations and rat and human proteins were solubilized in SDS gel loading buffer 3% SDS (w/v), 0·2 m Tris–HCl pH 6·8, 26% glycerol (w/v) with 2 m 2-mercaptoethanol and heated for 2 min at 100°C. Blots were probed with 1:5000 diluted rabbit anti-CYP2E1 or rabbit anti-CYP3A1 sera or different human sera diluted at either 1:300, 1:600 or 1:2500 in blocking buffer for 4 h with shaking. Following repeated washes, immunoblots were incubated with peroxidase-linked goat anti-rabbit IgG diluted 1:2000 or goat anti-human IgG Fc, goat anti-human IgM μ-chain and goat anti-human IgA α-chain diluted 1:20000 (Sigma Immunochemicals, Israel) for 2 h with shaking. For immunoblot re-probing experiments, the antibodies were removed from filters in stripping buffer 63 mm Tris, 2% SDS, 100 mm 2-mercaptoethanol by incubation at 65°C for 25 min. Following repeated washes in TBS–Tween buffer the filters were placed in blocking solution for 1 h at room temperature with shaking and then incubated with human sera and specific peroxidase-linked antibody as described above.
Statistical analysis
The graphed and tabulated values presented in this work are mean and standard error (s.e.m.). Unless mentioned otherwise all statistical analysis was performed with StatMost program using parametric unpaired Student’s t-test:
t = (X1 − X2)/s.d. √ (1/N1 + 1/N2).
RESULTS
Immunoblotting assessment of anti-CYP autoantibodies in sera of paediatric and adult patients on immunosuppressants
Sera of adult and paediatric drug exposures, OLT group, and paediatric controls were assessed for their immunoblot reactivities. We observed that 11 of 67 (16%) sera tested of paediatrics on CyA, five of 16 (31%) sera tested of paediatrics on CyA and switched to FK506 and three of 14 (21%) sera tested of paediatrics on FK506 gave positive reactions against human CYPs. By contrast, only two of 30 (7%) sera tested from adults on CyA or FK506 and four of 47 (8·5%) sera tested of paediatrics not on CyA or FK506 treatments gave positive reactions (Table 1). The positive sera reactions for each CYP450 isoform given in Table 1 show that the greatest number of positive reactions were among the Kid Tx group.
Table 1.
Autoantibodies against cytochrome P450s among children on immunosuppressive drugs
| Adults | Children | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CyA or FX | No drug | CyA | CyA switched to FX | FX | |||||||||||||||||||||
| Number CYP pos. | Number CYP pos. | Number CYP pos. | Number CYP pos. | Number CYP pos. | |||||||||||||||||||||
| Pos. | 2E1 | 3A4 | 1A2 | 2C9 | Pos. | 2E1 | 3A4 | 1A2 | 2C9 | Pos. | 2E1 | 3A4 | 1A2 | 2C9 | Pos. | 2E1 | 3A4 | 1A2 | 2C9 | Pos. | 2E1 | 3A4 | 1A2 | 2C9 | |
| Kidney transplant | 7 | 1 | 1 | − | − | 0 | − | − | − | − | 21 | 1 | 5 | 1 | 2 | 29 | 1 | 2 | 1 | − | 25 | 1 | 1 | − | − |
| Nephrotic/kidney transplant | − | − | − | − | − | 0 | − | − | − | − | 20 | − | 1 | − | − | 50 | − | 1 | − | − | 0 | − | − | − | − |
| Nephrotic/ | − | − | − | − | − | 20* | − | − | 1 | − | 11 | − | − | − | 1 | 0 | − | − | − | − | 0 | − | − | − | − |
| Liver disease transplant | − | − | − | − | − | 0 | − | − | − | − | 0 | − | − | − | − | 0 | − | − | − | − | 0 | − | − | − | − |
| Liver disease before | − | − | − | − | − | 6 | − | 1 | − | − | 0 | − | − | − | − | 0 | − | − | − | − | 0 | − | − | − | − |
| Controls | − | − | − | − | − | 8 | − | 1 | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Total | 7 | 1 | 1 | 0 | 0 | 8.5 | 0 | 2 | 2 | 0 | 16 | 1 | 6 | 1 | 3 | 31 | 1 | 3 | 1 | 0 | 21 | 1 | 3 | 0 | 0 |
| †IB intensity | 28(25–30) | 32(5–70) | 105(26–245) | 95(15–240) | 478–140 | ||||||||||||||||||||
The positive ECL-immunoblot IgG reactions against human cytochrome P450s 2E1, 3A4, 1A2 or 2C9 with 1:600 diluted sera from adults (n = 30) and children undergoing drug treatments cyclosporin A (CyA) (n = 67), CyA and tacrolimus FK506 (FK) (n = 16) or FK alone (n = 14). Pos, Population that gave positive anti-CYP reactivities in immunoblots.
The group included three patients on prednisone.
The immunoblot (IB) reactivities were quantified by laser scanning of the autoradiographs and densitometric analysis using Molecular Dynamics Image Quant software version 3.2. The values shown are the mean and range (parentheses) of band intensities in a.u./pmol P450.
Densitometric evaluation of the mean band intensities revealed reactions that were significantly stronger against CYP3A4 and CYP1A2 among the children on drug treatments CyA or switched from CyA to FK than the reactive sera from the paediatric control group or adult sera (Table 1). CYP3A4 reaction intensities of ≤50 arbitrary units (a.u.)/pmol were present in the controls and four of the 20 positive sera of children on drug treatments. Strong immunoblot band intensities of CYP2C9 for KidTx2023, 245 a.u/pmol, and patient with nephrotic syndrome 7817, 135 a.u./pmol, both on CyA, and of CYP2E1 for KidTx5349, 240 a.u/pmol, and KidTx 2167, 28 a.u./pmol both on FK, are in contrast to no reactivity against anti-CYP2C9 or anti-CYP2E1 reactivity found among the control groups. Among patients treated with CyA and switched to FK506, two patients gave positive immunoblots: KidTx with nephrotic syndrome 1384 against CYP3A4 and KidTx 7159 against CYP1A2. Both of these individuals had elevated serum transaminases and abnormal liver function tests and histological findings of fibrosis and inflammation in liver biopsies.
Paediatric patients in this study showed no clinical evidence of cytomegalovirus (CMV) or Epstein–Barr virus (EBV) and serological tests and polymerase chain reactions (PCR) of liver DNA were negative for hepatitis C (data not shown), that have been implicated as triggers for development of autoimmune hepatitis (AIH) [25].
Demonstration by immunoprecipitation that IgG autoantibodies in children with liver disease on CyA recognize CYP2C9 and CYP3A4 sequences
To verify that the sera reactive with purified recombinant human CYP2C9 and CYP3A4 (Table 1) were in fact representative of the presence of autoantibodies, the sera of children on drug exposures and controls were tested for their capacity to form immune complexes with 35S-methionine-labelled CYP2C9 and N-terminal truncated CYP3A4 produced by in vitro translation (Fig. 1). Serum from a paediatric kidney transplant patient on CyA (KidTx 2023) of high anti-h2C9 ELISA reactivity, A492 = 0.968 and serum from a liver transplant patient on FK506 (LivTx 621) A492 = 0.611, were both able to precipitate strongly the CYP2C9 and truncated CYP3A4 (Fig. 1a, lanes 7 and 8). Anti-h2C9-reactive sera from two paediatric nephrotics on CyA (Neph7817 and Neph7572) gave similar strong immunoprecipitation (Fig. 1, right upper bar graph), whereas control sera of low ELISA reactivity (A492 < 0·15) gave weak immunoprecipitation (Fig. 1a, lanes 9 and 10). Sera of paediatric liver disease patients displaying anti-3A4 reactivity (LivTx441, A492 = 0·58) gave immunoprecipitable band intensities of N-terminal truncated Asp123-CYP3A4 that were proportional to their reactivity in ELISA (Fig. 1a, lanes 1 and 2), whereas controls gave only weak immunoprecipitation (Fig. 1a, lanes 3 and 4). Immunoprecipitation experiments of these same sera using full length 35S-CYP3A4 his6 produced even stronger band intensities, but because hydrophobic 3A4 N-terminus sequences undergo self-aggregation and form precipitate outside the microsomal or lipid environment, the background in buffer elutions from Sepharose beads was higher than for the truncated Asp123-CYP3A4 construct (Fig. 1).
Figure 1.
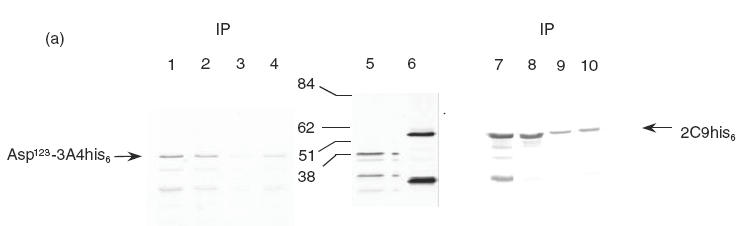
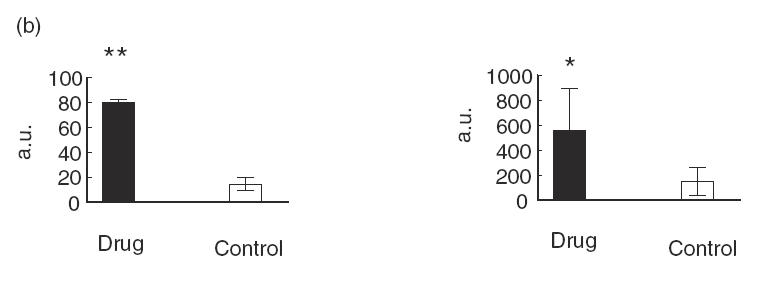
(a) SDS–PAGE and autoradiography of in vitro translated and immunoprecipitated 35S-Asp123CYP3A4his6 and 35S-CYP2C9his6. 35S-Asp123CYP3A4his6 and 35S-CYP2C9his6 were loaded in 25 μl of sample immunoprecipitate (IP) (lanes 1–4 and lanes 7–10) or 2·5 μl of the diluted reaction mixture (lanes 5 and 6) as described in Materials and Methods. Total 35S-Asp123CYP3A4his6 (lane 5) and 35S-CYP2C9his6 (lane 6) and immunoprecipitations with 1:150 dilution of human sera: drug treatments (lanes 1,2 and lanes 7,8) and controls (lanes 3,4 and lanes 9,10). Paediatric liver transplants on FK506: LivTx 441 (lane 1) and LivTx 621 (lanes 2 and 8); paediatric kidney transplant on CyA KidTx 2023 (lane 7) and control sera C5 (lanes 3 and 9) and C6 (lanes 4 and 10). The bar graphs (b) represent mean and s.d. of immunoprecipitated bands quantified by laser scanning of the autoradiographs and densitometric analysis using Molecular Dynamics Image Quant software version 3.2. Differences in reactivity between eight control subjects (□) and four patients on drug treatments (▪) is statistically significant at **P < 0·01 and *P < 0·05, Student’s unpaired t-test. The graphed values represent arbitrary units (a.u.) of band intensities taken from immunoprecipitations in two independent experiments.
The anti-CYP reactions of the liver disease patients were not accompanied by autoantibodies to FK506 immunophilin binding protein (FKB12), a candidate autoantigen previously investigated among liver transplant patients on FK506 [26].
Anti-human CYP autoantibodies of paediatric patients on FK506 or CyA recognize specific P450 isoforms
The IgG immunoblots seen with purified human recombinant CYP2E1, CYP3A4, CYP1A2 and CYP2C9 were specific because the reactivity was, with one exception (sera 7159), exclusively against one CYP isoform (Fig. 2). The sera showing positive reactions with purified recombinant CYPs were subjected to immunoblotting against a test panel of microsomal prepara-tions of recombinant human CYPs produced commercially (GENTEST): CYP2E1, CYP3A4, CYP1A2, CYP2C9 and CYP2D6. The specific pattern of anti-CYP reactivity was confirmed and gave no anti-2D6 reactions, thus making it unlikely that these individuals express anti-LKM type I autoantibodies (results not shown). Sera of LivTx 5718 against CYP3A4 and KidTx 2023 against CYP2C9 were tested on four preparations of human liver microsomes. LivTx 5718 produced a distinct immunoblot band corresponding to CYP3A4 in liver microsome preparations (Fig. 2c) and KidTx 2023 produced two specific bands, reaction towards CYP2C9 and CYP2C19, all microsomal preparations (Fig. 2d). This indicates that the sera have specific anti-CYP autoreactivity in complex mixtures of proteins. Furthermore, the LivTx 5718 serum against CYP3A4 only reacted with CYP3A4 in microsomes from lymphoblastoid cells heterologously expressed with CYP3A4, CYP2E1, CYP1A2 or CYP2C9 (Fig. 2a,c). Similarly, KidTx 2023 serum did not react with CYP3A4, CYP1A2 or CYP2E1 (Fig. 2a,d).
Figure 2.
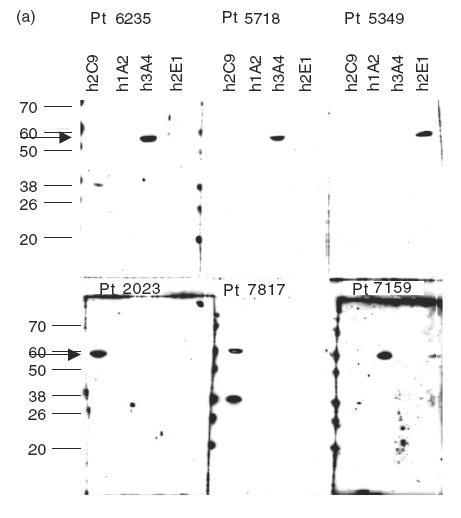
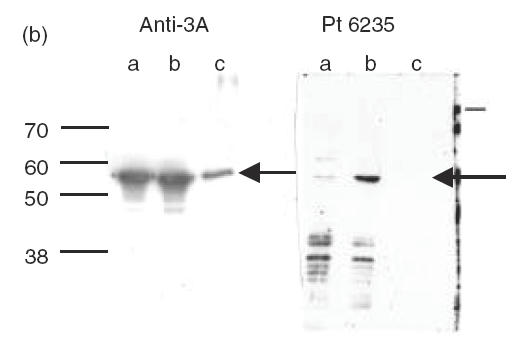
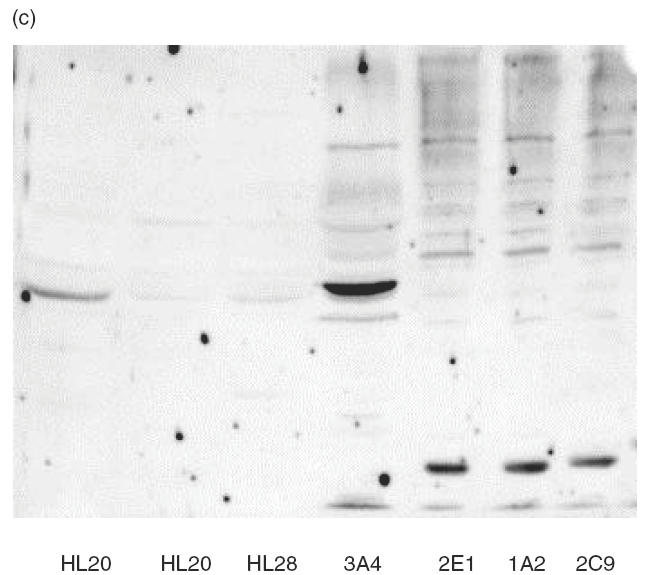
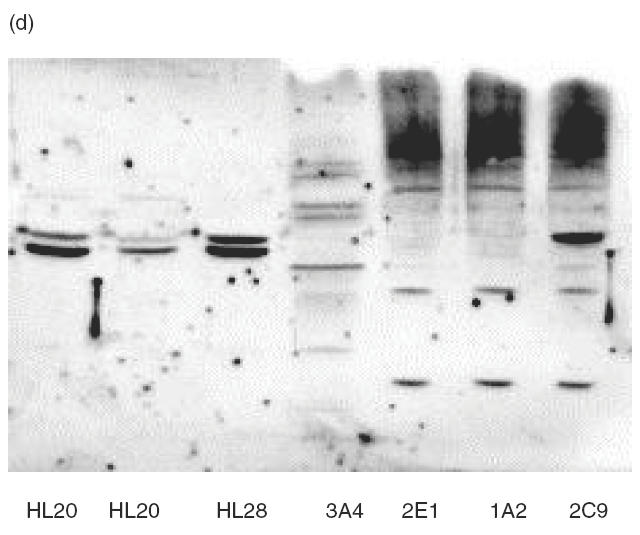
Immunoblots of human CYP450s with patient sera. Preparations of the purified recombinant human CYPs were loaded at 2·8 pmol CYP per lane (a), insect microsomal preparations of CY3A4 and CYP3A5 from baculovirus expression or purified rat CYP3A1, a = human CYP3A5, b = human CYP3A4, c = ratCYP3A1 were loaded at 5 g per lane (b), human liver microsomal samples (HL20, 21 and 28) and recombinant microsomal samples from lymphoblastoid cells were incubated with sera from Liver Tx patient 5718 (c) and Kidney Tx patient 2023 (d). Immunoblots were made with sera from the indicated paediatric patients (Pt) at 1:600 dilution in Tris-buffered saline and the ECL reactions were developed for 1 min on the same strip of Kodak X-omat photographic film (a). Immunoblot was made with rabbit anti-3A and Protein A–HRP or Pt 6235 and anti-human IgG–HRP with ECL reactions developed after 15-s and 2-min film exposures, respectively. Immunoblots for sera 5718 (c, 3A4-reactive) and 2023 (d, 2C-reactive) were made from gels loaded with 40 μg of microsomes in each lane.
The presence of anti-CYP3A4 antibodies is consistent with the fact that CYP3As are the major P450s responsible for metabolism of tacrolimus and cyclosporins. Kidney CYP3A5 constitutes a major source of extrahepatic CYP3A [27,28] and this CYP isoform might be a target for autoantibodies among renal transplant patients on postoperative immunosuppressive treatments or among patients with nephrotoxicity. Immunoblots were performed with CYP-positive sera on preparations of baculovirus microsomes containing either recombinant human CYP3A5 or recombinant human CYP3A4 under co-expression of human NADPH-cytochrome P450 reductase. Immunoblot reactivity of several sera was obscured by non-specific binding to denatured microsomes; however, the anti-CYP3A4+ sera of CyA KidTx 6235 (Fig. 2a) produced a strong band corresponding to CYP3A4, a weak reaction against CYP3A5, but no reaction against CYP3A1 (Fig. 2b). The identical pattern was observed using sera of FK506 LivTx 5718 (result not shown).
Because the catalysis of CyA or FK506 generates reactive metabolites that can bind to CYP enzyme or microsomal proteins [29,30], patient sera were examined for immunoblot reactivity against CYP3A4 and CYP3A5 in baculovirus microsomes that had been incubated with drugs under conditions favourable for drug bioactivation and in microsomes without NADPH generating system. The microsomes demonstrated enzymatic activity in hydroxylation of 5-OH testosterone and were capable of NADPH consumption, yet exposure to CyA or FK506 did not alter either the pattern or intensity of the patient sera reactivity in immunoblots (result not shown). Although no direct measurements were made to detect adduct formation per se, this finding suggests that the patient IgG CYP autoantibodies are not likely to bind protein adducts derived from CyA or FK506 metabolites.
Correlation between immunoblot reactivity of paediatric sera and age at onset and duration of FK506 or CyA exposure
In order to examine the relationship between immunoblot reactivities and drug exposure, the percentage of positive immunoblots was evaluated as function of age at onset of drug treatment, patient age on date of organ transplant, or record indicating date of first administration of immunosuppressant as well as duration of FK506 and CyA treatments, calculated as the time elapsed between age at onset and dates of individual sera donation (Table 2 and Fig. 3). The immunoblot reactivities demonstrate age-specific occurrence, and there is a significant bias of positive reactions among children < 3 years old (Fig. 3). Incidences of positive reactivity were found among children only after 6 months drug exposure at peaks of > 6 months and < 2 years and > 3 years and < 6 years (Fig. 3). The limitation inherent in this retrospective study is that patients were not randomized in their assignment to groups according to age and treatments, and for the paediatric kidney transplant patients we do not have data on the activity of individual reference sera taken prior to drug exposure.
Table 2.
Treatment of children with immunosuppressive drugs and formation of autoantibodies against cytochrome P450s
| Serum transaminases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at onset of treatment (year) | Duration of treatment (year) | Pos. population | Total population | |||||||
| Pos. pop. | Total pop. | Pos. pop. | Total pop. | ALAT (U/l) | ASAT (U/l) | γGT (μkat/l) | ALAT (U/l) | ASAT (U/l) | (GT (μkat/l) | |
| Kidney transplant | *5 ± 4 | 8 ± 5 | 4 ± 3 | 4 ± 3 | 720 | 800 | 1·6 | 230 | 400 | 0·24 |
| 70–2480 | 270–1980 | 0·18–10 | 60–2820 | 180–1280 | 0·15–9·6 | |||||
| Nephrotic/kidney transplant | 1·3 ± 0·5 | 9 ± 5 | 4·5 ± 2 | 5 ± 3 | ||||||
| Nephrotic/ | 2 | 6 ± 3 | 5·5 | 3 ± 2 | ||||||
| Liver disease transplant | 3·8 ± 1·3 | 8·6 ± 7 | 1 ± 0 | 3·7 ± 2·5 | ||||||
| Total | 4·5 ± 3·5 | 7·6 ± 6 | 4 ± 2·7 | 4 ± 3·2 | ||||||
The data shown were obtained from the children described in Table 1. Total = total patient population for which data were available. Age at onset of drug treatments between the patients of positive sera reactivity and the total patient population is statistically significant at
P = 0·1, Student’s unpaired t-test. The values of laboratory tests for serum transaminase are given as the mean and range. NA, Values not available.
Figure 3.
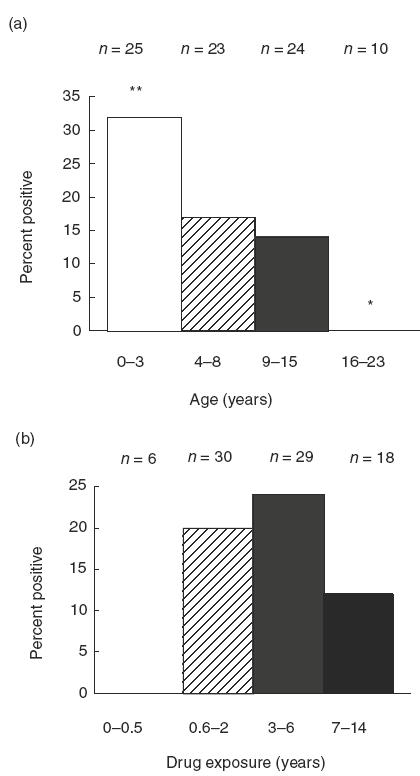
Effect of age and drug exposure on CYP450 autoantibodies. The percent of positive immunoblot reactions is plotted according to age at onset of FK506 or CyA treatments (a) and duration of drug exposure (b) determined from the difference in time between the date of organ transplant or first treatment and the date of sera sample donation. **P = 0·03; *P = 0·1, Pearson’s χ2 non-parametric analysis.
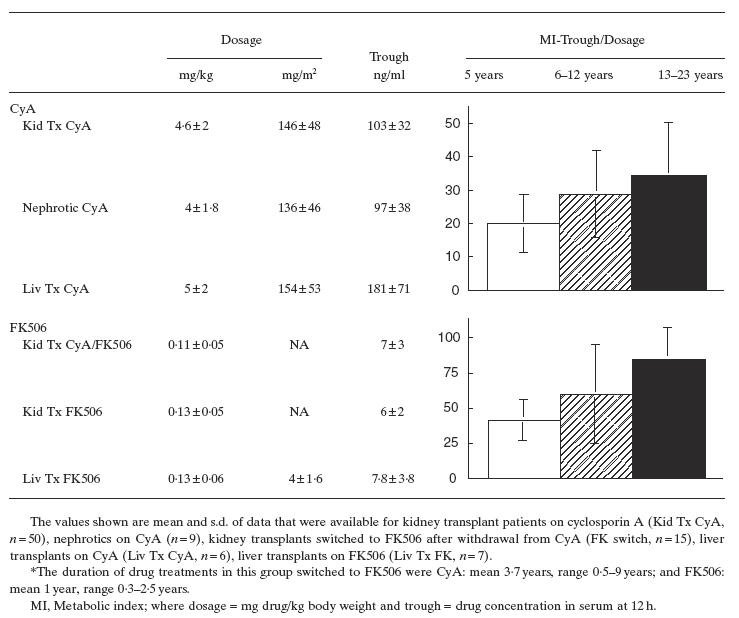
Monitoring of the drug dosage, trough levels (TL) and metabolic index (MI) in the serum at 12 h reveal that the patients of positive immunoblot reactivity showed slightly lower trough values and MI (CyA TL = 93 ± 55 ng/ml, MI = 24 ± 10) than the total patient population (CyA TL = 103 ± 32, MI = 26 ± 13). Interestingly, this trend is also seen for the one CYP2C9 immunoblot-positive nephrotic 7817 on CyA, TL = 77, MI = 12 that is 1 s.d. below the nephrotic population in general (Table 3). The extent to which these indices of drug clearance and/or metabolism are associated with CYP autoantibodies in patients of younger age at the onset of drug treatment (Fig. 3a) is supported by the finding that metabolic indices (MIs) of CyA and FK506 in the age group ± 5 years are 25–50% lower than the CyA and FK MIs in age groups 6–12 years and 13–23 years (Table 3). The CYP autoantibodies show no correlation to the administration of calcium blockers, β blockers and loop diuretic furosemide used for management of nephrotic syndrome and renal function, and there were no drug interactions that affect bioavailability of immunosuppressants (result not shown).
Table 3.
Increased drug metabolism of paediatric kidney and liver disease patients on immunosuppressants is age-dependent
DISCUSSION
There are to our knowledge no previous reports of antibodies to intact CYPs among subjects on immunosuppressive drugs. The results presented here indicate that postoperative CyA or FK506 treatment and use of CyA in autoimmune nephrosis are associated with IgG reactions against CYP2E1, CYP3A4, CYP1A2 and CYP2C9. The reactivity of IgG antibodies to CYP3A4 and CYP2C9 sequences in immunoblots were confirmed by immunoprecipitation experiments of 35S-Asp123-CYP3A4his6 and 35S-CYP2C9his6 produced by in vitro translation reactions.
Our findings of anti-CYP reactivities in patients suffering from transplant rejection and CyA toxicities support the previously proposed hypothesis that CYP autoantibodies, not necessarily against the major drug-metabolizing isoform, are associated with episodes of delayed and prolonged organ rejection that are unresponsive to steroids and immunosuppressive therapy [31]. The significance of CYP autoantibodies in this setting is indicated by the specific presence among paediatric transplant patients and the fact that although these patients are on immunosuppressive drugs known to interact with the target autoantigen, the CYP autoantibodies do not appear to bind protein adducts derived from CyA or FK506 metabolites.
Developmental ‘break points’ exist for many drug-metabolizing enzymes, and awareness of the ontogeny of specific CYP isoforms across the paediatric age range is of increasing importance in the appropriate pharmacologic management of children. The lower metabolic indices and trough values, indicating higher CyA and FK506 biotransformation for 0–3 and 4–8-year age groups relative to adolescents provide clinical data that are consistent with the rapid increase of CYP3A activities during infancy and the unstable pharmacokinetics of neonates and children < 5 years of age compared with adults [17,32]. A recent report of cyclosporin-induced remission of AIH in children emphasizes the need for only short-term treatment of 6 months duration to avoid lymphoproliferative disorders and renal complications [33], in keeping with therapeutic drug monitoring of neonates and children [17,34]. This recommendation is consistent with the age-specific efficacy of cyclosporin in vitro, which shows two-fold lower drug concentrations to inhibit peripheral blood monocyte cultures and seven-fold lower concentrations to inhibit IL-2 production among infants and 2–4-year-old children than among preadolescents (5–12 years) and adults (> 12 years) [35].
The CYP autoantibodies we found have no reactivity against human CYP2D6 in microsomal preparations and thus differ in origin from anti-LKM antibody type I found in AIH [25] as well as that of LKM1+ sera in hepatitis C viral infections of adults and children [36,37].
Annual monitoring of patient titres to CMV, EBV, Varicella and hepatitis C virus revealed no significant changes and none of the paediatric patients received blood transfusions. Because hereditary and congenital malformations were the underlying cause of kidney disease among the CYP450+ patients, and the few cases of acquired diseases, IgA nephropathy and glomerular nephritis, were CYP450− with the exception of one CYP3A4+ paediatric kidney transplant patient, autoimmune background and viral infection are unlikely factors to affect production of CYP450 antibodies. Although clinical events such as release of intracellular autoantigens by renal injury could challenge the immune system for the production of autoantibodies, the present study can not discern if the CYP autoantibody formation is a consequence of renal injury due to alloreactivity or chronic cyclosporin and tacrolimus treatments that are known to up-regulate transforming growth factor-beta and promote a persistent profibrogenic state that is closely associated with drug renal toxicity [38,39].
Our findings offer strong support to the conclusion that CYP autoantibody formation among children on immunosuppressants is correlated mainly to age at onset of drug treatment and, to a lesser extent, the duration of the treatment. Because cyclosporins and tacrolimus can cause serious nephrotoxic, hepatotoxic and neurotoxic side-effects associated with variations in CYP3A protein levels and activity, careful monitoring of both dosage and blood trough values in children is required to avoid the risk of organ rejection through under-dosage and toxic organ damage through over-dosage and accumulation of metabolites. Atypical autoantibodies, such as those against CYPs recognizing sequences restricted to a specific isoform, can serve as diagnostic markers of hepatic or renal dysfunction associated with cyclosporin or FK506 toxicity. However, the CYP autoantibodies among children on immunosuppressants are unlikely to be pathogenic or directly inhibit enzymatic activity. Concern should be that variability of individual P450 drug metabolism and CYP autoantibody formation under suppressed T-cell function may be an indicator for autoimmune or iatrogenic risk during paediatric immunosuppressive therapy.
Acknowledgments
We are indebted to Dr Jack Ragheb for discussions and critical reading of the manuscript and to Ms Åsa Nordling for invaluable help with immunoblotting experiments. Supported in part by The Swedish Medical Research Council and grants to S.D.L. received from Swedish Child Health Semariten stipendium and Anders Swärds Stiftelse, Stockholm.
REFERENCES
- 1.Uetrecht JP. Current trends in drug-induced autoimmunity. Toxicology. 1997;119:37–43. doi: 10.1016/s0300-483x(96)03594-9. 10.1016/s0300-483x(96)03594-9. [DOI] [PubMed] [Google Scholar]
- 2.Pumford NR, Halmes NC. Protein targets of xenobiotic reactive intermediates. Ann Rev Pharmacol Toxicol. 1997;37:91–117. doi: 10.1146/annurev.pharmtox.37.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Lecoeur S, Andre C, Beaune Ph. Tienilic acid-induced autoimmune hepatitis: anti-liver and anti-kidney microsomal type 2 autoantibodies recognize a three-site conformational epitope on cytochrome P4502C9. Mol Pharmacol. 1996;50:326–33. [PubMed] [Google Scholar]
- 4.Belloc C, Gauffre A, Andre C, Beaune Ph. Epitope mapping of human CYP1A2 in dihydralazine-induced autoimmune hepatitis. Pharmacogenetics. 1997;7:181–6. doi: 10.1097/00008571-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Leeder JS, Gaedigk A, Lu X, Cook VA. Epitope mapping studies with human anti-cytochrome P450 3A antibodies. Mol Pharmacol. 1996;49:234–43. [PubMed] [Google Scholar]
- 6.Eliasson E, Stål P, Lytton SD. Expression of autoantibodies to specific cytochromes P450 in a case of disulfiram hepatitis. J Hepatol. 1998;29:819–25. doi: 10.1016/s0168-8278(98)80264-x. [DOI] [PubMed] [Google Scholar]
- 7.Choudhuri K, Mieli-Vergani G, Vergani D. Cytochrome P4502D6: understanding an autoantigen. Clin Exp Immuol. 1997;108:381–3. doi: 10.1046/j.1365-2249.1997.4131311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemente MG, Obermayer-Straub P, Meloni A, et al. Cytochrome P450 1A2 is a hepatic autoantigen in autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1997;82:1353–61. doi: 10.1210/jcem.82.5.3913. [DOI] [PubMed] [Google Scholar]
- 9.Krohn K, Uibo R, Aavik E, Peterson P, Savilahti K. Identification by molecular cloning of an autoantigen associated with Addison’s disease as steroid 17α-hydroxylase. Lancet. 1992;339:770–3. doi: 10.1016/0140-6736(92)91894-e. [DOI] [PubMed] [Google Scholar]
- 10.Betterle C, Volpato M, Rees Smith B, et al. Adrenal cortex and steroid 21-hydroxylase autoantibodies in children with organ-specific autoimmune diseases: markers of high progression to clinical Addison’s disease. J Clin Endocrinol Metab. 1997;82:939–42. doi: 10.1210/jcem.82.3.3849. [DOI] [PubMed] [Google Scholar]
- 11.Wacher VJ, Silverman JA, Zhang Y, Benet LZ. Role of P glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J Pharmaceut Sci. 1998;87:1322–30. doi: 10.1021/js980082d. [DOI] [PubMed] [Google Scholar]
- 12.Lytton SD, Helander A, Zhang-Gouillon ZQ, et al. Autoantibodies against cytochromes P4503A and P4502E1 in alcoholics. Mol Pharmacol. 1999;55:223–33. doi: 10.1124/mol.55.2.223. [DOI] [PubMed] [Google Scholar]
- 13.Christians U, Sweing KF. Alternative cyclosporine metabolic pathways and toxicity. Clin Biochem. 1995;28:547–59. doi: 10.1016/0009-9120(95)00037-3. [DOI] [PubMed] [Google Scholar]
- 14.Su Q, Weber L, Lehir M, Zenke G, Ryffel B. Nephrotoxicity of cyclosporin A and FK506: inhibition of calcineurin phosphatase. Ren Physiol Biochem. 1995;18:128. doi: 10.1159/000173910. [DOI] [PubMed] [Google Scholar]
- 15.Ellouk-Achard S, Martin C, Duc HT, et al. FK506 (Tacrolimus) decreases the cytotoxicity of cyclosporin A in rat hepatocytes in primary culture: implication of CYP3A induction. Arch Toxicol. 1998;72:257–63. doi: 10.1007/s002040050499. 10.1007/s002040050499. [DOI] [PubMed] [Google Scholar]
- 16.Izuishi K, Wakabayashi H, Ohnishi T, Maeta H, Takashi M, Ichikawa Y. Effects of an immunosuppressive agent, tacrolimus (FK506), on the activities of cytochrome P-450-linked monooxygense systems in rat liver microsomes. Int J Biochem Cell Biol. 1997;29:921–8. doi: 10.1016/s1357-2725(97)00027-7. [DOI] [PubMed] [Google Scholar]
- 17.Loebstein R, Koren G. Clinical pharmacology and therapeutic drug monitoring in neonates and children. Ped Rev. 1998;19:423–8. doi: 10.1542/pir.19-12-423. [DOI] [PubMed] [Google Scholar]
- 18.Leeder S, Kearns GL. Pharmacogenetics in pediatrics: implications for practice in new frontiers in pediatric drug therapy. Pediatr Clin N Am. pp. 55–77. [DOI] [PubMed]
- 19.Gillam EM, Guo Z, Guengerich FP. Expression of modified human cytochrome P450 2E1 in Escherichia coli, purification, and spectral and catalytic properties. Arch Biochem Biophys. 1994;312:59–66. doi: 10.1006/abbi.1994.1280. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Gillam EM, Ohmori S, Tukey RH, Guengerich FP. Expression of modified human cytochrome P450 1A1 in Escherichia coli: effects of 5′ substitution, stabilization, purification, spectral characterization, and catalytic properties. Arch Biochem Biophys. 1994;312:436–46. doi: 10.1006/abbi.1994.1330. [DOI] [PubMed] [Google Scholar]
- 21.Eliasson E, Mkrtchian S, Halpert JR, Ingelman-Sundberg M. Substrate-regulated, cAMP-dependent phosphorylation, denaturation, and degradation of glucocorticoid-inducible rat liver cytochrome P450 3A1. J Biol Chem. 1994;269:18378–83. [PubMed] [Google Scholar]
- 22.Combalbert J, Fabre I, Fabre G, et al. Metabolism of cyclosporin A. IV. Purification and identification of the rifampicin-inducible human liver cytochrome P-450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metab Dispos. 1989;17:197–207. [PubMed] [Google Scholar]
- 23.Yasukochi Y, Masters BS. Some properties of a detergent-solubilized NADPH-cytochrome c (cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976;251:5337–44. [PubMed] [Google Scholar]
- 24.Laemelli UK. Cleavage of structural properties during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Manns MP, Obermayer-Straub P. Cytochromes P450 and uridine triphosphate-glucuronosyltransferases: model autoantigens to study drug-induced, virus-induced, and autoimmune liver disease. Hepatology. 1997;26:1054–66. doi: 10.1002/hep.510260438. [DOI] [PubMed] [Google Scholar]
- 26.Shinkura N, Ikai I, Egawa H, et al. Presence of anti-FKBP12 autoantibodies in patients with liver allografts: its association with allograft rejection. Transplantation. 1997;64:1336–42. doi: 10.1097/00007890-199711150-00017. [DOI] [PubMed] [Google Scholar]
- 27.Haehner BD, Gorski JC, Vandenbranden M, et al. Bimodal distribution of renal cytochrome P450 3A activity in humans. Mol Pharmacol. 1996;50:52–59. [PubMed] [Google Scholar]
- 28.Lohr JW, Willsky GR, Acara MA. Renal drug metabolism. Pharmacol Rev. 1998;50:107–41. [PubMed] [Google Scholar]
- 29.Sadrieh N, Thomas PE. Characterization of rat cytochrome P450 isozymes involved in the covalent binding of cyclosporin A to microsomal proteins. Toxicol Appl Pharmacol. 1994;127:222–32. doi: 10.1006/taap.1994.1156. [DOI] [PubMed] [Google Scholar]
- 30.Murray M. Drug-mediated inactivation of cytochrome P450. Clin Exp Pharmacol Physiol. 1997;7:465–70. doi: 10.1111/j.1440-1681.1997.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 31.Lohse AW, Obermayer-Straub P, Gerken G, et al. Development of cytochrome P450 2D6-specific LKM-autoantibodies following liver transplantation for Wilson’s disease—possible association with a steroid-resistant transplant rejection episode. J Hepatol. 1999;31:149. doi: 10.1016/s0168-8278(99)80175-5. [DOI] [PubMed] [Google Scholar]
- 32.Eichelbaum M, Burk O. CYP3A genetics in drug metabolism. Nature Med. 2001;2:285–6. doi: 10.1038/85417. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez F, Ciocca M, Velasco C, et al. Short term cyclosporine induces a remission of autoimmune hepatitis in children. J Hepatol. 1999;30:222–7. doi: 10.1016/s0168-8278(99)80065-8. [DOI] [PubMed] [Google Scholar]
- 34.Di Paolo S, Schena A, Morrone LF, et al. Immunologic evaluation during the first year of life of infants born to cyclosporine-treated female kidney transplant recipients: analysis of lymphocyte subpopulations and immunoglobulin serum levels. Transplantation. 2002;69:2049–54. doi: 10.1097/00007890-200005270-00013. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JD, Kearns GL. Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Therapeutics. 1999;66:66–7535. doi: 10.1016/S0009-9236(99)70055-X. [DOI] [PubMed] [Google Scholar]
- 36.Herzog D, Yamamamoto AM, Jara P, Maggiore G, Slares J, Alvarez F. Sera of children with hepatitis C infection and anti-liver-kidney microsome 1 antibodies recognize different CYP2D6 epitopes than adults with LKM+/HCV+ sera. J Pediat Gastroenterol Nutr. 1999;29:551–5. doi: 10.1097/00005176-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Kerkar N, Nadzic N, Davies E, et al. De novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351:409–12. doi: 10.1016/S0140-6736(97)06478-7. [DOI] [PubMed] [Google Scholar]
- 38.Pascual M, Swinford RD, Ingelfinger JR, Williams WW, Cosimi AB, Tolkoff-Rubin N. Chronic rejection and chronic cyclosporin toxicity in renal allografts. Immunol Today. 1998;19:514–9. doi: 10.1016/s0167-5699(98)01324-3. [DOI] [PubMed] [Google Scholar]
- 39.Mathieson PW. Cyclosporin: nephro-protective as well as nephrotoxic? Clin Exp Immunol. 2000;121:179–80. doi: 10.1046/j.1365-2249.2000.01289.x. 10.1046/j.1365-2249.2000.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


