Abstract
Streptococcus suis capsular type 2 is an important aetiologic agent of swine meningitis, and it has been highlighted as a cause of occupational disease leading to meningitis and fulminant sepsis in humans. The objective of the present work was to study the ability of S. suis type 2 to induce the release of tumour necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-8 and monocyte chemotactic protein one (MCP-1) by human monocytic THP-1 cells. The induction of these five cytokines was dose- and incubation time-dependent, and it was significantly enhanced by pre-treatment of cells with interferon gamma. IL-8 levels were markedly higher compared with those obtained with the other cytokines. However, elevated levels of MCP-1 and IL-6 were also observed. Levels of cytokine induced by heat-killed or live bacteria were similar. Pre-treatment of cells with anti-CD14 monoclonal antibodies suggested that this important host receptor is partially implicated in TNF, IL-1, IL-6 andMCP-1 production, while CD14-independent pathways seem to be responsible for IL-8 production after S. suis stimulation. In addition, blocking studies with anti-TNF and anti-IL-1 antibodies revealed that these cytokines are involved in amplification of the S. suis-induced cytokine cascade. When several different S. suis strains of human or porcine origin were compared, a very heterogeneous pattern of cytokine production was observed. Human strains did not exhibit a clear tendency to induce higher cytokine release by human THP-1 monocytes. The synergistic effect of the up-regulation of cytokines during S. suis meningitis may mediate many of the inflammatory reactions, including the sequestration of leucocytes at the site of infection.
Keywords: Streptococcus suis, meningitis, pro-inflammatory cytokines, THP-1 cells, CD14
INTRODUCTION
Streptococcus suis capsular type 2 is an important pathogen which has been associated with a wide variety of infections in swine such as meningitis, septicaemia, arthritis and pneumonia. Clinical presentation of S. suis infection may be as varied as asymptomatic bacteremia to fulminant systemic disease similar to Gram- negative (G–) sepsis. Meningitis is the most striking feature, and the presence of fibrin, oedema and cellular infiltrates in the meninges and choroid plexus are the histopathological characteristics most frequently observed [1]. Streptococcus suis has also been isolated from human cases of meningitis, endocarditis, septicaemia and toxic-shock syndrome. Streptococcus suis infection in humans is considered an occupational disease of increasing importance, and is considered to be the major cause of adult meningitis in Hong Kong [2,3].
The pathogenesis of the S. suis infection is still unclear, although it is accepted that transmission is via the respiratory route, after which the pathogen remains localized in the palatine tonsils of pigs. From there, bacteria may invade the blood, meninges or other tissues, possibly in close association with monocytes/macrophages [4]. Once in the central nervous system (CNS), the induction of an acute inflammatory exudate increases the volume of the cerebrospinal fluid (CSF), leading to increased intracranial pressure [5].
Although the basis for the earliest steps of the innate immune response to infection with Gram-positive (G+) bacteria is poorly understood, the host response to invasion appears to share similar characteristics with the response to G– micro-organisms. Invasion of the bloodstream by G+ and G– bacteria causes the sepsis syndrome characterized by the induction of cytokines and other inflammatory mediators [6,7]. The primary immune activator of G– bacteria is lipopolysaccharide (LPS). In contrast, no clearly identifiable constituent of G+ bacteria can be linked to the sepsis syndrome. The G+ cell wall consists of peptidoglycan (PGN), lipoteichoic acids (LTA) and various proteins, any of which could be involved in the activation of host cells leading to inflammation [7–10].
CD14, in addition to being a high affinity receptor for LPS, has been proposed as the first host pattern-recognition receptor involved in recognition of most of the above-mentioned bacterial components [9], and facilitates expression of inflammatory molecules, probably via activation of the Toll-like receptors [6,11]. Given the common dependence on many cell wall products on CD14, similar activation pathways have been hypothesized for both G+ and G– bacteria [6]. However, blockade studies with anti-CD14 antibodies reveal that there are also CD14- independent pathways responsible for cell stimulation by G+ bacteria [12,13].
The main cytokines involved in the regulation of inflammation are interferon gamma (IFN-γ), known as the chief macrophage-activating factor, interleukin-1 (IL-1), IL-6 and tumour necrosis factor alpha (TNF-α). Increased serum concentrations of IL-1, IL-6 and TNF-α (produced mainly by monocytes/macrophages) correlate with the severity of bacterial septicaemia [14]. In addition to these cytokines, chemokines are also involved in the initiation and propagation of inflammatory responses characterized by sequestration of leucocytes at the site of infection. IL-8 is the prototype member of the CXC chemokines, which have strong neutrophil chemotactic and activating properties, whereas monocyte chemotactic protein one (MCP-1) is the prototype member of the CC chemokine family which elicits primarily mononuclear cells. Monocytes/ macrophages are also a rich source of chemokines [15]. In addition to pro-inflammatory cytokines, high levels of MCP-1 and IL-8 have been observed in the CSF of patients with bacterial meningitis (i.e. Neisseria meningitidis, Streptococcus pneumoniae, Haemophilus influenzae), and they would be responsible for the chemotactic activity in the CNS [14,16,17].
Despite the well recognized association between several infectious diseases and the overproduction of pro-inflammatory cytokines, limited studies on the role of the inflammatory response in the pathogenesis of S. suis infections have been reported. Only one previous study has addressed S. suis cytokine activation of murine macrophages, and little is known about host receptors recognized by S. suis [18]. Moreover, the capacity of this important zoonotic meningeal pathogen to induce cytokine production by human cells has never been studied, and the interaction between strains of S. suis type 2 isolated from human cases and human host cells has not been studied before. This led us to investigate the induction of proinflammatory cytokines and chemokines TNF-α, IL-1β, IL-6, IL-8 and MCP-1 in human THP-1 monocytes stimulated with S. suis type 2. Since IFN-γ is the earliest cytokine detected at the site of infection and plays a critical role in cell priming and activation [19–21], the IFN-γ pre-activated cell model was compared with non-activated cells. The possible mechanism by which S. suis may stimulate monocytes (i.e. CD14-dependent or -independent pathways) was also addressed. In addition, we compared the ability of different S. suis type 2 strains of human or porcine origin to induce the release of cytokines by human THP-1 monocytes.
MATERIALS AND METHODS
Reagents
Cell culture media, foetal bovine serum (FBS), penicillin G (PenG) and streptomycin (Sm) were purchased from Gibco (Burlington, VT, USA); 2-mercaptoethanol (2-ME) was obtained from Bio-Rad (Mississauga, Ontario, Canada). Escherichia coli 0127:B8 LPS and polymyxin B sulphate (PmB) were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). Recombinant human IFN-γ was purchased from R & D Systems (Minneapolis, MN, USA).
Bacterial strains and growth conditions
The S. suis capsular type 2 virulent strain 31533, originally isolated from a case of porcine meningitis [22] and previously used for cytokine induction studies with murine macrophages [18], was used as the reference strain in this study. Strains of porcine or human origin used in the comparative study of cytokine production are listed in Table 1. Bacteria were maintained as stock cultures in 50% glycerol-Todd-Hewitt broth (THB; Difco Laboratories, Detroit, MI, USA) at – 80°C. Bacteria were grown overnight onto bovine blood agar plates at 37°C, and isolated colonies were used as inocula for THB, which were incubated for 18 h at 37°C. Working cultures for monocyte stimulation were produced by inoculating 10 ml of the overnight cultures into 200 ml THB with agitation at 37°C for 6 h until they reached the mid-log phase (540 nm, optical density of 0·4–0·5). Bacteria were washed twice in phosphate-buffered saline (PBS), pH 7·4, and diluted to approximately 2 × 109 colony-forming units (cfu)/ml in PBS. The final suspension was plated onto THB agar in order to accurately determine cfu/ml. Bacteria were then killed by heat treatment by incubating organisms at 60°C for 45 min (minimal experimental conditions required for killing S. suis) [18]. The killed cultures were subcultured onto blood agar plates at 37°C for 48 h to confirm the absence of viable organisms. Killed bacterial preparations were stored at 4°C and resuspended in cell culture media just before stimulation assays. In certain experiments, live bacteria (prepared as mentioned above) were used at initial infectious concentration of 104 cfu/ml. Heat-killed or live bacterial preparations at the concentrations indicated above did not cause cytotoxic effects to cultured cells, as evaluated by previously described lactate dehydrogenase (LDH) measurement (unpublished observations) [18,23,24].
Table 1.
Streptococcus suis capsular type 2 strains of porcine and human origins used in this study
| Strain | Origin | Virulence * | Geographic origin |
|---|---|---|---|
| 31533† | Diseased pig | V | France |
| S735‡ | Diseased pig | V | The Netherlands |
| D282 | Diseased pig | V | The Netherlands |
| 94–623 | Pig, healthy carrier | NV | France |
| TD10 | Pig, healthy carrier | NV | UK |
| 89–1591 | Diseased pig | V | Canada |
| 90–1330 | Diseased pig | NV | Canada |
| 89–999 | Diseased pig | V | Canada |
| Reims | Human; spondylodiscitis | NT | France |
| EUD95 | Human; meningitis | NT | France |
| Biotype 2 | Human; endocarditis | NT | France |
| HUD Limoge | Human; septic shock | NT | France |
| FRU95 | Human; meningitis | NT | France |
| LEF95 | Human; meningitis | NT | France |
| 96–52466 | Human; arthritis | NT | France |
| H11/1 | Human; meningitis | V | UK |
| AR770353 | Human; meningitis | NT | The Netherlands |
| AR770297 | Human; meningitis | NT | The Netherlands |
| 91–1804 | Human; endocarditis | NT | Canada |
| 94–3037 | Human; meningitis | NT | Canada |
| 98–3634 | Human; endocarditis | NT | Canada |
| 99–734723688 | Human; septicaemia | NT | Canada |
As indicated in the literature using experimental porcine models [22,52,53]. V: virulent; NV: non-virulent; NT: never tested. Strain H11/1: P. Norton, personal communication.
Strain used as reference in the present work, as well as in a previous work on S. suis cytokine stimulation [18]
ATCC 43765 S. suis type 2 reference strain.
Cell lines and cell culture
THP-1 human monocytic cell line, derived from an acute monocytic leukaemia (ATCC TIB-202, Rockville, MD, USA), was maintained in RPMI 1640 medium. Cell culture medium was supplemented with 10% heat-inactivated FBS, PenG (100 IU/ml), Sm (100 μg/ml) and 50 mm 2-ME, and cells were incubated at 37°C with 5% CO2.
Stimulation of THP-1 monocytes
For stimulation assays, 48 h cultures of THP-1 cells were washed once and resuspended in culture media at 106 cells/ml; 0·5 ml of this suspension was distributed in 24-well plates (FalconTM, Becton Dickinson, Bedford, MA, USA). Streptococcus suis strains (0·5 ml), diluted appropriately in culture medium, were added and tested in duplicate wells for each stimulation assay. Pre-activation and differentiation of monocytes was afforded by pre-treatment with IFN-γ (500 U/ml) for 48 h before induction. Pre-activated cells were then washed and resuspended in fresh medium as described above. Monocytes stimulated with LPS (1 μg/ml) served as positive controls. Monocytes with medium alone served as controls for spontaneous cytokine release. Cytokine induction plates were incubated at 37°C with 5% CO2. At different time intervals (see Results), culture supernatant fluids were harvested from individual wells, aliquoted, and frozen at – 20°C until cytokine determination. Each test of monocyte stimulation was done at least in triplicate.
Enzyme-linked immunosorbent assays (ELISA) for cytokines
TNF-α, IL-1β, IL-6, IL-8 and MCP-1 were measured by ELISA using pair-matched monoclonal antibodies (MoAbs) from R & D Systems, according to the manufacturer’s recommendations. Twofold dilutions of recombinant human TNF-α (3000 to 46 pg/ml), IL-1β (300 to 5 pg/ml), IL-6 (1500 to 3 pg/ml), IL-8 (600 to 5 pg/ml) or MCP-1 (500 to 8 pg/ml) were included as standard curves in each ELISA plate (NuncTM, VWR, Ville Mont Royal, Quebec, Canada). All cytokine standards were obtained from R & D Systems. Supernatant dilutions giving optical density readings in the linear portion of the appropriate standard curve were used to quantify the levels of each cytokine in the samples. Standard and sample dilutions were added in duplicate wells to each ELISA plate. All analyses were performed at least four times for each individual monocyte-stimulation assay.
Cytokine blockade
In selected experiments, we sought to assess whether bacterial cytokine production was the result of stimulation by other cytokines. To accomplish this objective, IFN-γ pre-activated THP-1 cells were cultured in the presence of mouse anti-human TNF-α (IgG1; 40 μg/ml), IL-1β (IgG1; 15 μg/ml) or MCP-1 (IgG2b; 5 μg/ml) MoAbs, or the combination of both TNF-α + IL-1β MoAbs. MoAbs were obtained from R & D Systems and were added in optimal blocking concentrations to ensure complete neutralization, as indicated by the manufacturer. Neutralizing MoAbs were added at the beginning of culture together with the bacterial stimulus (heat-killed 31533 strain at 109 cfu/ml) for 3 h or 24 h (depending on the cytokine being tested; see Results). Purified mouse monoclonal IgG1 and IgG2b (R & D Systems) used at similar concentrations of corresponding MoAbs served as isotype controls. Values were transformed to percentage of inhibition of values obtained for bacterial stimulus without treatment (100% of production) for comparison between experiments.
CD14 blockade
To evaluate the role of the CD14 receptor in cytokine induction by S. suis, IFN-γ pre-activated THP-1 cells were pre-incubated for 1 h at 37°C with anti-human CD14 MoAbs before adding the stimuli. Three different anti-CD14 MoAbs were used: clone MY4 (IgG2b; 10 μg/ml); clone IOM2, also named RMO52 (IgG2a; 20 μg/ml; Coulter Immunology, Mississauga, ON, Canada); and clone Leu-M3 (IgG2b; 5 μg/ml; Becton Dickinson, San Jose, CA, USA). Purified mouse monoclonal IgG2a and IgG2b (R & D Systems) used at similar concentrations of corresponding MoAbs served as isotype controls. The bacterial stimulus (heat-killed 31533 strain at 109 cfu/ml) or LPS (1 μg/ml), used as a reference to evaluate the effect of anti-CD14 MoAbs, was incubated in the presence of the anti-CD14 MoAbs for 3 h or 24 h (depending on the cytokine being tested; see Results). Values were transformed to percentage of inhibition of values obtained for bacteria or LPS without treatment (100% of production) for comparison between experiments.
Endotoxin contamination
All solutions and bacterial preparations used in these experiments were tested for the presence of endotoxin using a Limulus amebocyte lysate (LAL) gel-clot test (Pyrotell, STV, Cape Cod, Falmouth, MA, USA) with a sensitivity limit of 0·03 EU/ml. In some experiments, endotoxin contamination during stimulation of monocytes was controlled by parallel assays with PmB (10 μg/ml). Results from the LAL test and/or data from PmB treatment demonstrated that no significant levels of endotoxin contamination could be detected in different bacterial preparations (data not shown). Cell culture medium and IFN-γ solution contained less than 0·03 EU/ml. Thus, endotoxin levels represented <0·005 ng ml–1 endotoxin, a concentration below that known to cause macrophage activation [25].
Statistical analysis
Results were derived from linear regression calculations and expressed in pg/ml of cytokine. Results are presented as mean ± standard deviation (s.d.) of independent experiments. Differences were analysed for significance using the Student’s unpaired t-test (two-tailed P-value). A P-value <0·05 was used as the threshold for significance. Differences between the groups of strains of human and porcine origin, and differences among strains within the same group, were analysed for significance using general linear models (GLM) followed by Tukey-Kramer post-hoc tests for differences between strains. GLM was used for analysis of variance (anova), with the origin as one factor and the strain within origin as the other factor. The GLM procedure uses the method of least squares to fit general linear models. The SAS v8 software (SAS, Cary, NC, USA) was used for these analyses.
RESULTS
Kinetics of cytokine release by monocytes triggered by S. suis: effect of IFN-γ pre-activation of cells
Incubation of THP-1 cells without stimuli yielded a low basal level of cytokine expression. These values were used to correct data obtained after S. suis or LPS stimulation throughout this work. Incubation of cells with S. suis was associated with a time- dependent production of cytokines. TNF was the first cytokine detectable, peaking at 3 h and gradually declining thereafter (Fig. 1a and Fig. 2a), whereas IL-1, IL-6, IL-8 and MCP-1 increased progressively and reached peak concentrations at time points between 24 and 48 h (Fig. 1b–e and Fig. 2b–e). The kinetics of S. suis-induced cytokine release were similar in both non-activated and pre-activated cells. However, IFN-γ pre-activated THP-1 cells showed a markedly enhanced production of cytokines following incubation with either S. suis or LPS (P < 0·001) (Fig. 2). IL-8 levels were significantly higher compared with observed levels of other cytokines; IL-6and MCP-1 values were intermediate, and significantly lower levels of TNF and IL-1 production were observed (Fig. 1 and Fig. 2).
Fig. 1.
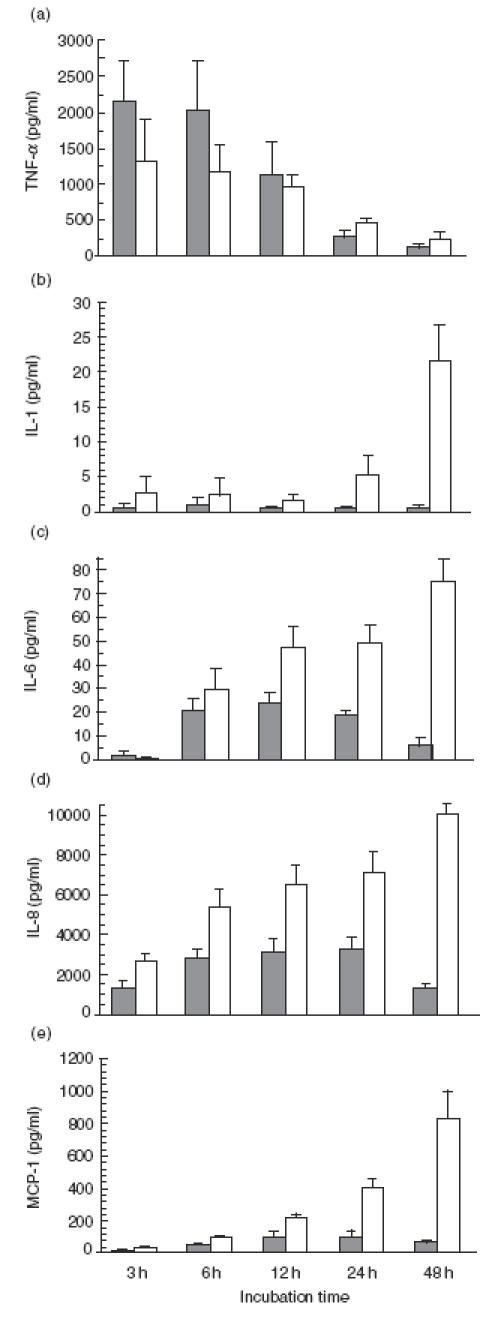
Time course of production of TNF-α (a), IL-1 (b), IL-6 (c), IL-8 (d) and MCP-1 (e) by non-activated THP-1 cells stimulated with heat-killed Streptococcus suis (□) strain 31533 (109 cfu/ml) or ( ) LPS (1 μg/ml, used as a positive control). Culture supernatant fluids were harvested at different time intervals and assayed for cytokine production by ELISA. Data are expressed as mean ± s.d. (in pg/ml). Values of basal expression of cytokines (cell culture medium alone, used as negative control) were corrected from data obtained after S. suis or LPS stimulation.
) LPS (1 μg/ml, used as a positive control). Culture supernatant fluids were harvested at different time intervals and assayed for cytokine production by ELISA. Data are expressed as mean ± s.d. (in pg/ml). Values of basal expression of cytokines (cell culture medium alone, used as negative control) were corrected from data obtained after S. suis or LPS stimulation.
Fig. 2.

Time course of production of TNF-α (a), IL-1 (b), IL-6 (c), IL-8 (d) and MCP-1 (e) by IFN-γ pre-activated THP-1 cells (500 U/ml for 48 h before induction) stimulated with heat-killed Streptococcus suis (□) strain 31533 (109 cfu/ml) or ( ) LPS (1 μg/ml, used as a positive control). Culture supernatant fluids were harvested at different time intervals and assayed for cytokine production by ELISA. Data are expressed as mean ± s.d. (in pg/ml). Values of basal expression of cytokines (cell culture medium alone, used as negative control) were corrected from data obtained after S. suis or LPS stimulation.
) LPS (1 μg/ml, used as a positive control). Culture supernatant fluids were harvested at different time intervals and assayed for cytokine production by ELISA. Data are expressed as mean ± s.d. (in pg/ml). Values of basal expression of cytokines (cell culture medium alone, used as negative control) were corrected from data obtained after S. suis or LPS stimulation.
Comparison of LPS- and S. suis-stimulated cytokines with non-activated THP-1 cells showed similar kinetics for TNF production with both stimuli (Fig. 1a), whereas levels of IL-1, IL-6, IL-8 and MCP-1 production after LPS stimulation were lower and slightly less sustained, declining after 24 h of incubation, than those obtained after S. suis stimulation (Fig. 1b–e). In contrast to those observed with non-activated THP-1 cells, similar kinetics and cytokine levels were shown after S. suis- or LPS-stimulation of pre-activated THP-1 cells (Fig. 2), except that levels of IL-8 induced by S. suis were much higher than those induced by LPS-stimulation (Fig. 2d).
Based on these experiments, IFN-γ pre-activated THP-1 cells were chosen as a model for subsequent studies. Supernatant fluids were harvested at 3 h after stimulation to analyse TNF production, whereas supernatant fluids harvested at 24 or 48 h after stimulation were used to measure induction of other cytokines.
Bacterial concentration-dependent cytokine release
IFN-γ pre-activated THP-1 monocytes were exposed to different concentrations of S. suis strain 31533. A high bacterial concentration was needed for maximal cytokine production. The two chemokines, IL-8 and MCP-1, showed the most sustained production. Levels first decreased at 108 cfu/ml, and then remained almost constant until 106 cfu/ml. Remarkably, IL-8 levels remained at 250 pg/ml at the lowest bacterial concentration tested in this study (5 × 104 cfu/ml). IL-1 and IL-6 levels gradually decreased and no production was observed at bacterial concentrations lower than 106 cfu/ml. TNF production quickly dropped when bacterial titre was decreased to 108 cfu/ml (Fig. 3).
Fig. 3.
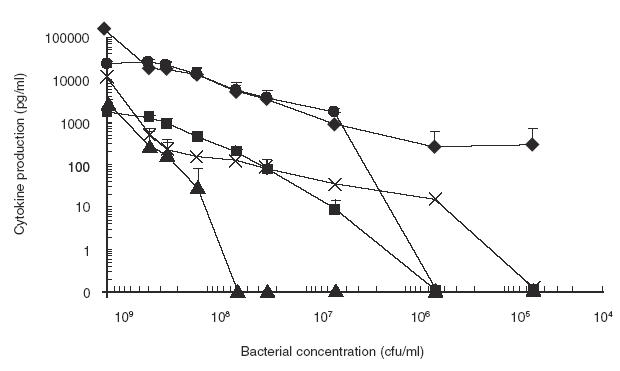
Effect of bacterial concentration on cytokine production. IFN-γ pre-activated THP-1 cells were stimulated with different concentrations of heat-killed Streptococcus suis strain 31533. TNF-α (▴), at 3 h of incubation, and IL-1 (▪), IL-6 (•), IL-8 (✦) and MCP-1 (×) at 48 h of incubation, were measured by ELISA titration of stimulated cell supernatant fluids. Data are expressed as mean ± s.d. (in pg/ml). Values of basal expression of cytokines (cell culture medium alone, used as negative control) were corrected from data obtained after S. suis stimulation.
Heat-killed versus live bacteria
In certain experiments, live bacteria at an initial infectious concentration of 104 cfu/ml were used to induce cytokine production by THP-1 cells. Since the number of bacteria rapidly increased from 104 to ˜108 cfu/ml after 3–4 h in cell culture plates, heat-killed bacteria at the latter concentration were used for comparison purposes. Under these conditions, levels of IL-6, IL-8 and MCP-1 production were similar for live and heat-killed bacteria (10739 ± 870 versus 10955 ± 1880; 114185 ± 23220 versus 100135 ± 14086; and 13523 ± 3064 versus 16828 ± 1431 pg/ml, respectively; P > 0·1). Although live bacterial preparations stimulated cells to release slightly higher levels of IL-1 than did heat-killed bacterial preparations, the difference was not significant (3221 ± 734 versus 2025 ± 872, respectively; P > 0·05). As indicated above, a 3 h stimulation time was used to analyse TNF production. However, TNF values were too low at the bacterial concentration used to reach any conclusion. Similar results were obtained when a higher initial infectious dose was used (data not shown).
Inhibition of S. suis-induced cytokines by anti-cytokine MoAbs: amplification pathways
In order to clarify to what extent TNF and IL-1 amplify the S. suis-induced cytokine network, IFN-γ pre-activated THP-1 cells were incubated with S. suis in the presence or absence of an excess of neutralizing anti-TNF MoAb, anti-IL-1 MoAb or a combination of both MoAbs, as indicated in Materials and Methods. Results are shown in Table 2. First, it was demonstrated that IgG isotype controls did not influence cytokine production (data not shown). Anti-TNF partially inhibited S. suis-induced IL-1 (P = 0·01) and IL-8 production (P = 0·005). The presence of the anti-IL-1 MoAb had no effect on IL-8 production, and no additive effect was observed when both MoAbs were used in combination. In contrast, anti-TNF or anti-IL-1 alone had no significant effect on IL-6 release, whereas the combination of both MoAbs resulted in partial inhibition of IL-6 production (P = 0·003). MCP-1 production was similarly inhibited by either anti-TNF or anti-IL-1 MoAbs. However, the combination of both MoAbs resulted in a highly significant increase of inhibition (P = 0·0001) (Table 2). TNF production was not affected by the anti-IL-1 MoAb (data not shown). These results suggest that TNF and IL-1 are involved in partial amplification pathways of S. suis-induced cytokine responses.
Table 2.
Effect of anticytokine MoAbs on Streptococcus suis-inducedcytokine release by IFN-γ pre-activated THP-cells †
| % of inhibition ‡ | ||||
|---|---|---|---|---|
| S. suis-induced | Anti-TNF | Anti-IL-1 | Anti-TNF + Anti-IL-1 | Anti-MCP |
| IL-1 | 33 ± 6* | NT | NT | 0 ± 0 |
| IL-6 | 17 ± 3 | 1 ± 1 | 31 ± 7* | 0 ± 0 |
| IL-8 | 31 ± 5* | 5 ± 4 | 28 ± 3* | 6 ± 4 |
| MCP-1 | 35 ± 3* | 35 ± 9* | 65 ± 4** | NT |
IFN-γ pre-activated THP-1 cells were cultured in the presence of antihuman TNF-α (40 μg/ml), IL-1β (15 μg/ml) or MCP-1 (5 μg/ml) MoAbs; or the combination of both TNF-α + IL-1β MoAbs. Neutralizing MoAbs were added at the beginning of culture together with the bacterial stimulus (heat-killed 31533 strain at 109 cfu/ml) for 24 h.
Data are mean ± s.e.m. NT: not tested.
Significant inhibition (by the Student’s unpaired t-test) respect to values for bacterial stimulus without treatment (100% of production).
P < 0·01;
P < 0·001.
Other than the chemotactic properties of MCP-1, this cytokine has been shown to induce the production of IL-1 and IL-6 by monocytes, suggesting an amplification pathway which might serve to sustain an inflammatory response [15]. This hypothesis was also tested in the S. suis-induced response, but the anti-MCP-1 MoAb did not affect the production of any cytokine tested (Table 2).
Role of CD14 receptor in S. suis-induced cytokines
We used three anti-human CD14 MoAbs (MY4, IOM2 and Leu-M3) to evaluate the role of CD14 in the S. suis-induced cytokine response. MY4 recognizes an epitope located between amino acids (aa) 34–44 [26]; the epitope recognized by IOM2 partially overlaps that of MY4 [27], whereas that recognized by Leu-M3 is specific for aa 135–146 on the CD14 molecule [26].
IFN-γ pre-activated THP-1 cells were subjected to pre- treatment with MoAbs for 1 h followed by stimulation with either S. suis at 109 cfu/ml or LPS at 1 μg/ml (Fig. 4). Sub-maximal doses of bacteria (5 × 108, 108 and 5 × 107 cfu/ml) or LPS (50 ng/ml) were also tested to determine whether the use of maximal doses of stimuli may mask antibody blocking effects. It was first determined that the same concentration of monoclonal isotype controls had no effect on LPS- or S. suis-induced cytokine production (data not shown). MY4 effectively blocked LPS-induced TNF, IL-1 and IL-6 (P < 0·001), and LPS-induced chemokines (P < 0·01), with both maximal (Fig. 4) or sub-maximal LPS doses (not shown). IOM2 significantly blocked LPS-induced TNF, IL-1 and IL-6 production (P < 0·001), but had no blocking effect on chemokine induction by a maximal LPS dose (Fig. 4). However, an increased blocking effect of this MoAb on IL-8 (45 ± 11% inhibition) and MCP (74 ± 8% inhibition) induction by a sub-maximal LPS dose was obtained (P < 0·01). When the effect of these two MoAbs was evaluated on S. suis-induced cytokines, a significant inhibition of TNF, IL-1 and IL-6 (P < 0·005) production was observed after pre-treatment of cells with MY4 MoAb. IOM2 also partially inhibited S. suis-induced TNF, IL-1 and IL-6 production (P < 0·01) (Fig. 4). Similar inhibitory patterns for TNF, IL-1 and IL-6 production were observed with both MoAbs after stimulation of cells with sub-maximal bacterial doses (data not shown). In contrast to LPS, neither MY4 nor IOM2 showed any effect on chemokine production by cells stimulated with S. suis at 109 cfu/ml (Fig. 4). Only MY4 was able to inhibit, to some extent, MCP-1 production by decreasing bacterial concentrations (data not shown). Maximal inhibition (38 ± 8%) was observed at a bacterial dose of 5 × 107 cfu/ml (P < 0·05). The level of IL-8 production was not affected by any of the MoAbs at any of the bacterial concentrations tested (P > 0·1, data not shown).
Fig. 4.
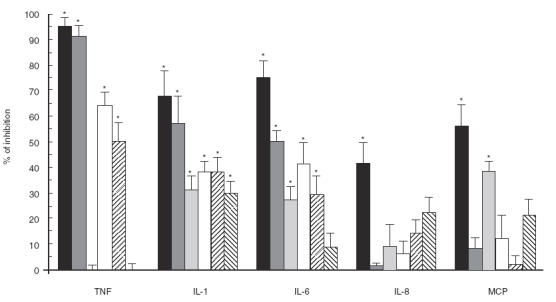
Effect of CD14 blockade on cytokine production. IFN-γ pre-activated THP-1 cells were pre-incubated for 1 h at 37°C with anti-human CD14 MoAb MY4 (10 μg/ml), MoAb IOM2 (20 μg/ml) or MoAb Leu-M3 (5 μg/ml), before adding the bacterial stimulus (heat-killed 31533 strain at 109 cfu/ml) or the LPS (1 μg/ml, used as a reference to evaluate the effect of anti-CD14 MoAbs). TNF-α, at 3 h of incubation, and IL-1, IL-6, IL-8 and MCP-1 at 24 h of incubation, were measured by ELISA titration of stimulated cell supernatant fluids. Data were transformed to percentage of inhibition with respect to values for bacteria or LPS without treatment (100% of production), and expressed as mean ± s.d. from four separate blockade experiments. (▪) LPS MY4; ( ) LPS IOM2; (
) LPS IOM2; ( ) LPS Leu-M3; (□) Streptococcus suis MY4; (
) LPS Leu-M3; (□) Streptococcus suis MY4; ( ) S. suis IOM2; (
) S. suis IOM2; ( ) S. suis Leu-M3. * P < 0·01 (versus the corresponding stimulus without treatment).
) S. suis Leu-M3. * P < 0·01 (versus the corresponding stimulus without treatment).
Figure 4 also shows results obtained with the third MoAb, Leu-M3. A completely different pattern of inhibition was observed with this MoAb compared with those described above. Leu-M3 partially inhibited the LPS-induced IL-1, IL-6 and MCP-1 (P < 0·001, P = 0·01 and P < 0·001, respectively), whereas it did not affect TNF or IL-8 induction (P > 0·1). On the other hand, Leu-M3 only partially inhibited the induction of IL-1 production by S. suis (P = 0·002).
Comparison between S. suis type 2 strains of porcine and human origin
Several strains of porcine or human origin (Table 1) were compared for their capacity to stimulate cytokine induction in IFN-γ pre-activated THP-1 cells. Although levels of cytokines induced by strains of porcine origin were, in general, more homogeneous than those induced by strains of human origin, Tukey-Kramer post-hoc tests revealed significant differences between strains within each group (P < 0·0001). In fact, no consistent effect on cytokine production could be attributed to the origin of the strain (Fig. 5). For example, no significant differences were observed in terms of production of IL-6 (P = 0·9) or MCP-1 (P = 0·8) induced by strains of different origins (Fig. 5c and Fig. 5e). While strains of human origin induced significantly higher levels of IL-1 and IL-8 production (P < 0·0001) (Fig. 5b and Fig. 5d), the opposite was observed for TNF production (P < 0·0001) (Fig. 5a). Interestingly, the strain Reims, isolated from a human case of spondylodiscitis [28], induced a much higher level of TNF, IL-1, IL-6 and IL-8 production compared with all other strains (P < 0·0001), but induced the lowest level of MCP-1 production (P < 0·001) (Fig. 5). It must also be noted that, in general, no association was observed between the virulence of the strains and the level of cytokines produced. These results suggest individual differences in the capacity of different strains of S. suis type 2 to induce cytokines.
Fig. 5.

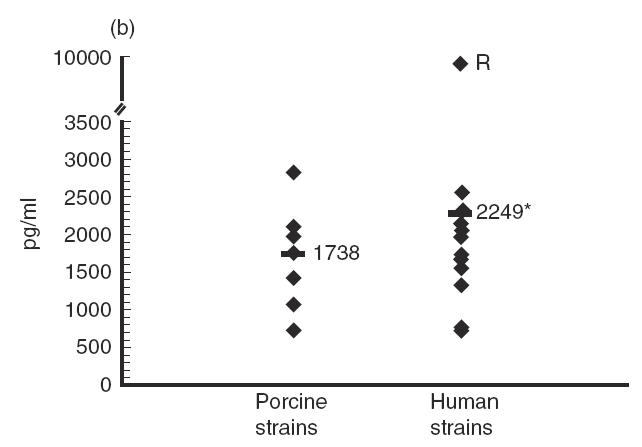
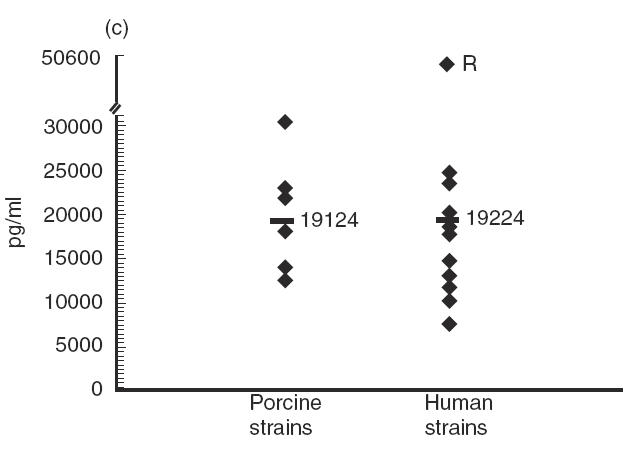
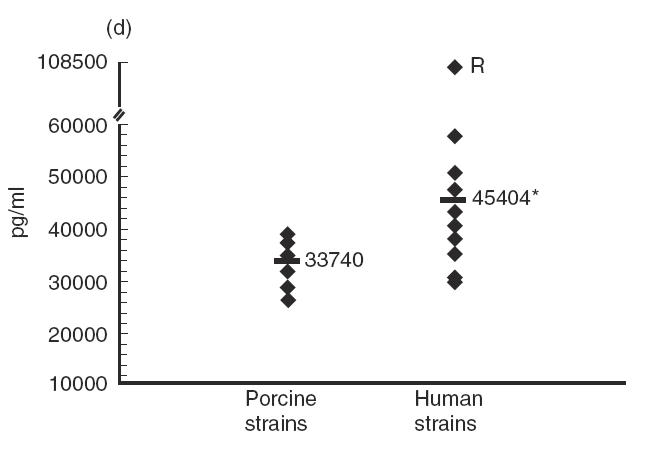
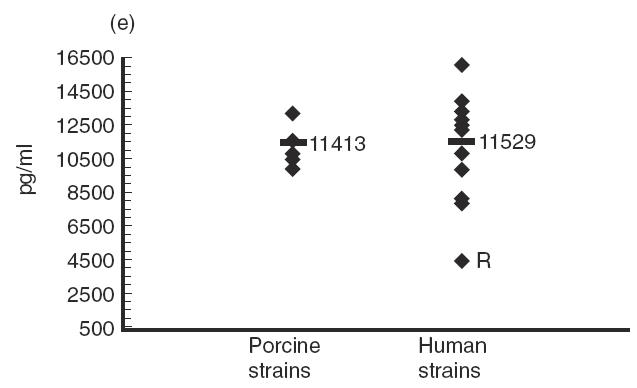
Comparative study of cytokine production by different Streptococcus suis strains. IFN-γ pre-activated THP-1 cells were stimulated by heat-killed (109 cfu/ml) S. suis type 2 strains of human or porcine origin (listed in Table 1). TNF-α (a) at 3 h of incubation, and IL-1 (b), IL-6 (c), IL-8 (d) and MCP-1 (e) at 48 h of incubation, were measured by ELISA titration of stimulated cell supernatant fluids. Points represent the mean production for each individual strain (in pg/ml). Lines represent the average cytokine production of each group of strains. *P < 0·0001 as calculated by the anova test. R indicates the S. suis human strain Reims [28].
DISCUSSION
The present work demonstrates for the first time that S. suis serotype 2 is able to interact with monocytes of human origin, inducing the release of large amounts of the pro-inflammatory cytokines TNF, IL-1, IL-6, IL-8 and MCP-1. The release of these cytokines was time- and dose-dependent. TNF is the first cytokine detected, but its levels drop much faster than those of other cytokines which remain at high levels for at least 48 h. These findings are consistent with the kinetics of TNF and IL-6 induction previously reported in murine macrophages exposed to S. suis [18], and to the reported kinetics of TNF, IL-1 and IL-6 production in THP-1 monocytes stimulated with heat-killed pneumococci [12]. Cauwels et al. [12] found that relatively high concentrations of LPS and intact pneumococci are required to induce substantial amounts of cytokines from THP-1 cells. The authors suggest that this may be attributed to the relative immaturity of THP-1 cells, including low levels of CD14 expression. Conflicting reports do exist in the literature regarding the capacity of uninduced THP-1 to release cytokines following stimulation with LPS or bacteria, and that pre-treatment of cells with vitamin D3 or IFN-γ is, in some cases, required [9,29]. In the present work, substantial levels of different pro-inflammatory cytokines were detected in supernatant fluids of uninduced THP-1 cells, although IFN-γ pre-treatment of THP-1 monocytes significantly increases the cytokine responses. In fact, in non-activated cells, S. suis induces higher cytokine levels than LPS, whereas levels are similar for both stimuli after IFN-γ pre-activation of cells. The effect of IFN-γ treatment on the expression of surface antigens (such as CD14, CD11 and CD18) and on functional activities (such as cell differentiation) may have important biological significance [30]. At the site of an immunoinflammatory response, the concentration of IFN-γ may reach very high levels. This IFN-γ may then act on infiltrating monocytes or maturing macrophages to maintain or to induce cytokine secretion in response to appropriate stimuli, such as bacterial antigens [19–21].
Important levels of IL-6, TNF and IL-1 were observed after S. suis stimulation of THP-1 cells. However, the release of these cytokines gradually decreased proportionately to bacterial concentrations, particularly in terms of TNF levels. This is in agreement with the reported large doses of heat-killed S. suis required for maximal TNF and IL-6 release by J774A1 macrophages [18]. TNF and IL-1 may act as initiators of meningeal inflammation by modulation of the brain blood barrier [17]. In vivo and in vitro evidence supports the concept that these cytokines play a positive role in local inflammatory reactions by amplifying leucocyte recruitment and by increasing the local production of chemokines by endothelial cells [17,31]. A role for pro-inflammatory cytokines in the initiation and amplification of meningeal inflammation has been suggested in S. suis infection [4]. In addition, these are the key mediators of toxic-shock syndrome [14,32] and, in this regard, recurrent septic shock due to S. suis has already been reported [33].
Streptococcus suis also induces extremely high and sustained levels of the two chemokines IL-8 and MCP-1, even at bacterial doses lower than 106 cfu/ml. Remarkably, IL-8 levels did not drop to zero, even at bacterial concentrations of 5 × 104 cfu/ml. Streptococcus suis experimental infection of specific pathogen-free pigs resulted in elevated bacteremia, with ˜105 cfu/ml recuperated from blood (unpublished observations). It has also been reported that meningeal inflammation is initiated when more that 105 pneumococci/ml are present in the CSF [34]. Thus, the sustained levels of chemokines induced at these bacterial concentrations are relevant, since chemokines are implicated in leucocyte trafficking across the vascular wall, and high levels of these chemokines are found in patients with bacterial meningitis [16]. Furthermore, IL-8 levels correlate with the number of neutrophils migrating into the CSF [17]. Neutrophils are the first leucocytes to appear at the onset of infection, but the cellular profile gradually changes to a predominantly mononuclear infiltrate [17]. The delayed and continued recruitment of monocytes mediated by chemotactic factors, such as MCP-1 derived from inflammatory cells, may also contribute to cellular damage [35]. In cases of bacterial meningitis caused by N. meningitidis, S. pneumoniae or H. influenzae, mononuclear cells are not prominent but may increase during the course of the disease [16]. Histopathological findings from diagnosed pigs with S. suis infection revealed meningeal infiltrates of a mixture of inflammatory cells. In some pigs the infiltrate was primarily neutrophilic while in others, the infiltrates contained mostly mononuclear cells [36]. Several reports of human cases of S. suis meningitis indicated turbid CSF, with high levels of leucocytes and variable percentages of neutrophils and monocytes [2,3,37]. Unfortunately, case reports are limited and data at later stages of the disease are not available, mainly due to the positive outcome of the patients after treatment.
It must be noted that heat-killed or viable S. suis induce similar levels of cytokine release in THP-1 cells. In the case of other bacterial species, such as Listeria monocytogenes, viable organisms are required to induce cytokine production [38] whereas this is not essential for other bacteria. For example, it has been reported that both living and heat-killed GBS induce TNF in murine macrophages [39], and that both viable and heat-killed Ehrlichia chaffeensis induce similar levels of IL-1β and IL-8 by human monocytes [40].
In vivo, it appears that a certain hierarchy exists wherein TNF induces IL-1 and IL-6, and a large number of other cytokines are induced by either IL-1 or TNF [14,15,19,32]. In the present work, blocking experiments demonstrated that TNF and IL-1 partially amplify the S. suis-induced cytokine cascade. Blockade experiments of cytokine release by human monocytes in response to group B Streptococcus (GBS) components revealed a TNF- independent induction of IL-1. However, IL-6 levels were significantly decreased by anti-TNF but not by anti-IL-1 antibodies [41]. The interactions between these bacterial-induced pro-inflammatory cytokines seem to be different for S. suis and GBS. In fact, important differences in the pathogenesis of the infections caused by these two pathogens have been reported [23,24,42].
The similarity between the kinetics of cytokine induction by S. suis and LPS, as well as our previous results with J774 macrophages [18], suggests that S. suis and LPS may use similar macrophage-signalling pathways. Since pre-activation of THP-1 cells with IFN-γ increases cytokine production by S. suis (this study) and CD14 expression (unpublished observations) [29,30], a role for CD14 in S. suis cytokine production may be suggested. Three anti-CD14 MoAbs were used to study the interaction between human THP-1 monocytes and S. suis, and LPS was used to validate the effect of these MoAbs. The inhibitory effect of the three anti-CD14 MoAbs on the production of the five cytokines under study varied, probably due to the different functional characteristics of the MoAbs. In fact, conflicting reports of the ability to block binding of LPS or subsequent activation by several available anti-CD14 MoAbs exist in the literature [26,43,44]. It has been reported that MoAbs MY4 and IOM2 inhibit both binding of LPS to CD14 (>95%) and LPS-mediated cell activation via CD14 (90%) [27,45]. MoAb Leu-M3 does not block LPS binding to CD14, and its use was intended as a negative control, as described in previous papers [26,27].
In the present work, S. suis-induced TNF, IL-1 and IL-6 release were significantly inhibited by MoAbs MY4 and IOM2, independent of the bacterial concentration used as stimulus. Conversely, a partial inhibitory effect was only observed with MY4 on S. suis-induced MCP-1 by decreasing the bacterial concentration. No inhibition of S. suis-induced IL-8 release was observed, regardless of the bacterial dose used. Similarly, van Furth et al. [46] reported that anti-CD14 inhibition of TNF release by monocytes following stimulation with H. influenzae is independent of the bacterial dose used, while inhibition of IL-10 production is inversely proportional to the bacterial concentration.
The region of CD14 that recognizes and binds LPS has been recently determined, and it is located in the 152 aa N-terminal region, more precisely between aa 51 and 64 [26]. Since MoAb MY4 and IOM2 epitopes partially overlap and are closely related to this region, the inhibitory effect of these two MoAbs can be due to impairment of binding of LPS to CD14, as previously reported [26,27,45]. The region of CD14 that interacts with intact S. suis is not known, but results of the present work suggest similarities with the LPS-binding site. The inhibitory effect of the these anti-CD14 MoAbs on S. suis cytokine induction could thus be explained by both impaired binding and/or reduced activation of monocytes during interaction of bacteria with CD14.
On the other hand, we confirmed that MoAb Leu-M3 does not block LPS-induced TNF production [47]. However, partial inhibition of IL-1, IL-6 and MCP-1 production induced by LPS, and IL-1 production by S. suis-stimulated THP-1 cells, was observed. Although it is known that Leu-M3 does not block the binding of LPS to CD14 [26,27], less information is available regarding the inhibition of cell activation by this MoAb. In fact, the CD14 epitope that recognizes MoAb Leu-M3 (aa 135–146) is completely outside the LPS binding region. Thus, the inhibitory effect of this MoAb can be explained by a reduced activation of monocytes during interaction of stimuli with CD14. Interestingly, an anti-CD14 MoAb (18E12) was also reported to inhibit LPS activation of cells (90%) without inhibiting LPS binding to CD14 [45,46]. Furthermore, Dziarski et al. [26] reported that MoAb Leu-M3 inhibits soluble PGN binding to CD14, which might explain the partial inhibition of the IL-1 response in cells stimulated by S. suis when this MoAb was present.
It has been shown previously that the cell wall of S. suis is the main component responsible for cytokine induction, and that the presence of the polysaccharide capsule affects IL-6 and TNF production by murine macrophages differently [18]. Binding of S. suis to monocytes and stimulation of cytokine production via CD14 may be mediated by PGN and LTA at the surface of the bacteria, as has been reported for other G+ bacteria such as GBS and S. pneumoniae [8,12]. Thus, it is possible that differences in the bacterial antigens may be responsible for the different cytokines induced and/or the interacting pathways used by S. suis to stimulate different cytokines, according to the different inhibitory patterns observed among cytokines and chemokines. Different cytokine induction patterns have also been reported for GBS, H. influenzae and S. pneumoniae [8,46].
Interestingly, total inhibition of cytokine production has not been achieved with anti-CD14 MoAbs. This was more evident when inhibition of chemokine production was attempted. The involvement of epitopes on the CD14 receptor not recognized by the three MoAbs used in the present study cannot be ruled out. On the other hand, a low but still significant production of cytokines is observed in non-activated THP-1 cells which are known to express low levels of CD14 [12]. These observations support the hypothesis that receptor(s) other than CD14 are used by S. suis to stimulate cytokine release by THP-1 monocytes. Since toll-like receptors have been shown to recognize several Gram+ bacterial species, and co-expression of CD14 and Toll-like receptor 2 synergistically enhances cell activation [6,11], a possible implication of Toll-like receptors, either in concert with CD14 or separately, in S. suis cell activation remains to be elucidated.
When several S. suis type 2 strains of human or porcine origin were compared, a large variability in their capacity to stimulate cytokine induction in THP-1 cells was observed. A clear tendency for human strains to induce higher cytokine release by human THP-1 monocytes could not be demonstrated. Although the differences in IL-8 and IL-1 levels between the two groups of strains were statistically significant, the results need to be interpreted with caution, due to the differences in cytokine induction reported for different strains. Despite the fact that pigs are of epidemiological importance to human infection [48], some cases of S. suis infection in persons not related to the porcine industry have also been reported [37]. Thus, the clinical relevance of potential species-specific differences in reactivity to bacterial strains remains unclear.
The concept of ‘virulence’ for S. suis has been debated in the literature [4,49]. In this study, strains are considered as ‘virulent’ or ‘non-virulent’ based on the development or not of clinical disease after experimental infection of piglets, respectively (Table 1). In the present work, no association could be observed between cytokine response and virulence of the strain. Interestingly, there are no observed differences between virulent and non-virulent strains in adhesion to different types of host cells (unpublished observations). Unlike other important streptococcal species, information on S. suis virulence factors, as well as molecules expressed at the surface, is limited [4]. Thus, differences in otherwise unidentified surface structures may explain the observed differences between strains. Giguère and Prescott [50] have reported that virulent and non-virulent Rodococcus equi strains induced similar levels of cytokine production in murine macrophages. They concluded that virulence is correlated with the ability to survive in macrophages but does not affect early cytokine release by macrophages. In this respect, using an experimental infection model, it was repeatedly observed that virulent S. suis strains are able to survive in circulation at high concentrations for more than 6 days, whereas non-virulent strains disappear from the circulation only 48 h after infection (unpublished observations). Wilson et al. [51] proposed that the capacity of bacteria or bacterial components to induce overproduction of cytokine in host cells can be viewed as a factor contributing to bacterial virulence.
In conclusion, S. suis may induce cytokine production by THP-1 cells in both a CD14-dependent and -independent manner, with TNF and IL-1 acting in amplification pathways. To the best of our knowledge, this is the first report of specific inflammatory cell receptor interactions implicated in the recognition of S. suis, a poorly studied pathogen. Additional studies on the signalling mechanisms involved in S. suis cytokine induction are currently in progress. A complete understanding of the interacting pathways would provide an important insight into disease progression and could contribute to the development of potential therapies for S. suis meningitis. Although effective antibiotics are available and the risk of mortality due to S. suis infection is low, high rates of neurological sequelae, such as permanent deafness, occur in patients who survive the initial infection.
Acknowledgments
We gratefully acknowledge S. Lacouture, J-M. Trudel and G. Vanier for technical assistance, and J. Moore for having edited the manuscript. We also wish to thank Dr M. Jacques and Dr M. Doré for critical reviews of the manuscript. We are also indebted to Dr M. Kobisch (Centre National d’Études Vétérinaires et Alimentaires, Ploufragan, France), Dr U. Vecht (DLO-Institute for Animal Science and Health, Lelystad, The Netherlands), Dr T. Alexander (University of Cambridge, UK), Dr L. Brasme (Centre Hospitalier Universitaire de Reims, France), Dr G. Grise (Hôpital des Feugrois, Elbeuf, France), Dr B. Cattier (Centre Hospitalier Universitaire Bretonneau, Tours, France), Dr B. François (Dupuytren Hospital, Limoges Cedex, France) and Dr P. Norton (Institute for Animal Health, Compton, UK) for providing some of the Streptococcus suis type 2 strains. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) grant # 0680154280, by the Fonds pour la Formation des Chercheurs et l’Aide à la Recherche du Québec (FCAR-équipe) grant # 99-ER-0214, and by the Canadian Research Network on Bacterial Pathogens of Swine.
REFERENCES
- 1.Higgins R, Gottschalk M. Streptococcal diseases. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Iowa State University. Ames: Diseases of swine; 1999. pp. 563–70. [Google Scholar]
- 2.Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988;10:131–7. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Chau PY, Huang CY, Kay R. Streptococcus suis meningitis. An important underdiagnosed disease in Hong Kong. Med J Aust. 1983;1:414–7. [PubMed] [Google Scholar]
- 4.Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol. 2000;75:59–71. doi: 10.1016/s0378-1135(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 5.Chanter N, Jones PW, Alexander TJ. Meningitis in pigs caused by Streptococcus suis – a speculative review. Vet Microbiol. 1993;36:39–55. doi: 10.1016/0378-1135(93)90127-s. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura A, Lien E, Ingalls RR, Tuomanen EI, Dziarski R, Golenbock DT. Cutting edge: Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 7.Verhoef J, Mattsson E. The role of cytokines in Gram-positive bacterial shock. Trend Microbiol. 1995;3:136–40. doi: 10.1016/s0966-842x(00)88902-7. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzola M, Mancuso G, Beninati C, et al. Human monocyte receptors involved in tumor necrosis factor responses to group B Streptococcal products. Infect Immun. 2000;68:994–8. doi: 10.1128/iai.68.2.994-998.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugin J, Heumann D, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 10.Wright SM. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 11.Lien E, Sellati TJ, Yoshimura A, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–25. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 12.Cauwels A, Wan E, Leismann M, Tuomanen E. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–60. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heumann D, Barras C, Severin A, Glauser MP, Tomasz A. Gram- positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–21. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whicher JT, Evans SW. Cytokines in disease. Clin Chem. 1990;36:1269–81. [PubMed] [Google Scholar]
- 15.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 16.Lahrtz F, Piali L, Spanaus K, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. doi: 10.1016/s0165-5728(97)00267-1. [DOI] [PubMed] [Google Scholar]
- 17.Sprenger H, Rösler A, Tonn P, Braune HJ, Huffmann G, Gemsa D. Chemokines in the cerebrospinal fluid of patients with meningitis. Clin Immunol Immunopathol. 1996;80:155–61. doi: 10.1006/clin.1996.0109. 10.1006/clin.1996.0109. [DOI] [PubMed] [Google Scholar]
- 18.Segura M, Stankova J, Gottschalk M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect Immun. 1999;67:4646–54. doi: 10.1128/iai.67.9.4646-4654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curfs JHAJ, Meis JFGM, Hoogkamp-Korstanje JAA. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–80. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haq AU, Rinehart JJ, Maca RD. The effect of gamma interferon on IL-1 secretion of in vitro differentiated human macrophages. J Leuk Biol. 1985;38:735–46. doi: 10.1002/jlb.38.6.735. [DOI] [PubMed] [Google Scholar]
- 21.Kubin M, Chow JM, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor α, and IL-1β production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–55. [PubMed] [Google Scholar]
- 22.Kobisch M, Gottschalk M, Morvan P, Cariolet R, Bénévent G, Joly JP. Experimental infection of SPF piglets with Streptococcus suis serotype 2. Journées Rech Porcine France. 1995;27:97–102. [Google Scholar]
- 23.Charland N, Nizet V, Rubens C, Kim KS, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–43. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalonde M, Segura M, Lacouture S, Gottschalk M. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology. 2000;146:1913–21. doi: 10.1099/00221287-146-8-1913. [DOI] [PubMed] [Google Scholar]
- 25.Martin CA, Dorf ME. Differential regulation of interleukin-6, macrophage inflammatory protein-1, and JE/MCP-1 cytokine expression in macrophage cell lines. Cell Immunol. 1991;135:245–58. doi: 10.1016/0008-8749(91)90269-h. [DOI] [PubMed] [Google Scholar]
- 26.Dziarski R, Tapping RI, Tobias PS. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–90. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 27.Couturier C, Haeffner-Cavaillon N, Caroff M, Kazatchkine MD. Binding sites for endotoxins (lipopolysaccharides) on human monocytes. J Immunol. 1991;147:1899–904. [PubMed] [Google Scholar]
- 28.Caumont H, Gerard N, Depernet B, Brasme L, Eschard JP, Etienne JC. Streptococcus suis L3–L4 spondylodiscitis in a butcher (letter) Presse Med. 1996;25:1348. [PubMed] [Google Scholar]
- 29.Raponi G, Ghezzi MC, Mancini C. The release of tumor necrosis factor alpha (TNF-α) by interferon gamma (IFN-γ) induced THP-1 cells stimulated with smooth lipopolysaccharide is inhibited by Mabs against HLA-DR and CD14 receptors on the effector cell. Microbiologica. 1997;20:1–6. [PubMed] [Google Scholar]
- 30.Perussia B, Dayton ET, Fanning V, Thiagarajan P, Hoxie J, Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983;158:2058–80. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 32.Cerami A. Inflammatory cytokines. Clin Immunol Immunopathol. 1992;62:S3–S10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 33.François B, Gissot V, Ploy MC, Vignon P. Recurrent septic shock due to Streptococcus suis. J Clin Microbiol. 1998;36:2395. doi: 10.1128/jcm.36.8.2395-2395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuomanen E, Tomasz A, Hengstler B, Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985;151:535–40. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]
- 35.Ehrlich LC, Hu S, Sheng WS, et al. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–8. [PubMed] [Google Scholar]
- 36.Sanford SE. Gross and histopathological findings in unusual lesions caused by Streptococcus suis in pigs. II. Central nervous system lesions. Can J Vet Res. 1987;51:486–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Leelarasamee A, Nilakul C, Tien-Grim S, Srifuengfung S, Susaengrat W. Streptococcus suis toxic-shock syndrome and meningitis. J Med Assoc Thai. 1997;80:63–8. [PubMed] [Google Scholar]
- 38.Mitsuyama M, Igarashi K, Kawamura I, Ohmori T, Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990;58:1254–60. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodrum KJ, Dierksheide J, Yoder BJ. Tumor necrosis factor alpha acts as an autocrine second signal with gamma interferon to induce nitric oxide in group B streptococcus-treated macrophages. Infect Immun. 1995;63:3715–7. doi: 10.1128/iai.63.9.3715-3717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee EH, Rikihisa Y. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–9. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Hunolstein C, Totolian A, Alfarone G, et al. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;65:4017–21. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura MA, Cléroux P, Gottschalk M. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol Med Microbiol. 1998;21:189–95. doi: 10.1111/j.1574-695X.1998.tb01165.x. [DOI] [PubMed] [Google Scholar]
- 43.Dentener MA, Bazil V, Von Asmuth EJU, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-α, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–91. [PubMed] [Google Scholar]
- 44.Wright SM, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 45.Viriyakosol S, Kirkland TN. A region of human CD14 required for lipopolysaccharide binding. J Biol Chem. 1995;270:361–8. doi: 10.1074/jbc.270.1.361. [DOI] [PubMed] [Google Scholar]
- 46.Van Furth AM, Verhard-Seijmonsbergen EM, Langermans JAM, Van Dissel JT, Van Furth R. Anti-CD14 monoclonal antibodies inhibit the production of tumor necrosis factor alpha and interleukin-10 by human monocytes stimulated with killed and live Haemophilus influenzae or Streptococcus pneumoniae organisms. Infect Immun. 1999;67:3714–8. doi: 10.1128/iai.67.8.3714-3718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landmann R, Knopf H, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–9. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol. 1999;37:362–6. doi: 10.1128/jcm.37.2.362-366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottschalk M, Higgins R, Quessy S. Dilemma of the virulence of Streptococcus suis strains. J Clin Microbiol. 1999;37:4202–3. doi: 10.1128/jcm.37.12.4202-4203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giguère S, Prescott JF. Cytokine induction in murine macrophages infected with virulent and avirulent Rhodococcus equi. Infect Immun. 1998;66:1848–54. doi: 10.1128/iai.66.5.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–9. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quessy S, Dubreuil JD, Caya M, Higgins R. Discrimination of virulent and avirulent Streptococcus suis capsular type 2 isolates from different geographical origins. Infect Immun. 1995;63:1975–9. doi: 10.1128/iai.63.5.1975-1979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vecht U, Arends JP, van der Molen EJ, van Leengoed LA. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am J Vet Res. 1989;50:1037–43. [PubMed] [Google Scholar]


