Abstract
Interleukin (IL)-15 is a recently discovered cytokine with the ability to stimulate the proliferation activity of Th1 and/or Th2 lymphocytes. Here, we investigated the involvement of IL-15 in the immune response to Leishmania infantum infection by studying patients with visceral leishmaniasis (VL). We found that IL-15 is produced by leishmanial antigen (LAg)-stimulated peripheral blood mononuclear cells (PBMC) from active VL patients at a significantly higher level than those produced by cells from healed VL subjects or healthy controls. A significant increase in IL-15 serum blood levels was also observed in acute VL patients compared with healed ones. Furthermore, recombinant IL-15 had an appreciable effect in vitro in reducing IL-4 and increasing the production of IL-12 in response to LAg, but it was ineffective in altering the production of interferon-γ (IFN-γ). The production of endogenous IL-15 in acute VL patients appeared to be insufficient to activate both IFN-γ and IL-12, as attested by the absence of modification of these two cytokines by neutralization experiments in the presence of anti-IL-15 monoclonal antibodies (MoAB). On the contrary, the neutralization of IL-15 increased IL-4 production. Together, these results indicate that endogenous IL-15 plays a role in the suppression of Th2-type cytokines, even though it does not enhance the production of Th1 cytokines in acute VL patients. Since IL-15, in the presence of anti-IL-4 MoAb, caused a further increase in IL-12 production and led to a significant production of IFN-γ, one of its indirect effects on Th1 cell activation could be due to the latter’s effect on Th2 cytokines such as IL-4. Therefore, our observations indicate that there is a potential for IL-15 to augment the T-cell response to human intracellular pathogens.
Keywords: IL-15, IL-4, IL-12, IFN-γ, leishmaniasis
INTRODUCTION
In the Mediterranean basin, active human visceral leishmaniasis (VL) is caused by the obligate intracellular protozoan parasite Leishmania infantum, leading to severe systemic visceral disease [1,2]. Leishmania parasites replicate in macrophages and challenge most of the immune cells in a complex manner [3,4]. The outcome of infection depends on appropriate CD4+ T-cell responses, and chronicity and high mortality are generally associated with the absence of an effective CD4+ T-cell function against the parasites [3,4]. This alteration in cell-mediated immunity is associated with a complex modification in the cytokine network which is well characterized in the murine model. In fact, in mice infected with Leishmania major, the critical role of interferon-gamma (IFN-γ) and interleukin (IL)-12 in Th1-cell development and the control of infection, and of IL-4 in Th2 development and exacerbation, is clear [3]. In humans infected with Leishmania donovani, the polarization of T-cell responses into Th1 and Th2 is not so clearly defined but also appears relevant. In fact, control of infection or complete recovery in human VL is associated with an increased production of IL-2 and IFN-γ [5–7]. Increased levels of mRNA encoding IL-10, IL-2 and IFN-γ in bone marrow aspirates [8] and lymph nodes [9] from African patients have been observed during active disease, whereas after healing, mRNA for IFN-γ and IL-2 were readily detectable [8]. Furthermore, the neutralization of IL-10 with specific monoclonal antibody (MoAb) restores T-cell proliferation and IFN-γ production in peripheral blood mononuclear cells (PBMC) from acute VL patients [10], and IL-12 shifts the responses towards a Th1 type determining the enhancement of IFN-γ production [11].
IL-15 is a recently described pleiotropic lymphokine derived from several cells and tissues, including placenta, skeletal tissue, muscle, kidney, lung, heart, fibroblasts, epithelial cells and monocytes [12]. The immunological activities of IL-15 are similar to those of IL-2, since IL-2 receptor (IL-2R) and IL-15R use the same β chain [13]; they include the stimulation of T-cell proliferation [14], B-cell maturation and isotype switching [15], the stimulation of natural killer (NK) cell proliferation and activation [13,16], the role as T-cell chemoattractant [17], and synergy with IL-12 in facilitating NK cell synthesis of IFN-γ and tumour necrosis factor alpha (TNF-α) [18]. Nevertheless, IL-15 has biological functions not shared with IL-2, which are a consequence of the fact that IL-15 uses a distinct α-chain from IL-2R [19,20]; it also presents a cytokine-specific receptor, IL-15RX, in selected cells (e.g. mast cells) [18] and, hence, induces novel signal transduction pathways [18,20,21].
IL-15 appears to enhance both Th1 responses by increasing IFN-γ production from NK and T cells [13,16,23], and Th2 responses augmenting IL-5 production [15,23]. However, IL-15 appears to contribute to the deviation of type 1/type 2 cytokine balance to type 1 predominance [24]. Recently, the ability of IL-15 to augment the local T-cell response to Mycobacterium leprae [23] and to enhance immune functions during HIV infection, restoring IL-12 production [25], has been described. Here, using cells from VL patients, we have studied the role of IL-15 during infection by evaluating its effect on the development of Th1 or Th2 type cytokine responses.
MATERIALS AND METHODS
Subjects
Six Sicilian adult patients with confirmed VL were studied. All patients had characteristic signs and symptoms of active VL, including irregular fever, hepatosplenomegaly, anaemia, leukopaenia, thrombocytopaenia and hypergammaglobulinaemia. The diagnosis was confirmed by the presence of amastigotes in spleen or bone marrow aspirate and also by a positive anti-leishmania titre evaluated by indirect immunofluorescence (>1/100) and by counterimmuno-electrophoresis. Length of illness before diagnosis was less than 6 weeks. Whole blood was collected at different stages of the disease: at the moment of diagnosis without specific treatment and after clinical recovery. The control group consisted of 10 blood donors from the same endemic area. Therapy was carried out with N-methyl-glucamine antimonate; the drug dosage was based on body surface area (bsa) according to the formula m2 = 0·13√kg2. The drug was given four times a day, intramuscularly, reaching the daily dose chosen.
Reagents and cytokines
Tissue culture medium consisted of RPMI-1640 (Euroclone Ltd, Devon, UK) supplemented with glutamine (2 mm), antibiotics, plus 10% heat-inactivated foetal calf serum (FCS, Euroclone). The FCS and RPMI were free of endotoxin at 0·03 EU (<10 ng/ml) by the limulus amebocyte lysate assay. Ficoll-Hypaque (Lymphoprep) was from Nicomed Pharm AS (Oslo, Norway). Soluble leishmanial antigen (LAg) was prepared by 10 cycles of freezing and thawing of a suspension of 2 × 108 parasites/ml phosphate-buffered saline (PBS). Recombinant human interleukin (Hu rIL)-10 (5 × 105 U/mg) and Hu rIL-15 (2 × 106 U/mg) were purchased from ImmunoKonctat (Frankfurt, Germany). Tissue culture plasticware was provided by Nunc (Roskilde, Denmark). Other reagents were purchased from Sigma. Monoclonal antibodies (MoAbs) anti-IL-10, anti-IL-4 and anti-IL-15 were obtained from ImmunoKontact.
Cell and culture conditions and in vitro stimulation of cytokine production
PBMC were incubated for 48 h with LAg (2 × 106 equivalent promastigotes) in the presence or absence of Hu rIL-10, Hu rIL-15, anti-IL-10 and anti-IL-15. Supernatant fluids were collected, filtered through 0·22 μm Millex Filters (Millipore S.A., Molshiem, France), and stored at – 70°C until tested.
Assay for cytokine determination
Cytokines in the sera and in the supernatant fluids were determined by enzyme linked immunosorbent assay (ELISA) commercial kits which employ the multiple antibody sandwich principle: IL-4 and IFN-γ (Immunotech, Marseille, France), IL-12 (the quantikine IL-12 immunoassay recognizing the IL-12 heterodimer) (R & D System, Minneapolis, MN, USA) and IL-15 (BioSource International, Comarillo, CA, USA).
Cell depletion
PBMC were depleted of T cells, monocytes, NK and B cells by negative selection using CD3, CD4, CD8, CD14, CD16 or CD19 MoAb, and magnetic beads coated with anti-mouse IgG (Dynal, Great Neck, NY, USA), as described [26]. Contaminating CD4+ or CD8+ T cells were <5%, as assessed by flow cytofluorometry (Becton Dickinson, Sunnyvale, CA, USA).
Statistical analysis
Standard deviation (s.d.) and standard error (s.e.) were calculated and statistical significance analysed by Student’s t-test by variance analysis (Student–Newmann–Keuls test); Mann–Whitney U-test was used to compare data between the two groups. P < 0·05 was considered statistically significant.
RESULTS
IL-15 levels in the sera and supernatant fluids from LAg-stimulated PBMC
The study of IL-15 activity in the sera of acute VL patients clearly indicated that IL-15 was significantly higher (P < 0·01) than that observed in healed patients and in healthy controls (Fig. 1a). Stimulation with LAg caused the induction of IL-15 by PBMC. As shown in Fig. 1(b), consistent levels of IL-15 were detected in both groups of patients, but IL-15 production was more significantly increased in PBMC from active VL patients compared with that from healed ones.
Fig. 1.
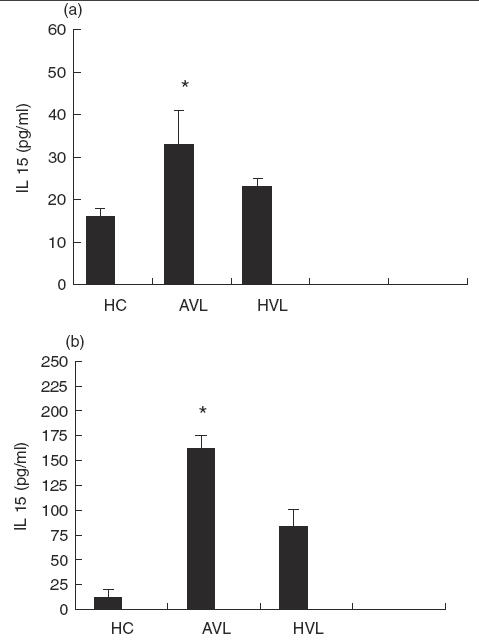
levels of IL-15 in the sera (a) and in the supernatant fluids (b) of six visceral leishmaniasis patients at the moment of diagnosis (AVL) and after recovery (HVL). Healthy controls (HC). Data are reported as mean ± s.e. *P < 0·01: significantly different from healed patients and healthy controls.
In accordance with other reports [12,14], we have observed that the main source of cells producing IL-15 is in the adherent PBMC population, since the elimination of CD14+ monocyte cells from PBMC of patients stimulated with LAg resulted in almost complete suppression of IL-15 production (Table 1). On the other hand, depletion of CD3+ T cells, CD19+ B cells or CD16+ NK cells had no significant effect on IL-15 secretion (Table 1). However, monocytes from the healthy controls and VL patients stimulated with LPS/IFN-γ were also able to produce IL-15, as already reported [12], and, furthermore, their depletion determined almost the complete reduction in IL-15 from PBMC. On the basis of these observations, we analysed the absolute number of CD14+ cells in VL patients. The results demonstrated that the absolute number of monocytes was significantly higher in healed patients than in acute ones (203 ± 15 versus 122 ± 40, P < 0·05). Therefore, our data indicate that in acute VL patients, there is no increase in the absolute number of monocytes producing IL-15, rather an increased capability of the cells to secrete IL-15.
Table 1.
Effect of cell-subset depletion on IL-15 production by PBMCof VL patient stimulated with LAg
| Cell depletion | IL-15 (pg/ml) |
|---|---|
| − | 170 ± 10* |
| -CD3 | 175 ± 15 |
| -CD4 | 180 ± 25 |
| -CD8 | 160 ± 19 |
| -CD14 | 20 ± 7† |
| -CD16 | 190 ± 15 |
| -CD19 | 159 ± 20 |
Data are expressed as mean ± s.e.
Significantly different from healthy undepleted PBMC (P < 0·01).
IL-12, IFN-γ and IL-4 production by PBMC from VL patients
In order to explore the effect of IL-15 on Th1 or Th2 cytokine production by PBMC from acute VL patients, we studied the ability of PBMC from acute and healed VL subjects to produce IL-12, IFN-γ and IL-4 in response to LAg (Table 2). The results indicate that acute VL patients were able to produce higher levels of IL-12 and IL-4 than those observed in healed subjects, even though the difference was insignificant. On the other hand, IFN-γ production was significantly reduced in the acute phase of disease compared with that detected in recovered patients, in line with previous reports [5,27].
Table 2.
IL-12, IFN-γ and IL-4 activity in supernatant fluids fromPBMC of VL patients before (A) and after (B) successful chemotherapy
Data are expressed as mean ± s.e. (pg/ml) (n = 6) and values determined by stimulating PBMC of all subjects with specific antigen.
Significantly different from healthy controls (P < 0·05).
Significantly different from acute VL patients (P < 0·05). C: healthy controls.
IL-15 effect on IL-12, IFN-γ and IL-4 production by PBMC from active VL patients
Since IL-15 synergizes with IL-12 in facilitating NK-cell synthesis of IFN-γ and TNF-α [19], it was of interest to determine whether IL-15 might affect IL-12 synthesis by LAg-stimulated PBMC from acute VL patients. Cells were cultured with LAg in the presence or absence of Hu rIL-15, anti-IL-15 MoAb, anti-IL-10 MoAb or control MoAb for 48 h, and culture supernatant fluids were tested for IL-12 content (Fig. 2a). IL-12 was significantly increased (P < 0·01) by the addition of Hu rIL-15. Similarly, the addition of neutralizing anti-IL-10 MoAb to LAg-stimulated cultures increased IL-12 production, confirming that IL-12 is negatively regulated by IL-10 [11]. The abrogation of endogenous IL-15 by neutralizing anti-IL-15 MoAb was without effect, demonstrating that in acute VL, IL-15 is beneficial for basal IL-12 expression, but it is not produced in an amount sufficient for optimal IL-12 production. Since IFN-γ is a key cytokine involved in macrophage activation and is at least partially dependent on IL-12, we examined whether IL-15 can regulate IFN-γ synthesis. We carried out these experiments again with PBMC from acute VL patients. As shown in Fig. 2(b), PBMC suspension from patients, but not from normal healthy individuals, produced significant levels of IFN-γ in response to LAg. The production of IFN-γ was modified neither by Hu rIL-15 nor by anti-IL-15 MoAb. Only IL-10 was inhibitory, as already reported [11]. Since IL-4 is associated with the progression of the disease, we investigated whether IL-4 production in PBMC from VL patients is influenced by IL-15. The addition of IL-15 determined the reduction of LAg-specific IL-4 production. Neutralizing anti-IL-15 MoAb augmented the production of IL-4, indicating that endogenous IL-15 is effective in down-regulating IL-4 synthesis (Fig. 2c).
Fig. 2.
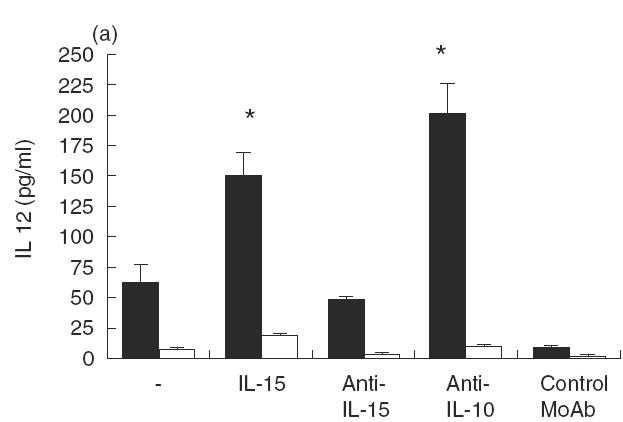
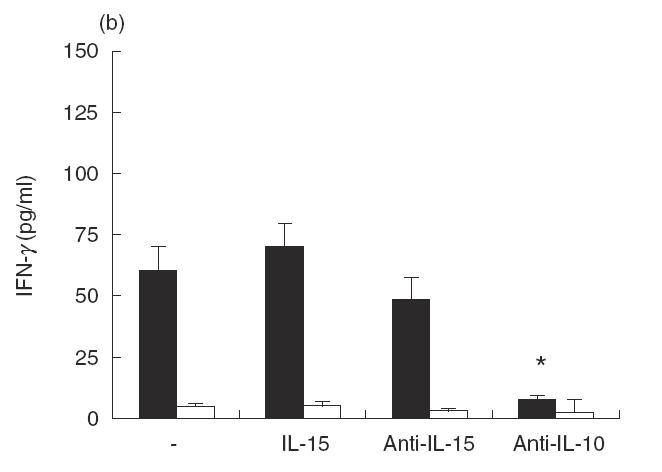
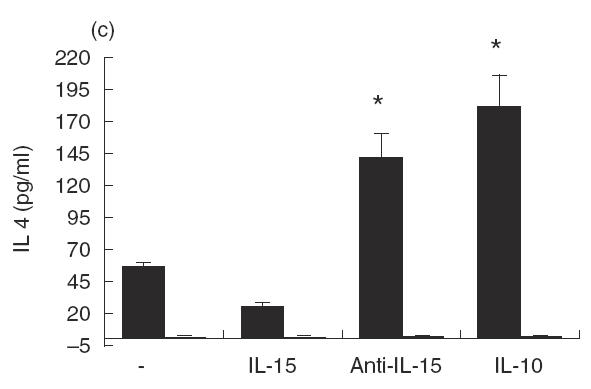
Effect of IL-15 on IL-12, IFN-γ and IL-4 production by PBMC from patients with active VL (▪). Cells (106/ml) were cultured for 48 h in the presence of LAg with or without Hu rIL-15 (1 ng/ml), anti-IL-15 MoAb (5 μg/ml), Hu rIL-10 (10 ng/ml), anti-IL-10 MoAb (25 μg/ml) or isotype-matched control MoAb. The culture supernatant fluids were analysed for IL-12 (a), IFN-γ (b) and IL-4 (c) secretion by ELISA. Normal healthy controls (□). Data from five persons (mean ± s.e.) from each group are shown. *P < 0·01: significantly different from cultures treated only with LAg.
Next, we investigated whether the effects of IL-15 on IL-12 production may be indirect and due to the latter’s effect on cytokines such as IL-4. We tested this hypothesis by culturing PBMC from acute VL patients with LAg and/or Hu rIL-15 in the presence of blocking antibodies to IL-4 (Fig. 3). Data clearly indicate that the addiction of IL-15 in the presence of anti-IL-4 MoAb caused a further increase in IL-12 production compared with that observed in the presence of IL-15 alone. Interestingly, Hu rIL-15 in the presence of MoAb against IL-4 induced a significant increase in IFN-γ production that was not observed in the presence of the IL-15 alone. These results appear to indicate that one of the mechanisms by which IL-15 exerts its effects could be the reduction of IL-4 production.
Fig. 3.
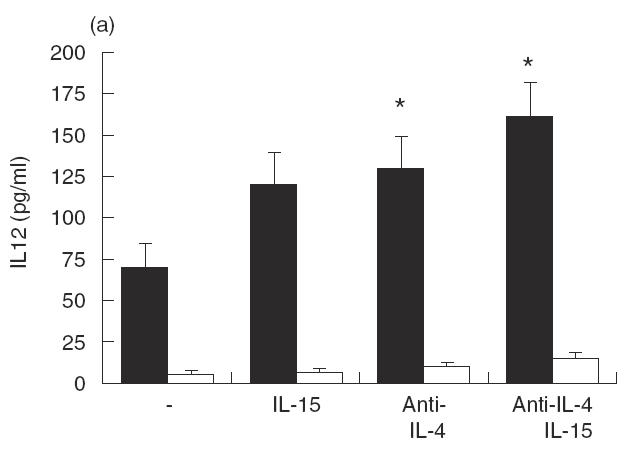
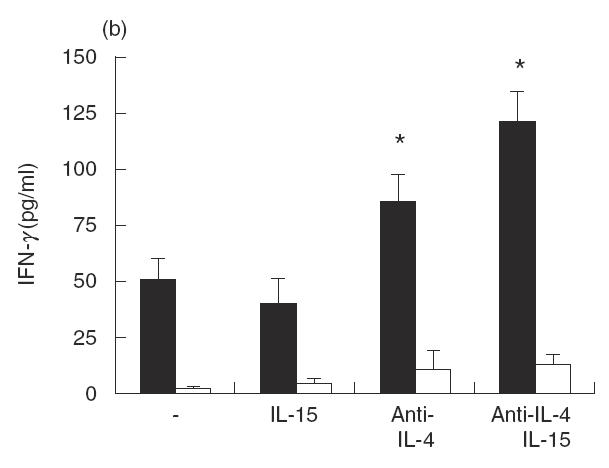
Effect of IL-15 on IL-12 and IFN-γ production by PBMC from active VL patients (▪) in the presence of anti-IL-4 MoAb. Cells (106/ml) were cultured for 48 h in the presence of LAg with or without Hu rIL-15 (1 ng/ml), anti-IL-4 MoAb (10 μg/ml) or isotype-matched control MoAb. The culture supernatant fluids were analysed for IL-12 (a) and IFN-γ (b) secretion by ELISA. Normal healthy controls (□). Data from three persons (mean ± s.e.) from each group are shown. *P < 0·01: significantly different from cultures treated only with LAg.
DISCUSSION
To our knowledge this is the first study to show that human VL PBMC produce IL-15 upon stimulation with LAg. We have found that IL-15 is produced by LAg-stimulated PBMC from active VL patients at significantly higher levels than those produced by PBMC from healed VL subjects or healthy controls. The main source of IL-15 are monocytes. Even though the absolute number of CD14+ cells is reduced in acute VL patients, the intrinsic capacity of monocytes for IL-15 production is higher than that observed in healed subjects. This could be due to the fact that IL-15 is most abundantly expressed in activated peripheral monocytes [22,28], and this is the case for acute VL circulating cells that are activated by the strong antigen load [5,6,10,29,30]. An increased level of IL-15 is also observed in the blood serum of active VL patients compared with healed ones. The ability of IL-15 to alter cytokine production of PBMC in response to LAg indicates that it could be associated with the regulation of immunity against VL.
The data on the effect of exogenous IL-15 on cytokine induction by PBMC from LV patients in response to LAg after short-term in vitro stimulation show that exogenous Hu rIL-15 is able to significantly increase IL-12 and decrease IL-4 production. However, Hu rIL-15 did not display relevant effects on IFN-γ synthesis. The ability of IL-15 to significantly increase the production of IL-12 heterodimer (IL-12/p70) by PBMC from acutely infected patients could have relevant significance in the disease pathogenesis. On the other hand, it has already been reported that the increase in IL-12/p70 is correlated with resistance against tuberculosis by M. leprae [31] and therefore, the potentiating effect of Hu rIL-15 on IL-12/p70 by PBMC from VL patients would suggest that IL-15 in VL disease could activate cell-mediated immunity, increasing IL-12 [32].
However, the observation that neutralization of IL-15 did not cause significant modifications in IL-12 production might indicate that endogenous IL-15 is beneficial but not necessary for basal IL-12 expression, or that endogenous IL-15 is insufficient to influence IL-12 activity. On the other hand, the amount of IL-15 produced in vivo or by stimulated PBMC from acute VL patients is very low compared with that added exogenously (32 ± 7 and 160 ± 18, respectively, versus 1000 pg/ml). The lack of effectiveness of endogenous IL-15 also on IFN-γ secretion indicates that in acute VL patients, circulating levels of IL-15 are unable to activate either Th1-associated cytokine critical for macrophage activation, like IFN-γ [3,4,11], or IL-12 that plays a central role in the induction and maintenance of the protective Th1 response [11,33,34].
However, the results of this study demonstrate that endogenous IL-15 could have a role in the suppression of the Th2-type cytokine response in acute VL patients, since the neutralizing antibodies against IL-15 were able to increase IL-4 secretion by PBMC. Since acute VL includes mixed Th1-like and Th2-like cells, with a dominance of Th2 cytokines [4,9,11], the ability of IL-15 to reduce Th2 responses and to up-regulate IL-12 production, which has a critical role in Th1 development [3], could give IL-15 a therapeutic potential. Furthermore, our data support the hypothesis of an indirect activity of IL-15 on IL-12, due to the latter’s effects on IL-4. In fact, in the presence of neutralizing antibody to IL-4, Hu rIL-15 was able to induce, in PBMC cultured for 48 h, strong production of IL-12 and IFN-γ. Thus, one of the mechanisms by which IL-15 exerts its effects appears to be the modulation of IL-4 production.
The ability of IL-15 to activate IFN-γ and IL-12 in the various experimental models and infectious diseases in different ways may reflect the peculiar capacity of various agents to induce IL-15 [35] and the local phenomena that take place in the infected tissues, where the modulation of IL-15 secretion begins [23]. In fact, it has been demonstrated that M. leprae was capable of eliciting mRNA for IL-15 in the lesions, but mRNA for IL-15 was more strongly expressed in immunologically-resistant tubercoloid patients than in unresponsive and susceptible lepromatous ones [23].
To summarize, we have shown that exogenous IL-15 could augment IL-12 synthesis and reduce IL-4 in PBMC suspensions from active VL patients, and endogenous IL-15 is able to moderate the Th2 response down-regulating IL-4-producing Th2 cells. Since the levels of IL-15 produced in vivo and in vitro by LAg stimulation are very low compared with those we use when we treat the cells with Hu rIL-15, the production of IL-15 during acute VL is probably useful but not enough to control the infection. Altogether, our data could suggest a possible use of IL-15, with other cytokines (IFN-γ and/or IL-12), to potentiate the conventional antimonial therapy of VL, especially as IL-15 has low toxicity in vivo [36].
Acknowledgments
We are grateful to Dr Sheila McIntyre for her help in the preparation of the manuscript. This work was supported by the Ministero Università Ricerca Scientifica e Tecnologica (MURST) 60% 1995–99.
REFERENCES
- 1.Marsden PD, Jones TC. Clinical manifestations, diagnosis and treatment of leishmaniasis. In: Chang KP, Bary RS, editors. Elsevier Science Publishers. Amsterdam: Leishmaniasis; 1985. pp. 183–98. [Google Scholar]
- 2.Gradoni L, Gramiccia M. Leishmania infantum tropism: strain genotype or host immune status? Parasit Today. 1994;7:264–8. doi: 10.1016/0169-4758(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 3.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Ann Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 4.Kemp M, Theander TG, Kharazmi A. The contrasting roles of CD4+ T cells in intracellular infections in humans: leishmaniasis as an example. Immunol Today. 1996;17:13–6. doi: 10.1016/0167-5699(96)80562-7. [DOI] [PubMed] [Google Scholar]
- 5.Cillari E, Milano S, Dieli M, et al. Reduction in the number of UCHL-1+ cells and IL-2 production in the peripheral blood of patients with visceral leishmaniasis. J Immunol. 1991;146:1026–30. [PubMed] [Google Scholar]
- 6.Carvalho EM, Badaro R, Reed SG, et al. Absence of gamma interferon and interleukin-2 production during active visceral leishmaniasis. J Clin Invest. 1985;76:2066–9. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks DL, Lai SL, Shrivastava SN, et al. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987;138:908–13. [PubMed] [Google Scholar]
- 8.Karp CL, El-Safi SH, Wynn TA, et al. In vivo cytokine profiles in patients with Kala-azar. J Clin Invest. 1993;91:1644–8. doi: 10.1172/JCI116372. Marked elevation of both interleukin-10 andinterferon-gamma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghalib HG, Piuvezam MR, Skeiky YAW, et al. Interleukin-10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho EM, Bacelar O, Brownell CE, et al. Restoration of IFN-gamma production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–56. [PubMed] [Google Scholar]
- 11.Ghalib HW, Whittle JA, Kubin M, et al. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 12.Bamford RN, Battiata AP, Burton JD, et al. Interleukin (IL)-15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I R region/IL-15 fusion message that lacks many upstream AUGs that normally attenuate IL-15 mRNA translation. Proc Natl Acad Sci USA. 1996;93:2897–902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson WE, Giri JG, Lindermann MJ, et al. Interleukin-(IL)-15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 15.Armitage RJ, MacDuff BM, Eisenman J, et al. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–90. [PubMed] [Google Scholar]
- 16.Carson WE, Ross ME, Baiocchi RA, et al. Endogenous production of interleukin-15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–82. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by IL-15. J Exp Med. 1995;181:1255–9. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamford RN, Tagaya Y, Waldmann TA. Interleukin-15. The Immunologist. 1997;5:52–6. [Google Scholar]
- 19.Borger P, Kauffman HF, Postma DS, et al. Interleukin-15 differentially enhances the expression of interferon-gamma and interleukin-4 in activated human (CD4+) T lymphocytes. Immunology. 1999;99:207–14. doi: 10.1046/j.1365-2567.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–53. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 21.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–63. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagaya Y, Bamford RN, De Filippis AP, et al. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329–36. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 23.Jullien D, Sieling PA, Uyemura K, et al. L-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–6. [PubMed] [Google Scholar]
- 24.Takeuchi E, Yanagawa H, Suzuki Y, Shinkawa K, Bando H, Sone S. Interleukin (IL-) 15 has less activity than IL-2 to promote type 2 cytokine predominance in tumor-associated mononuclear cells from lung cancer patients. Cytokine. 2001;13:119–23. doi: 10.1006/cyto.2000.0808. 10.1006/cyto.2000.0808. [DOI] [PubMed] [Google Scholar]
- 25.Chehimi J, Mason JD, Salvucci O, et al. IL-15 enhances immune functions during HIV infection. J Immunol. 1997;158:5978–87. [PubMed] [Google Scholar]
- 26.Estaquier J, Idziorek T, De Bels F, et al. Programmed cell death and AIDS: the significance of T cell apoptosis in pathogenic and non pathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–5. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cillari E, Vitale G, Arcoleo F, et al. In vivo and in vitro cytokines profiles and mononuclear cell subsets in Sicilian patients with active visceral leishmaniasis. Cytokine. 1995;7:740–5. doi: 10.1006/cyto.1995.0088. 10.1006/cyto.1995.0088. [DOI] [PubMed] [Google Scholar]
- 28.Cosman D, Kumaki S, Ahdieh M, et al. Interleukin 15 and its receptor. Chichester: John Wiley and Sons, Inc. pp. 221–9. T Cell Subsets in Infectious and Autoimmune Diseases Vol. 195.
- 29.Cenini P, Berhe N, Hailu A, et al. Mononuclear cell subpopulation and cytokine levels in human visceral leishmaniasis before and after chemoterapy. J Infect Dis. 1993;168:986–93. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- 30.Vitale G, Reina G, Mansueto S, et al. The significance of serum soluble IL-2 receptor as a marker for active visceral leishmaniasis in Sicilian patients. Clin Exp Immunol. 1992;90:219–22. doi: 10.1111/j.1365-2249.1992.tb07932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modlin RL, Barnes PF. IL-12 and human immune response to mycobacteria. Res Immunol. 1995;146:527–31. doi: 10.1016/0923-2494(96)83027-6. [DOI] [PubMed] [Google Scholar]
- 32.Parayath KE, Harrison TS, Levitz SM. Effect of interleukin (IL)-15 priming on IL-12 and interferon-gamma production by pathogen-stimulated peripheral blood mononuclear cells from human immunodeficiency virus-seropositive and seronegative donors. J Infect Dis. 2000;181:733–6. doi: 10.1086/315280. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen specific adaptive immunity. Ann Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 34.Adorini L. IL-12. Chem Immunol. 1997;68:1–205. doi: 10.1159/000058691. [DOI] [PubMed] [Google Scholar]
- 35.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–41. [PubMed] [Google Scholar]
- 36.Munger W, DeJoy SQ, Jeyaseelan R Sr, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165:289–93. doi: 10.1006/cimm.1995.1216. 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]


