Abstract
The aim of this study was to determine whether phagocytosis of necrotic or apoptotic cells affects antigen presentation by murine bone marrow-derived macrophages. After uptake of necrotic neutrophils, macrophages were able to stimulate significantly higher T cell proliferation in vitro against both the recall antigen albumin and the mitogen concanavalin A. No such effect was seen following phagocytosis of apoptotic neutrophils. Flow cytometry revealed that, within 4h of ingestion, macrophages that had taken up the necrotic cells expressed higher levels of CD40 than those that had phagocytosed apoptotic cells. Macrophage cultures pulsed with apoptotic, but not necrotic, neutrophils contained higher levels of transforming growth factor β1, but lower concentrations of tumour necrosis factor α, compared to untreated controls. Our interpretation of these results is that macrophages that have taken up necrotic neutrophils co-stimulate T cells with greater efficiency due to rapid CD40 up-regulation, whereas those that have ingested apoptotic cells are not only ineffective in co-stimulation, but also secrete inhibitory cytokine.
Keywords: antigen presentation, apoptosis, co-stimulatory molecules, macrophages, transforming growth factor β
INTRODUCTION
The induction and perpetuation of immune responses may be dependent on the immune system receiving ‘danger’ signals resulting from tissue damage [1,2] or infection [3,4], rather than tolerogenic stimuli associated with normal cell turnover. This view is supported by, for example, the poor immunogenicity of most protein antigens in the absence of adjuvant, and the extensive repertoire of quiescent autoreactive lymphocytes in healthy individuals [5–7]. One possibility is that the ability of antigen-presenting cells (APC) to stimulate responses is enhanced by the ingestion of necrotic cell debris, while the uptake of apoptotic cells exerts a neutral or inhibitory effect [1].
Although dendritic cells are important in presenting antigen, particularly to initiate primary immune responses, macrophages are the professional APC type most prominent in inflammatory sites and specialized for clearing necrotic and apoptotic material. Macrophages can act not only as professional APC, but can also perform either pro- or anti-inflammatory roles, dependent on the means by which they are activated [8,9]. Interferon-(IFN-)γ is the classical macrophage activating factor leading to synthesis and release of inflammatory mediators, enhanced phagocytosis together with up-regulation of MHC class II and co-stimulatory molecules [10,11]. However, other stimuli such as interleukin-(IL-)4 can initiate a state of ‘alternative activation’ with the development of anti-inflammatory characteristics [12]. It is now clear that macrophages develop distinct sets of properties in response to different cytokines, and that they subsequently become at least temporarily unresponsive to opposing stimuli [13]. This phenomenon of macrophage programming occurs in vivo in inflamed foci and presumably enables the cells to maintain an appropriately co-ordinated response when operating in a chaotic local environment [14].
Macrophages can be activated differentially not only by cytokines, but also by the uptake of dead cells [15]. Necrosis both precedes and follows inflammation, and the cellular debris generated is phagocytosed by macrophages, which become activated to secrete proinflammatory mediators in the process [16]. In addition, macrophages are able to recognize efficiently and ingest apoptotic cells via a number of specific mechanisms [17], including the CD36/vitronectin receptor (αvβ3) complex, scavenger receptors, the LPS receptor CD14, macrosialin CD68, exposure of phosphatidylserine on apoptotic cells, the ABC1 transporter and by poorly characterized lectin-dependent interactions. Not only do these specific uptake mechanisms prevent the release of noxious cellular contents, but the macrophage response to the phagocytosis of apoptotic cells is biased towards the release of anti-inflammatory mediators [18,19]. Although macrophages can act as a professional APC when appropriately activated, and stimulate CD4+ helper T cell responses [12], it is not known whether the phagocytosis of either necrotic or apoptotic cells affects this property. However, Gallucci et al. [2] have reported recently that dendritic cells can be activated to present antigen by the uptake of necrotic material, raising the possibility that there may be similar effects on other APC types.
Given the importance of macrophages in bridging the innate and adaptive immune systems in phagocytosis and their ability to be programmed for opposing functions, these cells are uniquely equipped for any immune discrimination between danger signals associated with cell damage and those arising from physiological cell turnover. The aim of this study was therefore to test the hypothesis that the uptake of necrotic, but not apoptotic cells, enhances the ability of macrophages to present antigen to T cells. The experiments utilized bone marrow derived macrophages (BMDM), which we have shown previously to be uncommitted cells in that they can be induced to develop mutually exclusive sets of properties when incubated with different cytokines [13]. Neutrophils were selected as the cell type to be rendered necrotic or apoptotic for uptake by the BMDM, since they are abundant but short-lived in acute inflammatory lesions. The effects of necrotic or apoptotic neutrophil ingestion on the ability of murine BMDM to stimulate antigen-specific or mitogen-driven T cell responses in vitro were compared, and the mechanism responsible for any change identified by measuring macrophage expression of MHC class II and co-stimulatory molecules, and the production of cytokines in cultures.
MATERIALS AND METHODS
Animals
BALB/c mice were supplied from sterile isolators by the Biological Service Unit at the University of Aberdeen.
Antigen and mitogen
The T cell mitogen concanavalin A (Con A) and the antigen ovalbumin (both from Sigma, Poole, Dorset, UK) were added to cultures at 20 μg/ml and 100 μg/ml, respectively, unless stated otherwise.
Immunization of mice
BALB/c mice were immunized with 0·2 mg ovalbumin in complete Freund’s adjuvant, followed by a second dose in incomplete Freund’s adjuvant 2–4 weeks later.
Isolation and culture of BMDM
Murine BMDM were obtained as described previously [13]. Briefly, bone marrow cells were flushed aseptically from the dissected femurs of female BALB/c mice to form a single cell suspension. The cells were cultured in Dulbecco’s MEM containing 10% L929 conditioned medium as a source of macrophage-colony stimulating factor (M-CSF). After 7 days in culture BMDM were dispensed into 24-well culture plates (Corning, New York, USA) at a concentration of 5 × 105 cells/well unless stated otherwise, and rested in medium without added M-CSF for 24 h prior to use in experiments.
Preparation of apoptotic and necrotic neutrophils
Neutrophils were isolated from fresh heparinized normal human blood by dextran sedimentation and Percoll centrifugation. Apoptosis was induced by ageing the neutrophils for approximately 24h [20]. More than 98% of these cells excluded trypan blue while apoptosis was verified by oil immersion light microscopy of May–Giemsa-stained cytospin preparations. Freshly isolated neutrophils were rendered necrotic by heating in a water bath at 56°C for 30 min, then for 30s in a 750-W microwave oven. All cells subjected to this treatment stained with trypan blue.
Uptake of apoptotic and necrotic neutrophils by macrophages
Apoptotic and necrotic cells were washed and re-suspended in RPMI at a concentration of 2·5 × 106/ml. One ml of either apoptotic or necrotic neutrophils was added to wells plated out with macrophages and allowed to interact for 30 min at 37°C in 5% C02 humidified atmosphere. The wells were washed in saline at 4°C to remove non-ingested neutrophils, and macrophage uptake of the remaining cells confirmed by inverted light microscopy. Macrophages prepared in this way were used in all the studies of antigen presentation.
Preparation of T cells
BALB/c splenic T cells were isolated under aseptic conditions using the method reported elsewhere [21]. T cell preparations of greater than 90% purity were obtained from single cell suspensions of macerated spleen by passage through glass bead affinity columns coated with murine IgG/rabbit antimurine IgG immune complexes [21]. The T cells were cultured at 1·25 × 106/ml in the alpha modification of Eagle’s medium (Gibco, Paisley, UK) supplemented with fresh 0·5% heat-inactivated (56°C for 30 min) BALB/c mouse serum and 5 × 10–5 M 2-mercaptoethanol (Sigma) [21].
Stimulation of T cell cultures with macrophages as APC
Proliferative responses of cultured T cells were measured as previously [21], using macrophages that had taken up dying neutrophils and been washed, or control macrophages that had not been preincubated with neutrophils, as the source of APC. Two ml of the T cell preparation were added to wells containing the adherent macrophages. Mitogenic proliferation was elicited by adding Con A to the mixed cultures of macrophages and T cells. To test their ability to induce recall antigenic responses after ingestion of neutrophils, macrophages were pulsed with ovalbumin for 1 h at 37°C in 5% C02 humidified atmosphere and washed immediately before the addition of T cells. The stimulated cultures were then incubated for 4 days under these conditions before proliferation was estimated from the incorporation of tritiated 3H] thymidine (Amersham, Bucks, UK) in triplicate 100 μl samples withdrawn from the wells, using a 1450 Microbeta Liquid Scintillation Counter (LKB Wallac). Results are presented either as the mean counts per minute (cpm) ± s.d. of the triplicate samples, or as stimulation index (SI), expressing the ratio of the mean cpm in stimulated versus unstimulated control cultures.
Flow cytometry
A benchtop FACSCalibur analyser (Becton Dickinson, Cowley, UK) was used and analysis performed with CELLQUEST software. FITC- or phycoerythrin-conjugated MoAbs against murine I-E/I-A, CD40, CD80 and CD86 were obtained from Cambridge Bioscience Ltd, Cambridge, UK.
Measurement of cytokine production in cultures
Sandwich ELISA were used to determine the concentrations of TNF-α, IFN-γ, IL-4, IL10 and active TGF-β1 in supernatants from macrophage/T cell cultures 5 days after stimulation. Capture antibody, biotinylated detection antibody and recombinant cytokine standards were obtained from Becton Dickinson (TNF-α and IL-10), Cambridge Bioscience (IFN-γ and IL-4) or R&D Systems, Abingdon, UK (TGF-β1). ELISA were performed as recommended by the manufacturers, whose data demonstrate that each of the antibody pairs is highly specific with no known cross-reactivity with other cytokines. The plates were developed by successive incubations with Extravidin-conjugated alkaline-phosphatase and the enzyme substrate p-nitrophenyl phosphate (both from Sigma), before the absorbence was read at 405 nm (Titertek Multiscan II plate reader, Labsystems, Basingstoke, UK). Cytokine levels were measured by interpolation from a standard curve generated with doubling dilutions of recombinant standard proteins. The lower limit of detection for typical assays was 5 pg/ml cytokine or less, and the coefficient of variation was less than 10%.
RESULTS
Effect of necrotic or apoptotic neutrophil uptake on the ability of macrophages to stimulate T cell proliferative responses
The influence of uptake of necrotic and apoptotic cells on the ability of macrophages to present antigen was assessed by measuring T cell proliferative responses to a mitogenic stimulus that is not dependent on antigen processing, and to a classic recall antigen.
First, murine BMDM that had taken up either necrotic or apoptotic neutrophils for 30 min were incubated with splenic T cells in the presence of the mitogen Con A. Figure 1 demonstrates that T cell proliferative responses to Con A were significantly higher in the presence of macrophages that had ingested necrotic neutrophils than in the presence of control macrophages that had not been preincubated with dying cells, or macrophages that had taken up apoptotic neutrophils. However, proliferation was similar in wells containing macrophages that had phagocytosed apoptotic neutrophils or control macrophages.
Fig. 1.
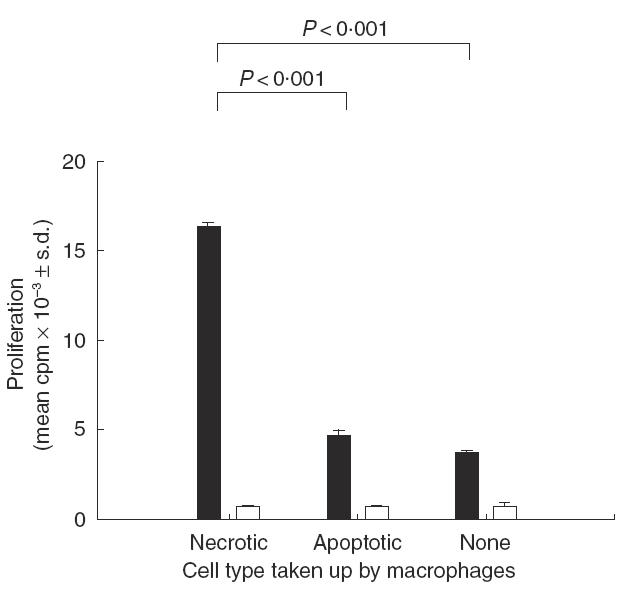
The effect of pulsing macrophages with necrotic or apoptotic neutrophils on the ability to induce splenic T cell proliferation against the mitogen Con A. Macrophages were allowed to take up dying neutrophils for 30 min and the non-ingested cells washed off before addition of the T cells and stimulation with Con A. The results in replicate cultures (n = 3) are summarized, and significant differences (P < 0·05) between proliferative responses are bracketed. Proliferation in wells containing T cells or macrophages alone was minimal (cpm <375). Stimulation: ▪, Con A; □, none.
Next, BMDM were preincubated with either necrotic or apoptotic neutrophils for 30 min and pulsed with ovalbumin for 1 h. They were then co-cultured together with splenic T cells from ovalbumin-immunized mice. Control macrophages that had not been preincubated with dying cells induced only very weak proliferation against ovalbumin (Fig. 2), but this response was enhanced significantly when the macrophages had previously ingested necrotic neutrophils. In contrast, uptake of apoptotic cells did not enhance proliferation, and indeed attenuated any response to the ovalbumin to below the background in unstimulated wells. The differential effects of necrotic and apoptotic cell uptake on the responses to ovalbumin were not dependent on the particular culture conditions chosen, since similar results were obtained when the concentrations of the macrophages or antigen were varied (Fig. 3). Analysing the results from a total of four experiments reveals that, in every case, prior incubation of macrophages with necrotic cells consistently increased the response to ovalbumin (P < 0·01), whereas uptake of apoptotic cells was associated with inhibition (P < 0·05).
Fig. 2.
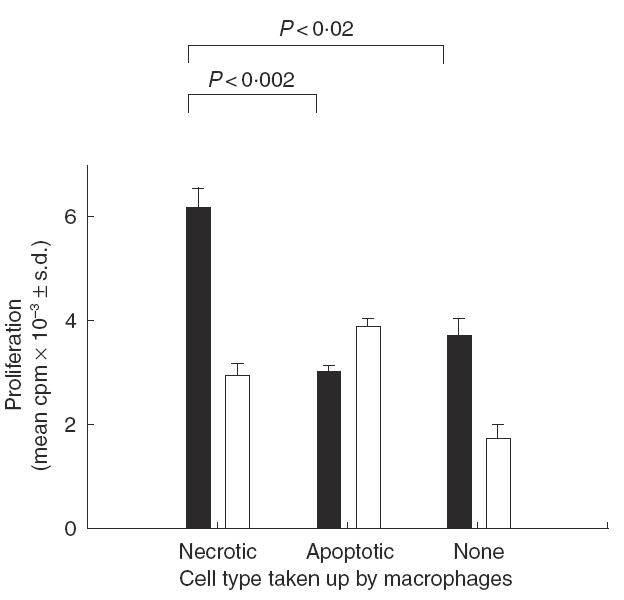
Representative experiment (n = 4) showing the effect of pulsing macrophages with necrotic or apoptotic neutrophils on the ability to induce splenic T cell proliferation by presenting the recall antigen ovalbumin. Macrophages were allowed to take up dying neutrophils for 30 min and the uningested cells washed off. The macrophages were then pulsed with ovalbumin for 1h, washed and the T cells added. Significant differences (P < 0·05) between proliferative responses are bracketed. Stimulation ▪, Ovalbumin; □, none.
Fig. 3.

The differential effects of necrotic or apoptotic neutrophil uptake on presentation of ovalbumin by macrophages are not dependent on the concentration of macrophages or antigen in cultures. Splenic T cell proliferation was stimulated by macrophages that had been plated out at 0·5 × 106 (a) and (b) or 106 (c) cells/ml and pulsed with the recall antigen ovalbumin at 10 (a) or 100 (b and c) μg/ml. The percentage changes in proliferation are relative to the corresponding responses elicited by macrophages that have not been incubated with dying neutrophils and are calculated from the stimulation indices of three replicate cultures.
Effect of necrotic or apoptotic neutrophil uptake on the expression of co-stimulatory and MHC class II molecules by macrophages
Differential regulation of macrophage co-stimulatory and/or MHC class II molecule expression could be one explanation for the contrasting effects of necrotic or apoptotic neutrophil uptake on antigen presentation. This possibility was addressed using flow cytometry to analyse expression of CD40, CD80, CD86 and MHC class II molecules by BMDM pulsed with necrotic or apoptotic neutrophils. The results summarized in Fig. 4 show that by 4 h, BMDM that had taken up necrotic neutrophils expressed significantly higher levels of CD40 than those that ingested apoptotic cells. The rapid divergence in CD40 staining by macrophages that had phagocytosed the two types of dying cell was reproducible in a total of six experiments. The effects of dying cell uptake on the levels of CD86 were less marked, but again the expression was significantly higher on macrophages that had ingested necrotic, compared with apoptotic, cells. Neither treatment altered the levels of CD80 or MHC class II.
Fig. 4.
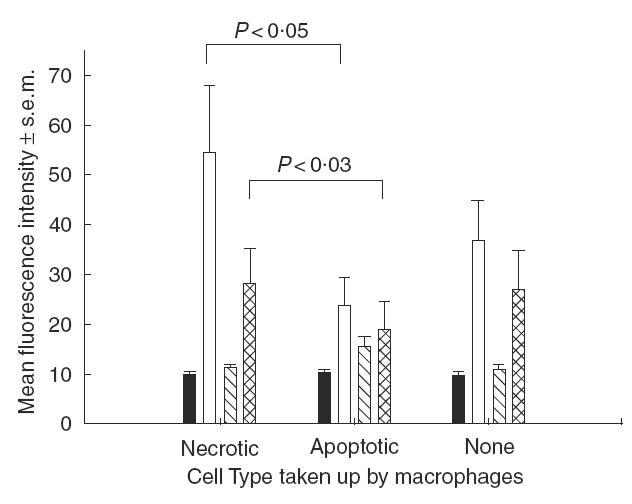
The effect of pulsing macrophages with necrotic or apoptotic neutrophils on the expression after 4h of MHC class II and the co-stimulatory molecules CD40, CD80 and CD86. The mean results of repeat experiments are summarized (MHC class II n = 2, CD40 n = 6, CD80 n = 2, CD86 n = 6). Significant differences (P < 0·05) between staining levels are bracketed. Staining: ▪, MHC class II; □, CD40;  , CD80;
, CD80; , CD86.
, CD86.
Effect of necrotic or apoptotic neutrophil uptake by macrophages on cytokine production
The inability of BMDM that have taken up apoptotic neutrophils to support recall T cell proliferative responses in vitro may be due to changes in cytokine secretion. Therefore, the levels of the proinflammatory cytokines tumour necrosis factor-(TNF-)α and IFN-γ, and the inhibitory cytokines IL-4, IL-10 and transforming growth factor-(TGF-)β1, were compared when BMDM had been preincubated with necrotic or apoptotic neutrophils. Neither treatment affected the levels of IFN-γ, IL-4 or IL-10 produced in cultures where these cytokines were detectable (results not shown). However, Fig. 5a shows that TGF-β1 levels were significantly higher than in controls after ingestion of apoptotic, but not necrotic cells. In contrast, although the concentration of TNF-α in cultures of control BMDM was variable, the levels were significantly lower when the macrophages had taken up apoptotic, but not necrotic neutrophils (Fig. 5b).
Fig. 5.

The effect of pulsing macrophages with necrotic or apoptotic neutrophils on the production of TGF-β1 (a) or TNF-α (b). For each cytokine, the duplicate results of at least three repeat experiments are summarized and significant differences (P < 0·05) between responses are bracketed.
DISCUSSION
The main finding reported here is that the uptake of necrotic, but not apoptotic, neutrophils enhances the ability of macrophages to act as APC by supporting T cell proliferative responses in vitro. This dichotomy is likely to be important in the stimulation and control of immune responses in vivo, since macrophages can act not only as professional APC [12] but are prominent cells in the removal of necrotic debris from inflammatory lesions, and equipped with receptors for the efficient recognition and clearance of apoptotic cells [17,22]. Furthermore, the effects on antigen presentation were seen after the ingestion of dying neutrophils, which are likely to be one of the major cell types that macrophages clear in vivo.
The results identify examples of two mechanisms that could account for the differential effects of necrotic or apoptotic neutrophil uptake on antigen presentation by macrophages. First, the type of death that cells undergo prior to ingestion affects macrophage expression of CD40 and other co-stimulatory molecules that are important in antigen presentation to helper T cells [23,24]. Thus, CD40 was rapidly and consistently expressed at higher level by macrophages that had taken up necrotic neutrophils, compared to those that had phagocytosed apoptotic cells, thereby enhancing the potential for interaction with T cells bearing the corresponding ligand, CD154. Such an early interaction would be expected to determine the effectiveness of macrophages as APC, as would the less marked differential regulation of CD86. The increased T cell proliferation against not only a recall antigen, but also the mitogen Con A, after necrotic cell uptake provides further evidence of the importance of changes in macrophage co-stimulation, rather than processing, in determining the magnitude of the responses.
The second mechanism potentially able to mediate the contrasting effects of necrotic or apoptotic neutrophil uptake on macrophage APC function is differential production of immunomodulatory cytokines. T cell cultures stimulated with macrophages pulsed with apoptotic, but not necrotic, neutrophils contained significant levels of TGF-β1, a cytokine with well-documented immunosuppressive and anti-inflammatory effects [25]. It has been shown previously that TGF-β1 is released by human macrophages in response to ingestion of apoptotic neutrophils and by murine macrophages that have taken up apoptotic Jurkat cells [19]. In the current work, control BMDM that had ingested no neutrophils were poor APC, so the effects of the TGF-β1 production on T cell proliferation in vitro were necessarily limited, but it is likely to have important down-regulatory effects on immune responses in vivo. Macrophage TGF-β1 would not only be an immune suppressant, inhibiting helper T responses in particular, but can also act as an autocrine factor to bias macrophages further towards an anti-inflammatory phenotype [18,19]. In particular, the inhibition of TNF-α production by macrophages after the uptake of apoptotic cells reported here has been attributed previously to such an autocrine effect of TGF-β1 [18,19].
Macrophages are protean cells with the ability to develop distinct sets of co-ordinated properties that provide appropriate responses to different microenvironments [12]. While it has been demonstrated previously that the cytokine milieu to which they are exposed can programme macrophages for these opposing functions [13], it is now becoming clear that the phagocytosis of dying cells can also exert differential effects. The uptake of necrotic cells results in classic activation, with the development of an inflammatory phenotype [16], whereas consequences of ingestion of apoptotic cells resemble those of alternative activation, including the production of the immunoregulatory cytokines TGF-β1 as reported here and by others [18,19], and/or IL-10 [12,17]. Furthermore, it has recently been reported that exposure to necrotic cells stimulates macrophage antitumour activity while uptake of apoptotic cells results in impairment of this defence [15]. The current work extends these findings by demonstrating that the type of death which a cell undergoes prior to phagocytosis can affect the ability of macrophages to activate helper T cells by presenting antigen.
The demonstration that uptake of necrotic, but not apoptotic, neutrophils increases the efficiency of macrophages as APC, together with similar results obtained using dendritic cells to present antigen [2], supports the view that the responsiveness of the immune system is heightened by ‘danger’ signals associated with inflammation [1]. The relative importance of these two APC types in detecting such signals is unclear, since, although it could be argued that dendritic cells would be needed to initiate any response, macrophages are key inflammatory cells that are specialized for phagocytosis. It has been accepted that innate recognition of infectious agents can enhance adaptive immune defences [3,4], and our results provide evidence that the response of macrophage APC to tissue damage can also exert an adjuvant effect. Such a mechanism would allow macrophages in an inflammatory site to perpetuate the immune response to potentially noxious agents, or to exacerbate autoimmune reactions. Conversely, following uptake of apoptotic cells the release of immunosuppressive cytokine by macrophages, and/or the presentation of autoantigen in the absence of co-stimulatory signals, could be important in maintaining immunological tolerance to self-tissues. Such a tolerogenic mechanism could also be exploited as a novel therapy in immune-mediated disease.
REFERENCES
- 1.Matzinger P. Tolerance, danger and the extended family. Ann Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 2.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nature Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 3.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–3. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA. The road less travelled by. The role of innate immunity in the adaptive immune response. J Immunol. 1998;161:539–44. [PubMed] [Google Scholar]
- 5.Vandenbark AA, Offner H, Reshef T, et al. Specificity of T lymphocytes lines for peptides of myelin basic protein. J Immunol. 1985;135:229–33. [PubMed] [Google Scholar]
- 6.Hooper DC. Self-tolerance for erythrocytes is not maintained by clonal deletion of T helper cells. Immunol Today. 1987;8:327330. doi: 10.1016/0167-5699(87)90005-3. [DOI] [PubMed] [Google Scholar]
- 7.Elson CJ, Barker RN, Thompson SJ, Williams NA. Immunologically ignorant autoreactive T cells, epitope spreading and repertoire limitation. Immunol Today. 1995;16:71–6. doi: 10.1016/0167-5699(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 8.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1998;101:1693–8. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams DO, Hamilton TA. Macrophages as destructive cells in host defence. In: Adams DO, Hamilton TA, editors. Inflammation: basic principles and clinical correlates. New York: Raven Press; 1998. pp. 471–9. [Google Scholar]
- 10.North RJ. The concept of the activated macrophage. J Immunol. 1978;121:806–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Murray HW. Interferon-gamma, the activated macrophage, the host defence against microbial challenge. Ann Inter Med. 1988;108:595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- 12.Goerdt G, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 13.Erwig L-P, Kluth DC, Walsh GM, Rees AJ. Initial cytokine exposure determines macrophages’ function and renders them unresponsive to other cytokines. J Immunol. 1998;161:1983–8. [PubMed] [Google Scholar]
- 14.Erwig L-P, Stewart KN, Rees AJ. Systemic IL-4 treatment does not alter macrophage programming but modulates macrophage function within nephritic glomeruli. Am J Path. 2000;156:295–301. [Google Scholar]
- 15.Reiter I, Krammer B, Schwamberger G. Differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. 1999;163:1730–2. [PubMed] [Google Scholar]
- 16.Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce release of thromboxane B2. J Leukoc Biol. 1992;52:269–73. [PubMed] [Google Scholar]
- 17.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–54. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, Bratton DL, Konowal K, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2 and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–72. [PubMed] [Google Scholar]
- 20.Erwig L-P, Gordon S, Walsh GM, Rees AJ. Previous uptake of apoptotic neutrophils or ligation of integrin receptors downmodulates the ability of macrophages to ingest apoptotic neutrophils. Blood. 1999;93:1406–12. [PubMed] [Google Scholar]
- 21.Perry FE, Barker RN, Mazza G, et al. Autoreactive T cell specificity in autoimmune hemolytic anemia of the NZB mouse. Eur J Immunol. 1996;26:136–41. doi: 10.1002/eji.1830260121. [DOI] [PubMed] [Google Scholar]
- 22.Savill JS, Wyllie AH, Henson JE, et al. Macrophage phagocytosis of ageing neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition of macrophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durie FH, Foy TM, Masters SR, Laman JD, Noelle RJ. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. p. 15. [DOI] [PubMed]
- 24.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 25.Letterio JJ, Roberts AB. TGF-β: a critical modulator of immune cell function. Clin Immunol Immunopathol. 1997;84:244–50. doi: 10.1006/clin.1997.4409. [DOI] [PubMed] [Google Scholar]


