Abstract
It is acknowledged that periodontitis results from the interaction of the host immune response with bacteria accumulating on the tooth surfaces. Although bacteria are essential, they are insufficient to cause the disease. Despite this knowledge it remains unclear why certain individuals are more susceptible to periodontitis than others. Therefore the present study investigated whether differences exist in the actual immune response between periodontitis patients and controls after stimulation of peripheral blood cells. Whole blood cell cultures (WBCC) were stimulated with LPS from Escherichia coli during 18 h and the release of prostaglandin E2 (PGE2), IL-1β, IL-6, IL-8, IL-10, IL-12p40, IL-12p70 and tumour necrosis factor-alpha (TNF-α) was measured. The levels of PGE2 were two-fold higher in the WBCC from periodontitis patients than from controls. In contrast, the levels of IL-12p70 in WBCC from patients were two-fold lower. Furthermore, WBCC from patients secreted lower levels of IL-1β and higher levels of IL-8 when compared with WBCC from controls. No differences were observed with respect to IL-6, IL-10, IL-12p40 and TNF-α production. It is known from the literature that LPS-stimulated WBCC reflect specifically the behaviour of the monocytes and that monocytes are peripheral precursors of antigen-presenting cells (APC). Therefore it is concluded that the monocytes in the present WBCC from periodontitis patients are responsible for the higher levels of PGE2 and lower levels of IL-12p70. Since it is has been shown that APC-derived IL-12p70 induces type (Th1) cells that promote cellular immunity, while APC-derived PGE2 induces type 2-helper (Th2) cells that promote humoral immunity, it is postulated that APC from periodontitis patients may have a bias in directing Th2 responses and thereby promoting the humoral immunity in periodontitis.
Keywords: periodontitis, monocytes, PGE2, IL-12p70, antigen-presenting cells
INTRODUCTION
Periodontitis is a chronic destructive inflammatory disease of the teeth-supporting tissues, i.e. connective tissue from the periodontal ligament and alveolar bone. This inflammatory condition will, if left untreated, eventually lead to loosened teeth and subsequent exfoliation. Periodontitis is a multifactorial disease in which host factors and environmental factors play an important role [1]. It is widely accepted that periodontitis results from interaction of the host’s defence mechanisms with bacteria accumulating on the tooth surface [2]. The prevalence of periodontal pathogens in the general population is moderate to high: 27% for Actinobacillus actinomycetemcomitans [3] to 100% for Fusobacterium nucleatum [4]. Despite this prevalence it is recognized that not everyone is equally susceptible to periodontitis [5,6]. Therefore, although bacteria are essential in the induction of the inflammatory response in the periodontal tissues, they are insufficient to cause destructive periodontal disease [1]. This implicates differences in susceptibility and intrinsic differences in the host immune response.
It is generally accepted that LPS, derived from Gram-negative bacteria that accumulate on the tooth surfaces, penetrate the periodontal tissues and subsequently recruit and activate immune cells [1]. Histological studies have shown that the immune response results in a periodontal lesion that consists of lymphocytes, monocytes/macrophages and plasma cells [2]. Triggering by (pro)inflammatory stress signals, like LPS, tissue cells as well as immune cells start to secrete inflammatory mediators such as cytokines, chemokines and prostaglandins. These released molecules may mediate the inflammatory response and the destruction of the periodontal tissues but may also affect the functional status of specific immune cells in the periodontal lesion. Such different effects of the induced mediators on the function of cells in the immediate neighbourhood determine the course of the immune response and hence the resistance or susceptibility to the disease [7].
In several diseases and inflammatory conditions LPS responsiveness of peripheral blood cells has been studied as a measure of the host immune capacity. Therefore the purpose of the present study was to investigate, in a whole blood cell culture (WBCC) system, differences in the non-specific cellular immune response to LPS between periodontitis patients and controls.
SUBJECTS AND METHODS
Selection of study subjects
Since smoking is recognized as an important risk factor in the pathogenesis of periodontitis, the possible influence of this environmental factor on the immune response was excluded [8]. Therefore a total of 19 non-smoking patients with chronic untreated periodontitis were recruited from patients who were referred to the Academic Centre for Dentistry Amsterdam (ACTA) for diagnosis and/or treatment. Non-smokers were defined as those who had never smoked or had ceased smoking more than 10 years ago. All patients showed on dental radiographs periodontal bone loss of >1/3 of the total length of the root on two or more teeth per quadrant. Nineteen control subjects, patients of ACTA referred for the treatment of dental caries, were matched for age and gender. The selected controls did not suffer from periodontitis and did not show loss of alveolar bone, as was confirmed on dental radiographs.
All participants were free from systemic diseases and had no clinical symptoms of bacterial, viral or parasitic infections at the time of the study. None of the subjects in the study had taken any form of medication that could affect their periodontal status, such as anti-inflammatory agents, antibiotics and immunosuppressants during at least the preceding 6 months. Approval by the Institutional Review Board on human studies was obtained and all subjects signed an informed consent.
To characterize further patients and controls, subgingival bacterial samples were taken and subsequently cultured in a standardized way as previously described [9] (Table 1).
Table 1.
Demographic, bacteriological and blood cell data of the two study groups
| Controls (n = 19) | Patients (n = 19) | |
|---|---|---|
| Age † (years) | 38 ± 5 | 37 ± 6 |
| No. Caucasians | 15 | 13 |
| No. females/males | 11/8 | 11/8 |
| No. subjects culture positive for ‡ | ||
| Actinobacillus actinomycetemcomitans | 5 | 8 |
| Porphyromonas gingivalis | 4 | 12* |
| Bacteroides forsythus | 8 | 18** |
| Prevotella intermedia | 15 | 18 |
| Fusobacterium nucleatum | 19 | 19 |
| Peptostreptococcus micros | 17 | 17 |
| Campylobacter rectus | 1 | 4 |
| No. (×109/l) of† | ||
| –Leucocytes | 5·89 ± 1·33 | 6·11 ± 1·73 |
| –Monocytes | 0·41 ± 0·12 | 0·36 ± 0·08 |
| –Lymphocytes | 1·82 ± 0·65 | 1·88 ± 0·59 |
| –Neutrophils | 3·50 ± 1·00 | 3·72 ± 1·41 |
| –Eosinophils | 0·11 ± 0·06 | 0·10 ± 0·05 |
| –Basophils | 0·04 ± 0·02 | 0·03 ± 0·02 |
Values are means ± s.d.
The sampling was carried out in total at four sites and the deepest sites of each quadrant of the dentition were selected.
P < 0·05;
P < 0·01 as analysed by Fisher’s exact test.
Whole blood cell cultures
Venous blood was collected by venepuncture in sodium heparin-containing blood collecting tubes (VT 100SH tubes; Venoject; Terumo Europe, Leuven, Belgium). The blood was diluted five-fold in endotoxin-free RPMI 1640 medium containing l-glutamine and 25 mm HEPES (Gibco BRL, Life Technologies B.V., Breda, The Netherlands) and was supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. The diluted whole blood was cultured in duplicate in non-pyrogenic 24-well flat-bottomed tissue culture plates (Costar, Cambridge, MA) in the absence or presence of LPS from Escherichia coli O127:B8 (Difco, Detroit, MI) in various concentrations for 18 h at 37°C. The period between the blood collection and the start of the incubation was 1·5 h. Following culturing, supernatants were collected and stored at −80°C until determination of cytokines and prostaglandin E2 (PGE2) concentrations.
In order to determine the most optimal LPS concentration for the stimulation of the WBCC, the blood samples were incubated with six different concentrations of LPS: 0, 0·01, 0·1, 1, 10 and 100 ng/ml.
Venous blood of all participants was also collected in an EDTA(K3) containing tube (Becton Dickinson Vacutainer System Europe, Meylan, France) for the determination of the leucocyte and the leucocyte differentiation counts, which were performed in standard automated procedures.
Cytokine and PGE2 determinations
The IL-1β, IL-6, IL-8, IL-10 and tumour necrosis factor-alpha (TNF-α) levels were measured in the supernatants using commercially available ELISAs (PeliKine Compact™ human ELISA kits; CLB, Amsterdam, The Netherlands) according to the manufacturer’s instructions. The sensitivity of the kits for the various cytokines used varied from 4 to 9 pg/ml.
Measurements of IL-12p70 and IL-12p40 levels were performed by a specific solid-phase sandwich ELISA as described previously [10]. The IL-12p70 ELISA detects biologically active IL-12. The detection limit of the IL-12 assays was 3 pg/ml for IL-12p70 and 20 pg/ml for IL-12p40.
PGE2 concentrations were determined using the ACE™ competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI), which had a detection limit of 15 pg/ml.
Statistical analysis
SPSS package (version 9.0 for Windows; Chicago, IL) was used for data analysis and box plot generation. Since the concentration of the released mediators showed a non-normal distribution, the data were log-transformed for statistical analysis. The influence of the demographic, bacteriological and blood cell count parameters, as listed in Table 1, on the cytokine and PGE2 secretion in the cultures was evaluated by using a forward linear regression analysis. Comparisons between patients and controls for the blood cell counts and the log-transformed cytokine and PGE2 concentrations were performed by means of the unpaired t-test and an analysis of covariance, respectively. P < 0·05 was considered significant.
RESULTS
The production of the inflammatory mediators IL-1β, IL-6, IL-8, IL-10, IL-12p40, TNF-α and PGE2 was measured in the supernatants of the WBCC after 18 h of stimulation with various concentrations of LPS from E. coli. For cells that were incubated without LPS, only in case of IL-8 were detectable levels of the cytokine found. Stimulation with increasing LPS concentrations induced a dose-dependent production of all the mediators tested and provided sigmoidal curves. The paths of all curves were similar for both the patient and the control groups. As the curves of the mediators reached a saturation level at 1 ng/ml LPS, it was decided to compare patients and controls for the production of mediators at this LPS dose. In this respect the only exception relates to IL-12p70. For this cytokine no dose–response curves could be obtained, since this cytokine is produced in low concentrations in LPS-stimulated conditions. Therefore, the production of IL-12p70 was only measured in the supernatants of the cell cultures that were stimulated with the highest level of LPS used, i.e. 100 ng/ml.
Demographic, bacteriological and blood cell data of the study groups are presented in Table 1. With regard to the blood cell data, analyses showed no differences between the periodontitis patients and the control subjects. The levels of mediators are presented in Fig. 1a,b. Linear regression analyses, including all the demographic, bacteriological, clinical and blood cell data, revealed that the number of monocytes, the age and the race of the subjects were significant confounders for the production of mediators. Therefore, in the comparisons between patients and controls these confounding factors were introduced as co-variants in the analyses of variance.
Fig. 1.
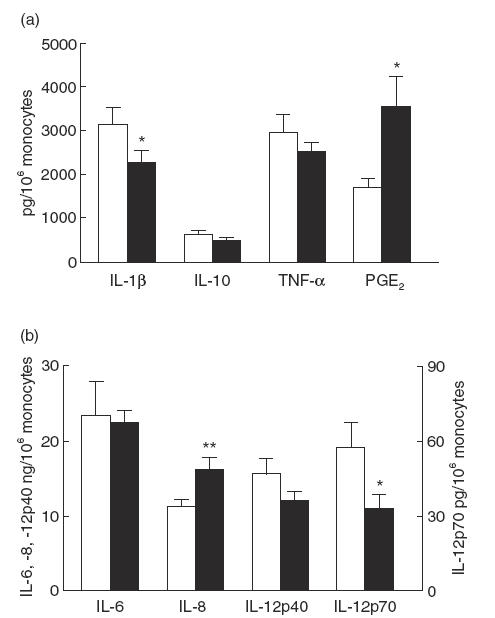
LPS-induced mediator production in 19 periodontitis patients and 19 controls. For the measurements of IL-1β, IL-6, IL-8, IL-10, IL-12p40, tumour necrosis factor-alpha (TNF-α) and prostaglandin E2 (PGE2) the whole blood cell cultures were stimulated with 1 ng/ml LPS from Escherichia coli for 18 h, whereas the cultures for IL-12p70 were stimulated with 100 ng/ml LPS from E. coli for 18 h. Data are means ± s.e.m. and are expressed as cytokine or PGE2 production per 106 monocytes. The individual data per subject were the mean of two different cultures. *P < 0·05; **P < 0·01 as determined by analysis of covariance, with age, race and number of monocytes as co-variants. □, Controls; ▪, patients.
Results showed no differences between the two study groups with regard to the levels of the released cytokines IL-6, IL-10, IL-12p40 and TNF-α in the supernatants (Fig. 1). However, the levels of IL-1β measured in the supernatants of the periodontitis patients were 1·5-fold lower than the levels measured in the supernatants of the controls. The periodontitis patients proved to produce two-fold higher concentrations of PGE2 in the supernatants in comparison with the control group (Fig. 1a). Within the patient group it was interesting to note that several patients secreted very high levels of PGE2: seven out of the 19 patients produced >5000 and up to 11 000 pg/106 monocytes, which was three- to six-fold higher than the mean of the control group. Furthermore, the mean levels of IL-8 in the supernatants of the periodontitis patients were 1·5-fold higher than those in the controls (Fig. 1b).
The concentrations of IL-12p70 were measured in 100 ng/ml LPS-stimulated cultures (Fig. 1b). IL-12p70 is the bioactive heterodimer that is regulated differently from IL-12p40, the biologically inactive form of IL-12 [11]. Detectable levels of IL-12p70 were found for 11 subjects in the control group and for 11 in the patient group. The assay revealed for these patients a mean concentration of 12·0 ± 6·6 pg/ml of IL-12p70, while the corresponding value for the control subjects was 24·4 ± 16·7 pg/ml. This showed that cells of the periodontitis patients produced half of the IL-12p70 levels of the controls.
DISCUSSION
In the present study the differences in the production of inflammatory mediators by innate immune cells from patients suffering from periodontitis and controls were studied. For this purpose, WBCC were stimulated with LPS from E. coli. The present data show that the production of PGE2 and IL-8 was elevated and the production of IL-12p70 and IL-1β was reduced in WBCC harvested from periodontitis patients. Concomitant production of IL-6, IL-10, IL-12p40 and TNF-α was comparable between patients and controls, indicating that the peripheral blood cells of patients with periodontitis showed a similar competence as control subjects to produce these latter cytokines.
It has been extensively studied and shown in parallel cultures of whole blood and freshly isolated monocytes as well as in kinetics that WBCC stimulated with LPS specifically reflect the behaviour of the monocytes [10,12–15]. It was shown that the purified monocytes but not CD14-depleted peripheral blood mononuclear cells (PBMC) or granulocytes were responsible for the production of cytokines following stimulation with LPS [10,14]. Furthermore, the reflected performance of monocytes in LPS-stimulated WBCC was found for relatively low levels of LPS, since the cytokine production by neutrophils required much higher amounts of LPS [15]. Therefore it is highly likely that the levels of the inflammatory mediators found in the WBCC in the present study reflect the behaviour of the monocytes.
In the present study LPS from E. coli was used as a non-specific stimulant to activate the peripheral blood cells. Escherichia coli-derived LPS was used to make valid comparisons possible with previous studies and studies outside the field of periodontology, since it is the most common and most widely studied source of LPS, and above all very reliable. In addition, not all patients and controls were colonized by the major periodontal pathogens. Furthermore, the findings in the present study corroborate the results of a study using freshly isolated monocytes purified from the peripheral blood of periodontitis patients and control subjects, which were also stimulated with E. coli-derived LPS [16]. Their results showed that the isolated monocytes from the periodontitis patients also produced higher levels of PGE2 and lower levels of IL-1β compared with controls, while the levels of TNF-α and IL-6 were comparable. Unfortunately, data on the release of the chemokine IL-8 and the cytokines IL-10, IL-12p40 and IL-12p70 were not available in that particular study.
Immunity depends on two major types of specific immune responses: cellular and humoral responses. The balance between these responses is orchestrated by cytokines produced by CD4+ Th cells. Th1 cells make the ‘type 1’ cytokine interferon-gamma (IFN-γ) and Th2 cells the ‘type 2’ cytokines IL-4 and IL-5 [17]. It is acknowledged now that factors associated with APC highly determine the tuning of the balance between type 1 and type 2 cytokines [18,19]. However, functional studies with APC harvested from the peripheral tissues are very difficult, if not impossible. Since studies on the role of APC are based on in vitro experiments with peripheral blood precursors, i.e. the monocytes, and which resemble the in vivo types [20–22], it is tempting to extrapolate the data of the present study towards the possible role of APC in periodontitis.
In fact, APC play a central role as they transfer information from an infected microenvironment to the T cells. This information is crucial to select the most effective immune response to the pathogenic antigens associated with that microenvironment [18]. APC provide T cells not only with an antigen-specific stimulatory signal and a series of costimulatory signals, but also with polarizing signals, i.e. soluble molecules. The expression of such polarizing APC factors is triggered by (pro)inflammatory stress (‘danger’) signals, such as microbial products like LPS, which may affect the APC either directly or indirectly via the activation of neighbouring tissue cells [19].
The most clearly defined factors determining Th1 and Th2 differentiation from a T-cell precursor are mediators present at the initiation of the immune response at the stage of ligation of the T-cell receptor [23,24]. In this respect IL-12 and PGE2 are important factors. IL-12 is a dominant factor in directing the development of Th1 cells producing high levels of IFN-γ [25–27]. PGE2 selectively inhibits IFN-γ production and is reported to favour the development of Th2 cells [28]. Furthermore, it has been proposed that the ratio of IL-12 to PGE2 produced by APC during T cell activation is highly predictive of the level of IFN-γ production by Th cells [29]. Because the present study showed both higher levels of PGE2 and lower levels of IL-12p70 in cultures of the patients, these results suggest a type 2-promoting phenotype of APC from patients with periodontitis. Accompanying this, a decreased type 1 response, i.e. reduced levels of IFN-γ produced by PBMC, has been reported for periodontitis patients [30,31]. This relationship between monocyte-derived PGE2 and IL-12 levels and decreased IFN-γ production by PBMC has also been shown for several other diseases, like atopic dermatitis, allergic asthma, rheumatoid arthritis and HIV infection [13,14,32–43].
In support of this suggested Th2-promoting phenotype of APC from periodontitis patients are the observations that T cells in periodontal lesions express higher levels of IL-4 and IL-5 mRNA, and produce more IL-4 and have a lower IL-2/IL-4 ratio compared with controls [44–46]. These Th2 cells, whose development is induced by IL-4, have been implicated in humoral immune responses due to their production of B-cell growth and differentiation factors [47]. Indeed, the infiltrate in the periodontal lesion seems to be dominated by B cells and plasma cells and T-cell regulated polyclonal B-cell responses are believed to be important in the pathogenesis of the progressive periodontal lesion [2,48].
The suggested Th2-promoting phenotype of APC from periodontitis patients implies either an intrinsic characteristic or a different priming of the monocytes, due to an altered tissue environment, i.e. the inflammatory periodontal lesion. This suggested Th2-promoting phenotype of APC from periodontitis patients may be an important factor in the susceptibility to the disease.
Acknowledgments
We would like to thank Dr A. J. van Winkelhoff of the Department of Oral Microbiology of the ACTA for the analysis of the microbiological samples. Professor Dr M. L. Kapsenberg of the Department of Cell Biology and Histology, Academic Centre of the University of Amsterdam, is kindly acknowledged for providing the IL-12p40 and IL-12p70 ELISAs and for his expertise in preparing the manuscript.
REFERENCES
- 1.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 2.Gemmell E, Marshall RI, Seymour GJ. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol. 1997;14:112–43. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 3.Müller HP, Zoller L, Eger T, Hoffmann S, Lobinsky D. Natural distribution of oral Actinobacillus actinomycetemcomitans in young men with minimal periodontal disease. J Periodontal Res. 1996;31:373–80. doi: 10.1111/j.1600-0765.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 4.Lie MA, Van der Weijden GA, Timmerman MF, Loos BG, van Steenbergen TJ, Van der Velden U. Oral microbiota in smokers and non-smokers in natural and experimentally-induced gingivitis. J Clin Periodontol. 1998;25:677–86. doi: 10.1111/j.1600-051x.1998.tb02505.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindhe J, Hamp SE, Loe H. Plaque induced periodontal disease in beagle dogs. A 4-year clinical, roentgenographical and histometrical study. J Periodontal Res. 1975;10:243–55. doi: 10.1111/j.1600-0765.1975.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 6.Loë H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14–46 years of age. J Clin Periodontol. 1986;13:431–45. doi: 10.1111/j.1600-051x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 7.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–42. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 8.Research Science Therapy Committee of the American Academy of Periodontology. Position paper: tobacco use and the periodontal patient. J Periodontol. 1999;70:1419–27. doi: 10.1902/jop.1999.70.11.1419. [DOI] [PubMed] [Google Scholar]
- 9.Petit MD, van Steenbergen TJ, Timmerman MF, de Graaff J, Van der Velden U. Prevalence of periodontitis and suspected periodontal pathogens in families of adult periodontitis patients. J Clin Periodontol. 1994;21:76–85. doi: 10.1111/j.1600-051x.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 10.Van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snijders A, Hilkens CM, Van der Pouw Kraan TC, Engel M, Aarden LA, Kapsenberg ML. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–12. [PubMed] [Google Scholar]
- 12.Van der Pouw Kraan TC, Boeije LC, Snijders A, Smeenk RJ, Wijdenes J, Aarden LA. Regulation of IL-12 production by human monocytes and the influence of prostaglandin E2. Ann NY Acad Sci. 1996;795:147–57. doi: 10.1111/j.1749-6632.1996.tb52663.x. [DOI] [PubMed] [Google Scholar]
- 13.Snijders A, Van der Pouw Kraan TC, Engel M, et al. Enhanced prostaglandin E2 production by monocytes in atopic dermatitis (AD) is not accompanied by enhanced production of IL-6, IL-10 or IL-12. Clin Exp Immunol. 1998;111:472–6. doi: 10.1046/j.1365-2249.1998.00516.x. 10.1046/j.1365-2249.1998.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Pouw Kraan TC, Boeije LC, de Groot ER, et al. Reduced production of IL-12 and IL-12-dependent IFN-gamma release in patients with allergic asthma. J Immunol. 1997;158:5560–5. [PubMed] [Google Scholar]
- 15.Cavaillon JM, Marie C, Pitton C, Fitting C. The production of TNF alpha and IL-8 in whole blood assays are differently regulated upon activation by LPS. Prog Clin Biol Res. 1995;392:433–9. [PubMed] [Google Scholar]
- 16.Shapira L, Soskolne WA, Sela MN, Offenbacher S, Barak V. The secretion of PGE2, IL-1 beta, IL-6, and TNF alpha by adherent mononuclear cells from early onset periodontitis patients. J Periodontol. 1994;65:139–46. doi: 10.1902/jop.1994.65.2.139. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 19.Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P. The role of antigen-presenting cells in the regulation of allergen-specific T cell responses. Curr Opin Immunol. 1998;10:607–13. doi: 10.1016/s0952-7915(98)80077-0. [DOI] [PubMed] [Google Scholar]
- 20.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–6. [PubMed] [Google Scholar]
- 21.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel MM, Vanhee DD, Postmus PE, Hoefsmit EC, Beelen RH. Functional and phenotypic differences of monocyte-derived dendritic cells from allergic and nonallergic patients. J Allergy Clin Immunol. 1998;101:90–95. doi: 10.1016/S0091-6749(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 23.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 24.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 26.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 28.Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–9. [PubMed] [Google Scholar]
- 29.Hilkens C, Snijders A, van der Vermeulen HMP, Wierenga E, Kapsenberg M. Accessory cell-derived interleukin-12 and prostaglandin E2 determine the level of interferon-gamma produced by activated human CD4+ T cells. Ann NY Acad Sci. 1996;795:349–50. doi: 10.1111/j.1749-6632.1996.tb52689.x. [DOI] [PubMed] [Google Scholar]
- 30.Sigusch B, Klinger G, Glockmann E, Simon HU. Early-onset and adult periodontitis associated with abnormal cytokine production by activated T lymphocytes. J Periodontol. 1998;69:1098–104. doi: 10.1902/jop.1998.69.10.1098. [DOI] [PubMed] [Google Scholar]
- 31.Petit MD, Van Wassenaar A, Van der Velden U, van Eden W, Loos BG. Depressed responsiveness of peripheral blood mononuclear cells to heat-shock proteins in periodontitis patients. J Dent Res. 1999;78:1393–400. doi: 10.1177/00220345990780080401. [DOI] [PubMed] [Google Scholar]
- 32.Chan S, Henderson WR, Jr, Li SH, Hanifin JM. Prostaglandin E2 control of T cell cytokine production is functionally related to the reduced lymphocyte proliferation in atopic dermatitis. J Allergy Clin Immunol. 1996;97:85–94. doi: 10.1016/s0091-6749(96)70286-5. [DOI] [PubMed] [Google Scholar]
- 33.Chan SC, Kim JW, Henderson WR, Jr, Hanifin JM. Altered prostaglandin E2 regulation of cytokine production in atopic dermatitis. J Immunol. 1993;151:3345–52. [PubMed] [Google Scholar]
- 34.Chehimi J, Starr SE, Frank I, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruchaud A, Despont JP, Roth A, Dayer JM. Phagocytosis, bactericidal capacity, and PGE2 production of monocytes in systemic lupus erythematosus and rheumatoid arthritis. Diagn Immunol. 1984;2:203–12. [PubMed] [Google Scholar]
- 36.Fernandez-Cruz E, Gelpi E, Longo N, et al. Increased synthesis and production of prostaglandin E2 by monocytes from drug addicts with AIDS. AIDS. 1989;3:91–96. doi: 10.1097/00002030-198902000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Foley P, Kazazi F, Biti R, Sorrell TC, Cunningham AL. HIV infection of monocytes inhibits the T-lymphocyte proliferative response to recall antigens, via production of eicosanoids. Immunology. 1992;75:391–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Jakob T, Huspith BN, Latchman YE, Rycroft R, Brostoff J. Depressed lymphocyte transformation and the role of prostaglandins in atopic dermatitis. Clin Exp Immunol. 1990;79:380–4. doi: 10.1111/j.1365-2249.1990.tb08099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jujo K, Renz H, Abe J, Gelfand EW, Leung DY. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992;90:323–31. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- 40.Murray HW, Rubin BY, Masur H, Roberts RB. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:883–9. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- 41.Schröder HC, Begin ME, Klocking R, et al. Avarol restores the altered prostaglandin and leukotriene metabolism in monocytes infected with human immunodeficiency virus type 1. Virus Res. 1991;21:213–23. doi: 10.1016/0168-1702(91)90034-s. [DOI] [PubMed] [Google Scholar]
- 42.Seitz M, Deimann W, Gram N, Hunstein W, Gemsa D. Characterization of blood mononuclear cells of rheumatoid arthritis patients. I. Depressed lymphocyte proliferation and enhanced prostanoid release from monocytes. Clin Immunol Immunopathol. 1982;25:405–16. doi: 10.1016/0090-1229(82)90205-7. [DOI] [PubMed] [Google Scholar]
- 43.Seitz M, Napierski I, Augustin R, Hunstein W, Kirchner H. Reduced production of interferon alpha and interferon gamma in leukocyte cultures from patients with active rheumatoid arthritis. Scand J Rheumatol. 1987;16:257–62. doi: 10.3109/03009748709102926. [DOI] [PubMed] [Google Scholar]
- 44.Tokoro Y, Matsuki Y, Yamamoto T, Suzuki T, Hara K. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol. 1997;107:166–74. doi: 10.1046/j.1365-2249.1997.d01-880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki K, Nakajima T, Hara K. Immunohistological analysis of T cell functional subsets in chronic inflammatory periodontal disease. Clin Exp Immunol. 1995;99:384–91. doi: 10.1111/j.1365-2249.1995.tb05562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manhart SS, Reinhardt RA, Payne JB, et al. Gingival cell IL-2 and IL-4 in early-onset periodontitis. J Periodontol. 1994;65:807–13. doi: 10.1902/jop.1994.65.9.807. [DOI] [PubMed] [Google Scholar]
- 47.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 48.Seymour GJ, Powell RN, Davies WI. The immunopathogenesis of progressive chronic inflammatory periodontal disease. J Oral Pathol. 1979;8:249–65. doi: 10.1111/j.1600-0714.1979.tb01826.x. [DOI] [PubMed] [Google Scholar]


