Abstract
It has been suggested that the humoral immune system plays a role in the pathogenesis of non-specific interstitial pneumonia (NSIP). Although some circulating autoantibodies to cytoskeletal protein(s) have been suggested, the antimyofibroblast antibody has not been investigated in patients with idiopathic pulmonary fibrosis (IPF) and NSIP. The purpose of this study is to evaluate the existence of antimyofibroblast antibody in the sera of patients with IPF and NSIP. The MRC5 cell line was used as a model of myofibroblast. The anti-MRC5 cell antibody was characterized in a patient with NSIP using Western blotting. Since we found that one of the anti-MRC5 antibodies was an antivimentin antibody, we established an enzyme-linked immunosorbent assay (ELISA) to measure the levels of antivimentin antibody in the sera of patients with IPF (n = 12) and NSIP (n = 23). Initially, two anti-MRC5 cell antibodies were detected in the sera of patients with NSIP, one of which was characterized as the antivimentin antibody by Western blotting. The other was characterized as an antivimentin fragment antibody. We established an ELISA to measure the antivimentin antibody and found significantly higher levels in patients with IPF and NSIP than in normal volunteers. One of the anti-MRC5 cell antibodies in the serum of a patient with NSIP was against vimentin. The serum levels of antivimentin antibody were increased in patients with IPF and NSIP compared with that of normal volunteers. These results suggest that the antivimentin antibody may be involved in the process of lung injury in IPF and NSIP.
Keywords: antibody, idiopathic pulmonary fibrosis, MRC5, non-specific interstitial pneumonia, vimentin
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) and non-specific interstitial pneumonia (NSIP) are inflammatory lung diseases of unknown aetiology, characterized by the presence of varying degrees of inflammatory cell infiltration of the interstitium and fibrosis within the alveolar walls, resulting in accumulation of fibroblasts. In addition, it has been suggested that NSIP is observed frequently as a pathological classification of the lung involvement in patients with connective tissue diseases [1,2].
The myofibroblast, a cell having ultrastructural features of fibroblasts as well as smooth muscle cells, was described first in granulation tissue [3]. It has been suggested that myofibroblasts play a role in wound contraction and in the retractile phenomena observed during fibrotic diseases [4]. Skalli et al.[5] identified four types of fibroblast/myofibroblast which express: only vimentin (a cells); vimentin, α-smooth muscle actin (α-SMA) and desmin (b cells); vimentin and α-SMA (c cells); and vimentin and desmin (d cells).
Yoshinouchi et al.[6] have suggested that the active contraction of myofibroblasts plays a role in the remodelling of the lung in pulmonary fibrosis. They have also reported that the appearance of myofibroblasts is a characteristic finding in rheumatoid arthritis (RA) associated with interstitial pneumonia and in the acute stage of IPF [3]. It has also been reported that many myofibroblasts in both the Masson bodies of bronchiolitis obliterans organizing pneumonia (BOOP) and the fibroblastic foci of usual interstitial pneumonia (UIP) are of the c type, containing vimentin and α-SMA [7].
Vimentin, known as the intermediate filament polypeptide, is present typically in cells of mesenchymal origin, e.g. fibroblasts [8]. Vimentin surrounds the nucleus and extends out to the cell periphery [9] and is responsible for cell elongation, regulation of cell attachment, subcellular organization and signal transduction from plasma membrane to the nucleus [10].
We have demonstrated previously the presence of anticytokeratin 8 [11], anticytokeratin 18 [12], and anticytokeratin 19 [13] autoantibodies in sera of patients with pulmonary fibrosis. As cytokeratins are also known as the intermediate filament polypeptides of epithelial cells, and as myofibroblasts which express vimentin as well as α-SMA are infiltrated in the lungs of patients with IPF [6] and NSIP (personal communication), we hypothesized that antimyofibroblast antibodies (especially an antivimentin antibody) might be detected in the sera of patients with IPF and NSIP.
MATERIALS AND METHODS
Subjects
Thirty-five patients diagnosed with interstitial pneumonia were studied from 1990 to 2000. Of the 35 patients, 12 had IPF and 23 had NSIP (17 were idiopathic NSIP and six were associated with polymyositis/dermatomyositis). The diagnoses of interstitial pneumonia were made on clinical, radiological, physiological and histological grounds. The criteria used included history of exertional dyspnoea and cough, fine crackles on physical examination, compatible findings on the chest radiograph (diffuse basal reticulonodular shadowing), physiological abnormalities of restrictive lung defects including decreased diffusing capacity and abnormal PaO2 at rest and/or with exertion. In all patients high resolution computed tomographic scanning of the lungs (HRCT) was performed. None of the patients received immunosuppressive treatment such as corticosteroid or cyclophosphamide. Pulmonary function tests, %vital capacity (VC) and forced expiratory volume in 1 s (FEV1) were performed in all patients.
Histological confirmation was obtained in all cases by open lung biopsy. IPF (pathologically UIP) was diagnosed according to the criteria of the International Consensus Statement [11], and NSIP was diagnosed according to the pathological criteria of Katzenstein et al.[1]. Finally, among the 35 patients, 12 were diagnosed with IPF three women, median age 68 (range 47–78)] and 23 with NSIP 15 women, median age 62 (range 44–74), 17 patients with idiopathic NSIP and six patients with polymyositis/dermatomyositis)]. We also studied 16 normal subjects (seven1 women) with a median age of 63 years.
The protocols for this study were approved by the institutional review board for human studies and informed written consent was obtained from the subjects.
Blood samples
Peripheral venous blood samples without ethylenediamine tetra-acetic acid (EDTA) were obtained before breakfast. After centrifugation at 1000 g for 10 min at 4°C the serum was frozen and stored at −70°C until used. Arterial blood oxygen and carbon dioxide tensions (PaO2 and PaCO2) were measured with a blood gas analyser. In a preliminary experiment, a 53-year-old female was found to have a high titre of the antivimentin antibody by Western blotting. Therefore, to evaluate antibodies against the MRC5 cell line, the serum of this patient was used as a positive control.
Cell lines
MRC5 cell lines were used as a model for myofibroblasts. MRC5 was obtained from the Japanese Type Culture Collection. MRC5 cells were grown in Dulbecco's modified Eagle's medium (DMEM, Nissui, Tokyo, Japan) supplemented with 10% fetal calf serum (Trace Scientific Ltd, Melbourne, Australia) at 37°C under 5%CO2/95%air. To confirm that MRC5 cells have characteristics of myofibroblasts, immunohistochemical staining by antihuman monoclonal antibodies against vimentin as well as against α-SMA was performed. MRC5 cells were stained immunohistochemically, employing the avidin–biotin peroxidase complex method (Dako LSAB kit-peroxidase, Dako Corp., Kyoto, Japan) using mouse monoclonal antibodies to vimentin (Dako, 1:50 dilution) as well as anti-α-SMA (Dako, 1:200 dilution). In order to retrieve and increase the immunoreactivity, microwave pretreatment (with 0·01 m citrate buffer at 95°C for 5 min) was performed before antivimentin staining. No pretreatment was performed before anti-α-SMA staining.
SDS-PAGE electrophoresis and Western blotting
SDS-polyacrylamide gel electrophoresis was performed according to Laemmli's method [12] with a slight modification. The lysate of MRC5 cells were mixed with sodium dodecyl sulphate (SDS 2·0%) and heated (100°C, 5 min). Commercially available recombinant human vimentin (Progen Biotechnik GmbH, lot 907019, Heidelberg, Germany) was also used as the positive control for vimentin. The sample was then applied to a 10/20% SDS polyacrylamide gel, electrophoresed (60 mA, 60 min), fixed in 50% methanol, 10% acetic acid and stained with Coomassie Blue. Standard molecular weight markers were purchased from Oriental Yeast Co. Ltd (Tokyo, Japan) and consisted of multiple synthetic peptides with molecular weights of 14800, 28201, 41603, 55004, 68406 and 81807.
Proteins were transferred electrophoretically onto a nitrocellulose membrane [13] and detection was performed by immunoblotting; using the serum from one patient (53-year-old female with NSIP; a positive control as described previously) and peroxidase conjugated mouse monoclonal antihuman IgG antibody (Southern Biotechnology Associates, Inc., lot J560-X070, Birmingham, AL, USA), and then stained with 4 CN PLUS for chromogenic detection of horseradish peroxidase (NEM Life Science Products, Boston, MA, USA). We also used the peroxidase conjugated goat antihuman IgA (Sigma Chemical Co., lot 89H9150, St Louis, MI, USA) as a second antibody.
The positive controls of Western blotting for vimentin were also performed using a mouse monoclonal antihuman vimentin antibody (CIT605, Lot 1194B, YLEM s.r.l., Rome, Italy) as the first antibody, and peroxidase conjugated goat antimouse IgG antibody (Santa Cruz Biotechnology, Lot F050, CA, USA) as the second antibody.
In order to identify the existence of an antibody against α-SMA, Western blotting using mouse monoclonal antihuman α-SMA (PDM003, Lot B116, YLEM s.r.l., Rome, Italy) was also performed.
Digestion of recombinant human vimentin by recombinant human caspase 3
We also evaluated the existence of antibodies against vimentin fragments. Vimentin fragments were prepared by incubating recombinant human vimentin (1 mg/ml, 10 μl) with recombinant human caspase 3 (1 U/ml, 25 μl, Chemicon International, Inc., lot 20021112, CA, USA) in 37°C, for 48 h. Then, the same amount of sodium dodecyl sulphate (SDS 2·0%) was added to this solution and heated (100°C, 5 min) for Western blotting.
Enzyme-linked immunosorbent assay for antivimentin autoantibody in human sera
An enzyme-linked immunosorbent assay (ELISA) was established to measure levels of antivimentin antibody in human serum samples. Serum (diluted 1:100) was added to wells coated with recombinant human vimentin (4 μg/ml) at 4°C and left overnight. After incubation for 1h and three washings with PBS containing 0·05% Tween 20, the solid phase-bound antihuman vimentin autoantibody was incubated further for 1h with peroxidase-conjugated mouse antihuman IgG antibody (Southern Biotechnology Associates, Inc., lot J560-X070, diluted 1:1000). After further washing the wells with 0·05% PBS-Tween20, 100 μl of freshly prepared substrate (TMB Peroxidase EIA Substrate Kit, Bio-RAD Laboratories, Hercules, CA, USA) were added to each well. After 5 min incubation at room temperature, the reaction was stopped by adding 100 μl of 1 N H2SO4. Optical densities were measured at 450 nm in a double-beam spectrophotometer. The assay was calibrated using a standard solution of the patient's serum (diluted at 1:50, 1:100, 1:200, 1:400, 1:800 and 1:1600) in which the antivimentin antibody had been demonstrated by Western blotting. Data are expressed as mean values from duplicate determinations.
In 23 patients with NSIP, correlations between each parameter were evaluated. To evaluate correlations between antivimentin antibody levels and other factors, we also measured the parameters as follows. In serum, we measured elastase: α1-proteinase inhibitor complex (E-PI), hepatocyte growth factor (HGF), C-reactive protein (CRP), lactate dehydrogenase (LDH) and immunoglobulin G (IgG). In BALF, E-PI, HGF, LDH, IgG, albumin and cell differentiations were measured. In peripheral venous blood, white blood cell numbers (WBC) and the erythrocyte sedimentation rate (ESR) were measured. Arterial blood gas values (PaO2 and PaCO2) were also measured. In addition, as a pulmonary function test, percent vital capacity (%VC) and forced expiratory volume (FEV1·0%) were measured.
E-PI concentration was determined using enzyme-linked immunosorbent assay (ELISA) kits (Diagnostica Merck, Darmstadt, Germany). HGF was measured by ELISA with monoclonal and polyclonal antibodies against human HGF (Otsuka Assay Laboratories, Tokushima, Japan). LDH in BALF was measured for enzyme activity (Wroblewski–LaDue method). Tumour markers were measured by radioimmunoassay. Albumin in BALF was measured by ELISA (Albuwell; Exocell Inc., Philadelphia, PA, USA). Data in BALF were corrected by albumin (each datum was divided by albumin).
Statistical methods
The results are expressed as mean (s.e.) values. Comparisons of values between groups were analysed with the Mann–Whitney U-test. Correlations were evaluated by the Pearson's correlation coefficient, and Fisher's r to Z method was used to calculate the P-values. A P-value of less than 0·05 is considered significant.
RESULTS
Immunohistochemical staining of MRC5 cells showed that vimentin as well as α-SMA were clearly stained in the cytoplasm, confirming that MRC5 cells had characteristics of myofibroblasts (data not shown).
Western blotting analysis using monoclonal antivimentin antibodies as well as the patient's serum against the lysate of MRC5 cells and recombinant human vimentin is shown in (Fig. 1). Four bands were found in MRC5 cells using the antivimentin monoclonal antibody; however, only two bands (MW 57 000 and 48 000) were demonstrated in MRC5 cells using the patient's serum. Since the patient's serum reacted strongly with recombinant human vimentin and the molecular weight was the same with recombinant human vimentin, one band which had a molecular weight of 57 000 was identified as vimentin.
Fig. 1.
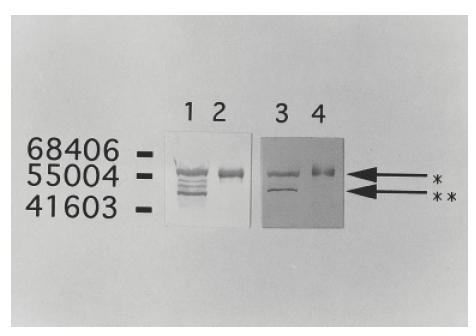
Western blotting analysis using serum from a patient. Lane 1, mouse antihuman vimentin monoclonal antibody against MRC5; lane 2, mouse antihuman vimentin monoclonal antibody against human recombinant vimentin; lane 3, patient's serum against MRC5; lane 4, patient's serum against human recombinant vimentin. Although only a single band (arrow *) was detected in vimentin, two bands (arrow * and **) were demonstrated in MRC5 stained by the serum but four bands were demonstrated in MRC5 stained by the antivimentin monoclonal antibody.
Western blotting analysis using a patient's serum against MRC5 cell line and human recombinant vimentin showed that the patient's serum had a stronger reaction when using antihuman IgG, whereas there were no positive reactions when antihuman IgA antibodies were used (Fig. 2).
Fig. 2.
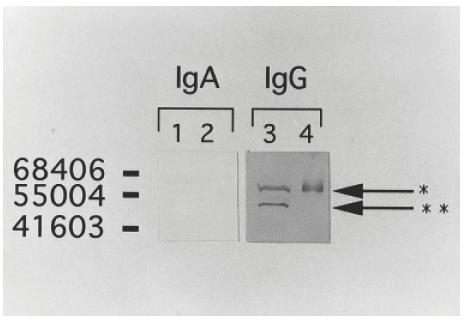
Western blotting analysis using serum from a patient against lysates of MRC5 cell lines and human recombinant vimentin incubating in antihuman IgA (lane 1 and 2) and IgG (lane 3 and 4) antibodies. Lane 1, MRC5; lane 2, vimentin; lane 3, MRC5; lane 4, vimentin. The patient's serum reacted strongly only with antihuman IgG, but did not react with antihuman IgA.
Western blotting analysis using a patient's serum against MRC5 cells, human recombinant vimentin and human recombinant vimentin treated with caspase 3 showed that the patient's serum reacted with two proteins derived from MRC5 cells. The positive band, which had a higher molecular weight (approximately 57 000), corresponded to vimentin. As the molecular weight was the same with the vimentin fragment; which was formed from human recombinant vimentin digested by caspase 3, the other band which had a lower molecular weight (approximately 48 000) seemed to correspond to the vimentin fragment (Fig. 3). The patient's serum also reacted with the new band which had a molecular weight of 70 000 and formed through the digestion of recombinant human vimentin by caspase 3 (as the molecular weight was larger than vimentin, this protein was assumed to be a complex of vimentin fragments).
Fig. 3.
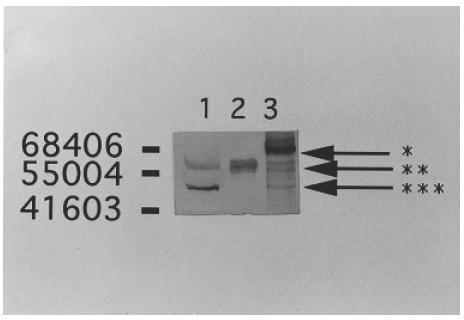
Western blotting analysis using the patient's serum against MRC5 (lane 1), recombinant human vimentin (lane 2), and recombinant human vimentin mixed with recombinant human caspase 3 (lane 3). The patient's serum reacted strongly with the human recombinant vimentin digested by caspase 3 (arrow *), suggesting that the band was the complex of some vimentin fragments. The positive band which had a molecular weight just over 55kD (arrow **) corresponded to vimentin. The positive band which had a lower molecular weight (arrow ***) seemed to correspond to the second band of lane 1 (vimentin fragment).
(Figure 4) shows standard curves of ELISA which detect antivimentin antibody in the patient's serum. Using this ELISA, it was possible to measure the antivimentin antibody in the patients' serum samples. Mean (± s.e.) levels of antivimentin antibodies in sera from patients with NSIP (0·33 (0·04), when compared with the control patient's serum which was determined to be 1·0) were significantly higher than those of normal volunteers (0·11 (0·01), P < 0·0001), and patients with IPF (0·17 (0·04), P < 0·001). In addition, levels of antivimentin antibodies in sera of patients with IPF were significantly higher than those of normal volunteers (P < 0·002) (Fig. 5).
Fig. 4.
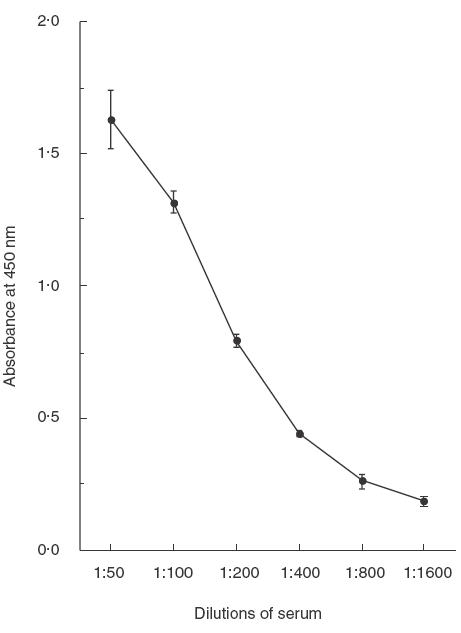
Standard curve of ELISA revealing antivimentin antibody in patient's serum. The concentration of recombinant vimentin to coat wells is 4 μ g/ ml.
Fig. 5.
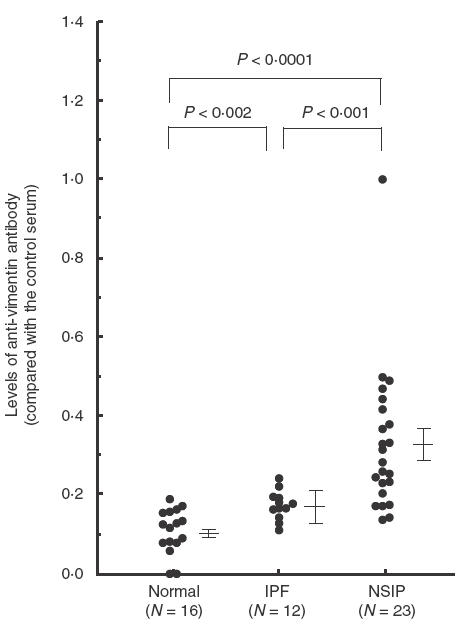
Levels of antivimentin antibodies in serum samples from patients with IPF, NSIP and normal volunteers. Mean (s.e.) levels of antivimentin antibodies in the sera of patients with NSIP (0·33 (0·04)) were significantly higher than those of normal volunteers (0·11 (0·01), P < 0·0001, Mann–Whitney U-test) and patients with IPF (0·17 (0·04), P < 0·001).
In patients with NSIP, antivimentin antibody levels in sera had a significant positive correlation with serum IgG (R = 0·51, P < 0·05), IgG in BALF (R = 0·59, P < 0·05), and %lymphocytes in BALF (R = 0·52, P < 0·05).
DISCUSSION
In this study, we have shown the antivimentin antibody to be one of the IgG antibodies against myofibroblasts (MRC5 cells) in patients with IPF and NSIP. Measurement of this IgG antibody by ELISA showed that the level of antivimentin antibodies in serum from patients with IPF and NSIP were significantly higher than in normal volunteers.
To date, autoantibodies to different classes of intermediate filaments have been detected in various human sera. Some have been found to be directed against vimentin. Vimentin has also been shown to be a target for an autoimmune reaction, not only in bacterial [17], parasitic [18] and viral infections [15], but also in rheumatic diseases [20–22]. Senecal et al.[19] have reported that the antivimentin antibody is detected when clinical and serological characteristics are typical of both systemic lupus erythematosus and progressive systemic sclerosis. Uchida et al.[24] have reported that the antivimentin antibody was detected in 11 of 68 patients with RA (16·2%) and in four of 19 patients with polymyositis/dermatomyositis (21·1%). In RA patients, although there was no association of this antibody with age, sex, age at onset or course of the disease, patients with the antivimentin antibody show higher frequencies of pulmonary fibrosis. In addition, Suzuki et al.[25] have reported that 50% of RA patients with the antivimentin antibody and 83% of PSS patients with this antibody have lung fibrosis. These results suggest that the antivimentin antibody is closely associated with the occurrence of pulmonary fibrosis.
The significance of the antivimentin antibody in the pathogenesis of IPF and NSIP should be discussed. In NSIP lung, vimentin is expressed in fibroblasts and myofibroblasts [6]. Zhang et al.[26] have reported that the proliferation of human bronchial myofibroblasts significantly increases after epithelial cell damage. The unmasking of hidden antigens after cell injury during the inflammatory process of disease may lead to sensitization and antibody production [27]. Then, the resulting antibody–antigen interaction with immune complex formation could have a significant role in the perpetuation of the disease processes, either by direct injury of fibroblasts, myofibroblasts, or via local macrophage activation as they are cleared by phagocytosis. In the present study, levels of antivimentin antibody correlated with serum IgG, IgG in BALF and %lymphocytes in BALF, suggesting that levels of antivimentin antibody in serum was caused by local production of the antivimentin antibody in alveoli.
It has been postulated that post-translational modifications and relocalization of proteins during apoptosis lead to presentation of these molecules to the immune system in such a way that normal mechanisms of tolerance are bypassed [28].
Although the initiating events in autoantibody production remain unknown, there is increasing evidence that apoptotic cells may serve as reservoirs of autoantigens [28]. Normal mice immunized intravenously with syngeneic apoptotic thymocytes develop low levels of autoantibodies, whereas similar mice immunized with non-apoptotic syngeneic splenocytes generally do not develop autoantibodies [29]. In addition, human keratinocytes induced to undergo apoptosis demonstrate clustering of autoantigens into discreet blebs at the cell surface membrane [30]. Moreover, cultured keratinocytes exposed to ultraviolet B/ultraviolet A irradiation (a known apoptotic stimulus) demonstrate enhanced binding of autoantibodies directed against Sm, RNP, Ro and La to the cell surface membrane [31]. Such evidence raises the possibility that apoptotic cells might present self-antigens to the immune system in a manner that bypasses normal mechanisms of tolerance [28].
Furthermore, Gensler et al. have demonstrated that immunization of normal mice with apoptotic Jurkat cells results in the formation of antibodies targeting multiple autoantigens or autantigen complexes, including Ku, rRNPs, snRNPs and vimentin [28]. These findings are consistent with the hypothesis that apoptosis can contribute to the development of autoimmunity.
Although the mechanism of induction of autoantibodies against vimentin in IPF and NSIP still remains obscure, proteolysis of vimentin might play a role. Prasad et al.[32] have reported apoptosis-associated proteolysis of vimentin in human prostate epithelial tumour cells and demonstrated that vimentin is subjected to limited proteolysis in the apoptotic cells as has been observed with cytokeratin. In addition, intact vimentin and some of its proteolytic fragments correspond to ubiquitinated polypeptides which are formed during the process of apoptosis [33]. In the present study, we characterized not only one protein which had a molecular weight of 57 000 as vimentin, but also another antigen; the vimentin fragment produced through the digestion of vimentin by caspase 3. Therefore, caspase 3 mediated proteolysis during the process of apoptosis might be the main mechanism of antibody formation. However, to evaluate the mechanism of formation of the antivimentin antibody, the specific epitope detected by the antivimentin antibody in patients' sera should be performed. Alcover et al.[34] have reported that antivimentin antibodies interact preferentially with a specific domain of the protein, a peptide with a molecular weight of 30 kilodalton close to the amino-terminal of the vimentin molecule. Since we identified several patients who had high titres of the antivimentin antibody, epitope analysis should be performed in future studies.
In conclusion, our data demonstrate that the antivimentin antibody was formed in some patients with IPF, idiopathic NSIP and NSIP associated with polymyositis/dermatomyositis. In addition, levels of antivimentin antibody in sera increased in patients with IPF and NSIP. These results suggest that antivimentin antibodies may have played a significant role in the process of lung injury in pulmonary fibrosis. Therefore, the significance of the antivimentin antibody in terms of disease severity, stage, prognosis and possible response to treatment should be evaluated in future studies.
REFERENCES
- 1.Katzenstein A, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18:136–47. [PubMed] [Google Scholar]
- 2.Fujita J, Yamadori I, Suemitsu I, et al. Clinical features of non-specific interstitial pneumonia. Respir Med. 1999;93:113–8. doi: 10.1016/s0954-6111(99)90300-1. [DOI] [PubMed] [Google Scholar]
- 3.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt-Graff A, Desmouliere A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 1994;425:3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- 5.Skalli O, Schurch W, Seemayer T, et al. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989;60:275–85. [PubMed] [Google Scholar]
- 6.Yoshinouchi T, Ohtsuki Y, Ueda R, et al. Myofibroblasts and S-100 protein positive cells in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated interstitial pneumonia. Eur Respir J. 1999;14:579–84. doi: 10.1034/j.1399-3003.1999.14c16.x. 10.1034/j.1399-3003.1999.14c16.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249–56. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- 9.Steinert PM, Roop DR. Molecular and cellular biology of intermediate filaments. Ann Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- 10.Owen PJ, Johnson GD, Lord JM. Protein kinase C-delta associates with vimentin intermediate filaments in differentiated HL60 cells. Exp Cell Res. 1996;225:366–73. doi: 10.1006/excr.1996.0187. [DOI] [PubMed] [Google Scholar]
- 11.Dobashi N, Fujita J, Ohtsuki Y, et al. Detection of anti-cytokeratin 8 antibody in the serum of patients with cryptogenic fibrosing alveolitis and pulmonary fibrosis associated with collagen vascular disorders. Thorax. 1998;53:969–74. doi: 10.1136/thx.53.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobashi N, Fujita J, Murota M, et al. Elevation of anti-cytokeratin 18 antibody and circulating cytokeratin 18: anti-cytokeratin 18 antibody immune complexes in sera of patients with idiopathic pulmonary fibrosis. Lung. 2000;178:171–9. doi: 10.1007/s004080000020. [DOI] [PubMed] [Google Scholar]
- 13.Fujita J, Dobashi N, Ohtsuki Y, et al. Elevation of anti-cytokeratin 19 antibody in sera of the patients with idiopathic pulmonary fibrosis and pulmonary fibrosis associated with collagen vascular disorders. Lung. 1999;177:311–9. doi: 10.1007/pl00007649. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretherton L, Toh BH, Jack I. IgM autoantibody to intermediate filaments in Mycoplasma pneumoniae infections. Clin Immunol Immunopathol. 1981;18:425–30. doi: 10.1016/0090-1229(81)90135-5. [DOI] [PubMed] [Google Scholar]
- 18.Bohme MW, Evans DA, Miles MA, et al. Occurrence of autoantibodies to intermediate filament proteins in human visceral leishmaniasis and their induction by experimental polyclonal B-cell activation. Immunology. 1986;59:583–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Kataaha PK, Mortazavi-Milani SM, Russell G, et al. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985;44:446–9. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcover A, Molano J, Renart J, et al. Antibodies to vimentin intermediate filaments in sera from patients with systemic lupus erythematosus. Arthritis Rheum. 1984;27:922–8. doi: 10.1002/art.1780270812. [DOI] [PubMed] [Google Scholar]
- 21.Kurki P, Helve T, Virtanen I. Antibodies to cytoplasmic intermediate filaments in rheumatic diseases. J Rheumatol. 1983;10:558–62. [PubMed] [Google Scholar]
- 22.Senecal JL, Oliver JM, Rothfield N. Anticytoskeletal autoantibodies in the connective tissue diseases. Arthritis Rheum. 1985;28:889–98. doi: 10.1002/art.1780280808. [DOI] [PubMed] [Google Scholar]
- 23.Senecal JL, Rothfield NF, Oliver JM. Immunoglobulin M autoantibody to vimentin intermediate filaments. J Clin Invest. 1982;69:716–21. doi: 10.1172/JCI110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida K, Kondo H, Kashiwazaki S. The clinical significance of antibodies to vimentin intermediate filaments in patients with rheumatoid arthritis. Ryumachi. 1987;27:356–63. [in Japanese] [PubMed] [Google Scholar]
- 25.Suzuki T, Kondo H, Kashiwazaki S. Antibodies to vimentin and cytokeratin in patients with connective tissue diseases. Jpn J Clin Immunol. 1989;12:184–91. [Google Scholar]
- 26.Zhang S, Smartt H, Holgate ST, et al. Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: an in vitro co-culture model of airway remodeling in asthma. Lab Invest. 1999;79:395–405. [PubMed] [Google Scholar]
- 27.Mayet WJ, Press AG, Hermann E, et al. Antibodies to cytoskeletal proteins in patients with Crohn's disease. Eur J Clin Invest. 1990;20:516–24. doi: 10.1111/j.1365-2362.1990.tb01895.x. [DOI] [PubMed] [Google Scholar]
- 28.Gensler TJ, Hottelet M, Zhang C, et al. Monoclonal antibodies derived from BALB/c mice immunized with apoptotic Jurkat T cells recognize known autoantigens. J Autoimmun. 2001;16:59–69. doi: 10.1006/jaut.2000.0464. 10.1006/jaut.2000.0464. [DOI] [PubMed] [Google Scholar]
- 29.Mevorach D, Zhou J, Song X, et al. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythramatosus are clustered in two populations of surface blebs on cultured keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golan TD, Elkon KB, Gharavi AE, et al. Enhanced membrane binding of autoantibodies to cultured keratinocytes of systemic lupus erythematosus patients after ultraviolet B/ultraviolet A irradiation. J Clin Invest. 1992;90:1067–76. doi: 10.1172/JCI115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad SC, Thraves PJ, Kuettel MR, et al. Apoptosis-associated proteolysis of vimentin in human prostate epithelial tumor cells. Biochem Biophys Res Commun. 1998;249:332–8. doi: 10.1006/bbrc.1998.9137. 10.1006/bbrc.1998.9137. [DOI] [PubMed] [Google Scholar]
- 33.Soldatenkov VA, Prasad S, Voloshin Y, et al. Sodium butyrate induces apoptosis and accumulation of ubiquitinated proteins in human breast carcinoma cells. Cell Death Differ. 1998;5:307–12. doi: 10.1038/sj.cdd.4400345. [DOI] [PubMed] [Google Scholar]
- 34.Alcover A, Hernandez C, Avila J. Human vimentin autoantibodies preferentially interact with a peptide of 30kDmol.wt, located close to the amino-terminal of the molecule. Clin Exp Immunol. 1985;61:24–30. [PMC free article] [PubMed] [Google Scholar]


