Abstract
Wegener’s granulomatosis (WG) is characterized by a predominance of the type 1 T-helper cell (Th1) response. We have studied monocytic cytokine expression in untreated patients and in patients who did not respond to prior methotrexate or trimethoprim-sulphamethoxazole therapy, i.e. patients with active disease. Intracytoplasmic IL-12 and TNF-α expression was significantly increased in WG compared with healthy controls. IL-8 expression was not increased. Two and 12 weeks of daily standard oral cyclophosphamide and corticosteroid (CYC + GC) treatment induced a stable remission of the disease. Elevated IL-12 and TNF-α expression of monocytes was normalized. The active metabolite of CYC was shown to down-regulate IL-12 mRNA in vitro. Monocytic cytokines, especially IL-12, may have a role in the early determination and skewing of the immunoregulatory response towards a Th1 profile. It appears that CYC + GC exerts its effect by normalizing the Th1-driving cytokine pattern, and CYC may maintain this mode of action. Normalization of the skewed cytokine pattern may be a prerequisite and an indicator of inducing a remission in WG.
Keywords: Wegener’s granulomatosis, cyclophosphamide, IL-12, TNF-α
INTRODUCTION
Wegener’s granulomatosis (WG) is a rare disease characterized by granulomatous lesions and a small vessel vasculitis. Involvement of multiple organs and a pulmonary–renal syndrome are found in generalized WG. The outcome of WG was usually fatal prior to the use of immunosuppressive drugs. T cells and monocytes have an important role in the pathogenesis of (WG). CD4+ T cells, monocyte-derived tissue macrophages, giant cells and neutrophils are found in significant numbers in granulomatous lesions in WG. Granuloma formation and vasculitis may be initiated and sustained by specialized, cytokine-producing T cells, activated monocytes and macrophages [1–5]. An increased production and release of IFN-γ by activated CD4+ T cells indicates a predominance of a Th1-like response in generalized WG [1–3]. T cells were shown to produce TNF-α in WG [3]. Monocytes play an important role in the early determination of the cytokine profile. Monocytes from WG patients secrete increased amounts of IL-12, a cytokine driving the T-cell response towards a Th1 pattern [3]. Furthermore, an enhanced TNF-α gene expression has been reported in monocytes [6], suggestive of additional mechanisms of innate immunity in the immunopathogenesis of WG.
In a recent study, Comabella et al[7] demonstrated that pulse cyclophosphamide and methylprednisolone therapy was able to normalize elevated IL-12 production by monocytes and increased IFN-γ production of T cells in multiple sclerosis [7]. Standard oral cyclophosphamide (CYC) and corticosteroids (GC) are still the mainstay in the treatment of WG patients with new, full-blown active disease or a major relapse of WG [8]. In this report, we demonstrate, that CYC + GC therapy normalizes monocytic IL-12 and TNF-α production in WG. In vitro experiments supported the view that the active metabolite of CYC down-regulates IL-12 mRNA. These findings suggest that normalization of the Th1-driving cytokine pattern may be a prerequisite and an indicator of achieving a stable remission in WG. Furthermore, future anti-cytokine therapies will have to be directed towards normalizing the skewed immune response in WG.
MATERIALS AND METHODS
Patients and treatment regimen
Six patients with generalized, biopsy-proven WG were studied in a longitudinal study. All patients met the criteria of the American College of Rheumatology [9] and the Chapel Hill Consensus Conference definition for WG [10]. All patients were C-ANCA and PR3-ANCA positive. The disease extension and vasculitis activity were described by the Disease Extension Index (DEI) and Birmingham Vasculitis Activity Score as outlined elsewhere. In brief, the DEI is the equivalent of the current organ involvement in WG, whereas the BVAS considers clinical features and laboratory data to give a measure of vasculitis activity [11,12]. All patients had active disease. In two of the patients, WG was newly diagnosed. Four patients had a major relapse. Two patients were treated with methotrexate (MTX) and one patient with trimethoprim-sulphamethoxazole (T/S) prior to the relapse. Another patient had been in full remission and had no treatment prior to his relapse. After the diagnosis of relapse, or in newly diagnosed WG, all patients received standard oral CYC + GC therapy for the induction of remission, starting with 2 mg/kg of CYC and 1 mg/kg of GC. The GC dose was subsequently tapered. The male/female ratio was 3/3, and the age was 58 ± 14·7 years (mean ± s.e.m.). Informed consent was obtained from all patients. Nine healthy donors were also investigated (M/F = 2/7; age = 38·3 ± 12·9 years).
Antibodies
Anti-human CD45/CD19/CD16/CD56/CD3 (PerCP/APC/PE/ FITC) and anti-human CD45/CD3/CD8/CD4 (PerCP/FITC/ PE/APC) four colour reagents and IgG2a/IgG1 two colour (FITC/PE) reagents, APC conjugated anti-human CD14, CD4, PE-conjugated mouse IgG1, FITC anti-human CD69 and APC-conjugated anti-human CD8, Cy-Chrome anti-human CD3, PE-conjugated mouse anti-human TNF-α, rat anti-human IL-12, IL-8 and rat IgG2a were obtained from Becton Dickinson (Heidelberg, Germany).
Cell culture
Heparinized peripheral blood (150 µl) was diluted with 850 ¼l RPMI 1640 supplemented with 10% FCS, 5 mml-glutamine, 50 U/ml penicillin and 50 mg/ml streptomycin (all from Sigma, Munich, Germany). To determine cytoplasmic expression of cytokines in monocytes, cells were cultured either in medium alone (basal condition) or with LPS (100 ng/ml, Sigma) as a positive control, for 20 h. Monensin was also added to the medium. Cells were incubated at 37°C in a humidified atmosphere in a 5% CO2 incubator.
Intracytoplasmic staining and flow cytometry
Cells were pooled and washed twice (250 g, 5 min, 4°C) in staining buffer (PBS without Ca2+ and Mg2+ (Gibco BRL, Karlsruhe, Germany), 0·1% BSA, 0·1% sodium azide (Sigma), pH = 7·4). Cells were stained in 100 µl staining buffer containing fluorochrome-conjugated monoclonal antibodies for cell surface antigens (each antibody was tested previously to determine the optimal concentration). Following incubation on ice for 30 min in the dark, two more washing steps with staining buffer were performed. The resuspended cells were fixed and lysed in 2 ml diluted FACS Lysing Solution (Becton Dickinson) for 10 min at room temperature. Cells were washed twice in staining buffer. The pellet was resuspended in 100 µl permeabilization buffer (PBS without Ca2+ and Mg2+, 0·1% BSA, 0·1% sodium azide, 0·1% saponin (Sigma), pH = 7·4) containing a previously determined optimal concentration (0·25–1·0 ¼g/100 µl) of fluorochrome-conjugated anti-cytokine antibodies for intracellular antigens, or appropriate negative (isotype) controls. Incubation was performed at 4°C for 30 min in the dark. After incubation, cells were washed twice in permeabilization buffer. Resuspended cells in staining buffer were further analysed by flow cytometry, or were resuspended in storage buffer (1% formaldehyde in PBS) and reserved for future use (2 days) at 4°C in the dark.
Four-colour flow cytometric analysis was performed using a FACSCalibur™ flow cytometer (Becton Dickinson). Data were acquired with CELL-Quest™ software (Becton Dickinson). Monocytes were gated for analysis based on light scattering properties and on CD14 staining. Data for 1000 monocytes were collected. Positively and negatively stained populations were calculated by quadrant dot plot analysis determined by isotype controls.
In vitro experiment using Mafosfamide
Mafosfamide, an active cyclophosphamide metabolite, was a kind gift of Astamedica AG (Frankfurt, Germany). Mafosfamide, i.e. 4-(2-sulphonatoethylthio)]-cyclophosphamide, was used because it undergoes rapid spontaneous hydrolysis in vitro with liberation of 4-hydroxy-cyclophosphamide. This imitates metabolic activation of cyclophosphamide. Cyclophosphamide undergoes metabolic activation to the active compound of 4-hydroxy-cyclophosphamide by hepatic cytochrome P450 mediated hydroxylation in vivo. PBMC (1 × 106/ml) were cultured in six-well plates for 1 h with or without Mafosfamide at 10–9, 10–7 and 10–5m. Afterwards, cells were washed, pelleted and frozen at –70°C for further analysis.
IL-12 RT-PCR
Analysis of IL-12 p35 messenger RNA (mRNA) expression by PBMC was performed by RT-PCR. Total RNA was isolated using a phenol-chloroform based kit (Roth, Karlsruhe, Germany) according to the manufacturer’s instructions. RNA was reverse transcribed using 0·5 ¼m oligo (pdT)12–18, 1 ¼l RNaseOUT (Life Technologies, Karlsruhe, Germany) and 200 U Superscript II reverse transcriptase (Life Technologies). Complementary DNA (cDNA) was subjected to non-quantitative PCR. The reaction volume was 25 ¼l, with 20 pmol each primer (Life Technologies), 200 µm dNTPs (peqlab, Erlangen, Germany) and 0·5 U Taq polymerase (Life Technologies). GAPDH served as internal control. Amplified products were separated on a 1·5% agarose gel and stained with ethidium bromide. A 100 bp ladder (Life Technologies) was included as marker.
Statistical analysis
Data are presented as mean values ± s.e.m. For comparison of WG patients and healthy controls, the Mann–Whitney U-test was used. For comparison during follow-up of WG patients before and after CYC and GC therapy, the Wilcoxon signed rank test was employed. Correlations were analysed using Spearman’s rank correlation.
RESULTS
Patients’ outcome
All patients achieved a stable remission within 3 months of CYC + GC treatment. DEI decreased from 7·4 ± 0·9 to 2·4 ± 0·4 (P < 0·05). BVAS improved from 24·0 ± 3·1 to 4·6 ± 0·8 (P < 0·05). The dose of GC could be tapered from doses of 50–70 mg/day after initiation of the therapy to 5 mg/day in five patients and 7·5 mg/day in one patient without disease activity recurring at 3 months.
IL-12 and TNF-α expression in monocytes of WG patients before treatment
In active, generalized WG, intracytoplasmic expression of IL-12 in monocytes was found in a higher percentage of cells compared with healthy controls under basal conditions (mean 25%versus 5%, P < 0·01).
Following LPS stimulation, intracytoplasmic IL-12 expression was also found in a higher percentage of monocytes in WG compared with healthy controls (34%versus 18%), but the difference was not significant.
We also detected higher intracytoplasmic TNF-α expression in monocytes of WG patients compared with healthy controls without LPS stimulation (17%versus 4%, P < 0·05). Upon LPS stimulation, intracytoplasmic TNF-α expression in monocytes was still higher in WG compared with healthy controls (44%versus 31%), but as for IL-12, the difference was not significant. A very high correlation was found for intracytoplasmic IL-12 and TNF-α expression under basal conditions (r = 0·975; P≤ 0·05). Figure 1 shows representative stainings of surface and intracellular markers of the fraction of the (gated) CD14+ monocyte population of a WG patient.
Fig. 1.
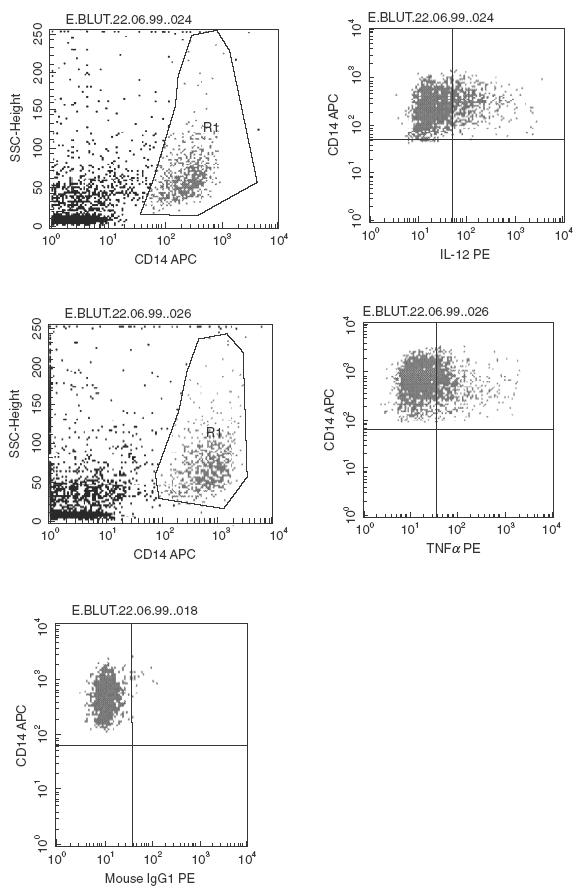
Representative quadrant dot plot analysis of peripheral blood CD14+ monocytes of a WG patient. Monocytes were gated for analysis based on light scattering properties and on CD14 staining. The fraction of positively-stained monocytes displaying intracytoplasmic IL-12 and TNF-α was calculated by quadrant dot plot analysis determined by isotype controls. An isotype control for an intracytoplasmic cytokine staining is also shown.
Normalization of IL-12 and TNF-α expression in WG patients after treatment
To investigate the influence of CYC + GC treatment on intracytoplasmic cytokine expression of monocytes in WG, an early (2 weeks) and a late (12 weeks) time point was chosen for examination. Basal monocytic intracytoplasmic IL-12 expression (25%versus 2%) and basal intracytoplasmic TNF-α expression (17%versus 2%) of WG patients were normalized to the levels of healthy controls following 14 days of CYC + GC (P < 0·05). Moreover, after 12 weeks, basal intracytoplasmic IL-12 and TNF-α expression remained at a low level (2%versus 4% and 2%versus 3%, respectively) (Fig. 2,Table 1).
Fig. 2.
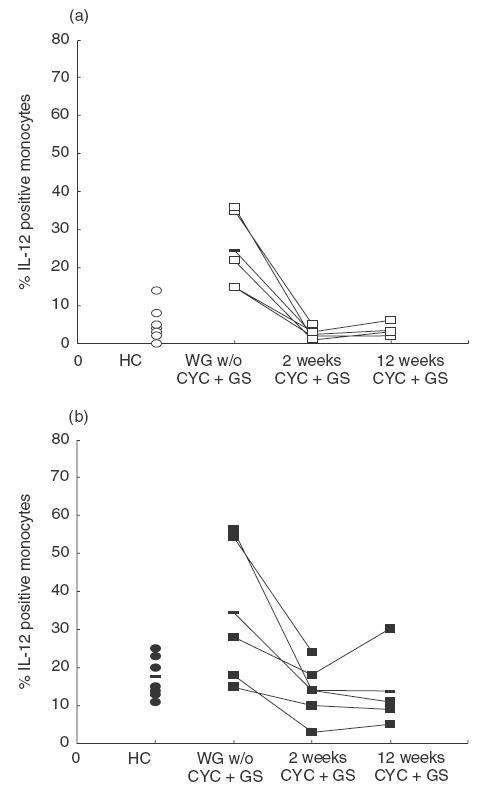
(a) Basal and (b) LPS-stimulated (20 h) cytoplasmic IL-12 expression in active, generalized WG patients and healthy controls. The number of IL-12 positive monocytes was significantly elevated compared with healthy controls without, but not with, LPS stimulation (P < 0·05 and P > 0·05, respectively). Two and 12 weeks of treatment with CYC and GC normalized the increased IL-12 expression. The percentages of IL-12-staining monocytes are indicated for individuals in each group and means are indicated by a horizontal line.
Table 1.
Comparison of cytoplasmic IL-8, IL-12 and TNF-α expression of CD14+ monocytes in generalized WG and healthy controls using FACS analysis. IL-12 and TNF-α, but not IL-8, expression was significantly reduced by CYC + GC therapy. Data are expressed as mean ± s.e.m. of the percentage of CD14+ monocytes displaying intracytoplasmic cytokine expression
| IL-8 | IL-12 | TNF-α | |
|---|---|---|---|
| Healthy controls (n = 9) | |||
| Basal condition | 84·9 ± 3·4 | 4·9 ± 1·4 | 4·1 ± 1·7 |
| LPS stimulation | 95·1 ± 2·8 | 17·7 ± 1·7 | 31·4 ± 6·4 |
| WG patients before CYC = GC therapy (n = 6) | |||
| Basal condition | 82·4 ± 12·7 | 24·6 ± 4·6* | 17 ± 5·8* |
| LPS stimulation | 97·4 ± 0·9 | 34·2 ± 8·8 | 43·6 ± 12·2 |
| WG patients after 2 weeks of CYC = GC therapy (n = 6) | |||
| Basal condition | 24·6 ± 4·6 | 2·0 ± 0·75* | 1·6 ± 0·75* |
| LPS stimulation | 34·2 ± 8·8 | 13·8 ± 3·6 | 16·6 ± 4·7 |
| WG patients after 12 weeks of CYC + GC therapy (n = 6) | |||
| Basal condition | 41·2 ± 17·4 | 3·5 ± 0·87* | 3 ± 1·7* |
| LPS stimulation | 67·8 ± 22·4 | 13·8 ± 5·6 | 18·5 ± 9·4 |
Significant changes (P-value < 0·05 or 0·01) of monocytes displaying intracytoplasmic cytokine expression in WG patients before and after 2 and 12 weeks of standard oral cyclophosphamide (CYC) and corticosteroid (GC) therapy. WG = Wegener’s granulomatosis.
Cytoplasmic IL-8 expression before and after treatment
Intracytoplasmic IL-8 expression of monocytes under basal and stimulated conditions was not statistically different between WG and healthy controls before and after treatment.
Down-regulation of IL-12 mRNA by the active metabolite of cyclophosphamide in WG patients in vitro
GAPDH mRNA was present in both samples, as shown in Fig. 3. With regard to IL-12 p35, we observed expression of mRNA for both healthy controls and WG patients. However, at 10–5m Mafosfamide, there was no signal for IL-12 p35 expression in the PBMC of WG patients, indicating down-regulation of previously up-regulated IL-12 mRNA, whereas in the healthy control sample a weak expression was still detectable.
Fig. 3.
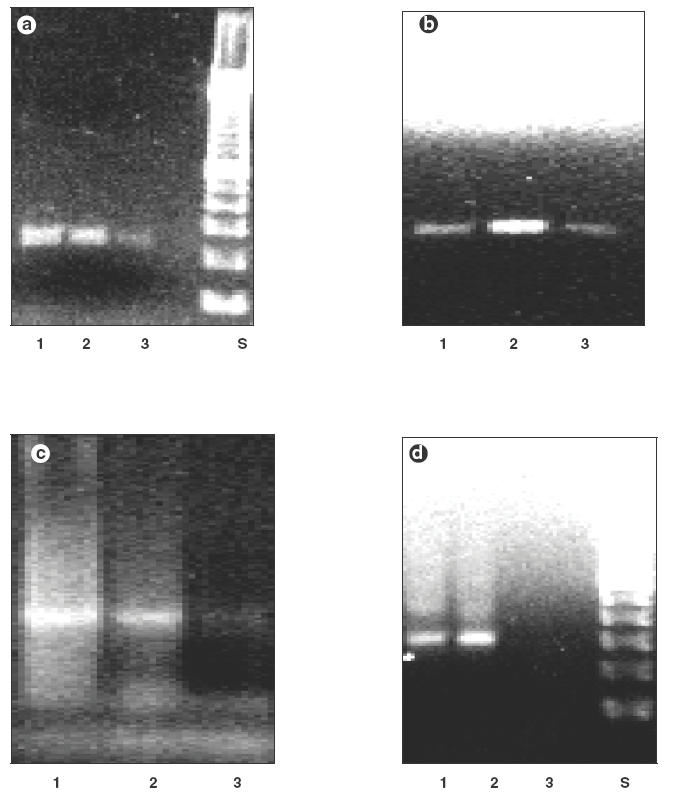
GAPDH mRNA (233 bp) expression by in vitro Mafosfamide-treated PBMC (lane 1: no Mafosfamide; lane 2:10–9m Mafosfamide; lane 3:10–5m Mafosfamide; S: 100 bp standard) of a healthy control (a) and a WG patient (b). IL-12 p35 mRNA (295 bp) expression by in vitro Mafosfamide-treated PBMC (lane 1: no Mafosfamide; lane 2:10–9m Mafosfamide; lane 3:10–5m Mafosfamide) of a healthy control (a) and a WG patient (d). We observed a down-regulation of IL-12 p35 mRNA at the highest concentration of Mafosfamide by the PBMC of the WG patient, whereas the IL-12 p35 signal for the PBMC from the healthy control was still detectable.
DISCUSSION
We found elevated cytoplasmic IL-12 and TNF-α expression in monocytes during active, generalized WG compared with healthy controls. Following LPS stimulation, the difference in cytoplasmic IL-12 and TNF-α expression became less prominent between WG and healthy controls due to the induced higher IL-12 and TNF-α levels in the controls. Monocytic IL-8 was not found to be over-expressed. In three of the six patients, previous treatment with either MTX or T/S had not resulted in normalized monocytic cytokine IL-12 and TNF-α expression, and had not been able to control disease activity. These three patients relapsed while being treated with MTX or T/S. Standard oral CYC + GC treatment resulted in a significant and persistent reduction of intracytoplasmic IL-12 and TNF-α expression. IL-12 mRNA in PBMC of WG patients was found to be down-regulated in the presence of an active metabolite of CYC in vitro.
An excellent qualitative correlation has been shown between flow cytometric (FACS) results of cytoplasmic cytokine expression and the level of cytokine production measured by conventional techniques such as ELISA. Furthermore, flow cytometric analysis allows the detection of single cell expression of cytokines in phenotypically well characterized cell subsets. In contrast, cytokine production measured in bulk cultures of PBMC does not allow exact allocation of cytokine expression to distinct, phenotypically characterized single cell subsets [13]. Ludviksson et al.[3] recently demonstrated monocytic IL-12 production by ELISA [3], and our results on intracytoplasmic IL-12 expression in monocytes of WG are in agreement with their study. Furthermore, we also detected IL-12 mRNA in PBMC of patients with active WG. Taken together, these findings indicate ongoing IL-12 production of activated monocytes in WG. Given the importance of IL-12 in priming the Th1 response [14], the data suggest that monocytic IL-12 may initiate skewing of the immune response towards a predominant Th1 response in WG.
In this report we found increased monocytic TNF-α expression, which was suppressed by standard oral CYC + GC. Deguchi et al.[6] reported enhanced TNF-α gene expression in monocytes [6]. The study by Ludviksson et al.[3] failed to detect increased TNF-α production measured by ELISA in monocytes, whereas T cells were found to be a source of TNF-α secretion [3]. However, as outlined above, FACS allows intracytoplasmic detection of cytokines [13], indicative of at least some TNF-α production in monocytes, which may have been missed by ELISA measurement of TNF-α secretion. Enhanced TNF-α gene expression [6] and cytoplasmic TNF-α expression in monocytes indicates a role for monocytes as a source of TNF-α. Against this background, treatment with anti-TNF-α antibodies or TNF-α receptor antagonists may appear to be beneficial in WG. CYC and GC related severe adverse effects such as infections, myelotoxicity and osteoporosis may be avoided by more specific anti-cytokine therapies.
Cytokine primed monocytes have been demonstrated to release IL-8 upon stimulation with ANCA in vitro[15]. All our patients were C-ANCA and PR3-ANCA positive. However, we did not find increased cytoplasmic IL-8 expression in monocytes during active WG. Our data suggest that in vivo, monocytic IL-8 production may be down-regulated during the course of WG.
Oral CYC + GC has been used as a standard therapy for the induction of remission in systemic vasculitis for many years. Profound suppression of B-cell function with decreased antibody production has been reported as a mechanism of action, implying that CYC acts predominantly on humoral immunity [16]. GC may inhibit the production of several cytokines, including IFN-γ[17]. Pulse CYC and methylprednisolone therapy has been found to normalize elevated monocytic IL-12 production and increased IFN-γ production by T cells in multiple sclerosis [7]. We extended this observation by demonstrating that monocytic IL-12 and TNF-α expression remain low when GC doses are tapered after 3 months. Furthermore, IL-12 mRNA of PBMC in WG patients was found to be down-regulated in the presence of 10–5m mafosfamide, an active cyclophosphamide metabolite. Thus, clinical data and in vitro findings may support the view that CYC alone was able to maintain the beneficial effect on the cytokine profile at a later stage when GC doses were tapered. Normalization of monocytic IL-12 and TNF-α production, with subsequent resolution of the Th1-driving cytokine pattern, points to a specific mode of action of CYC and is related to the induction of remission in WG. Normalization of the skewed cytokine pattern may be a prerequisite and an indicator of inducing a remission in WG.
Acknowledgments
This work was supported by a grant from the German Academic Exchange Service to GK and the German research society fund SFB 367/A8 to PL, AM and WLG.
REFERENCES
- 1.Csernok E, Szymkowiak C, Wang G, Paulsen J, Gross WL. T-cell cytokine profiles in patients with Wegener’s granulomatosis. (Abstract) Arthritis Rheum. 1995;38:S375. [Google Scholar]
- 2.Csernok E, Trabandt A, Müller A, et al. Cytokine profiles in Wegener’s granulomatosis: predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheum. 1999;42:742–50. doi: 10.1002/1529-0131(199904)42:4<742::AID-ANR18>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Ludviksson BR, Sneller MC, Chua KS, et al. Active Wegener’s granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160:3602–9. [PubMed] [Google Scholar]
- 4.Müller A, Trabandt A, Glöckner-Hofmann K, et al. Localized Wegener’s granulomatosis: predominance of CD26 and IFN-γ expression. J Path. 2000;192:113–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH656>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Muller Kobold AC, Kallenberg CGM, Cohen Tervaert JW. Monocyte activation in patients with Wegener’s granulomatosis. Ann Rheum Dis. 1999;58:237–45. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deguchi Y, Shibata N, Kishimoto S. Enhanced expression of the tumor necrosis factor/cachetin gene in peripheral blood mononuclear cells from patients with systemic vasculitis. Clin Exp Immunol. 1990;81:311–4. doi: 10.1111/j.1365-2249.1990.tb03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comabella M, Balashov K, Issazadeh S, Smith D, Weiner HL, Khoury SJ. Elevated IL-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J Clin Invest. 1998;102:671–8. doi: 10.1172/JCI3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross WL. New concepts in treatment protocols for severe systemic vasculitis. Curr Opin Rheumatol. 1999;11:41–6. doi: 10.1097/00002281-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 10.Jennette CJ, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 11.DeGroot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol. 2001;55:31–8. [PubMed] [Google Scholar]
- 12.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 13.Picker LJ, Singh MK, Zdraveski Z, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 14.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–9. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 15.Ralston D, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. J Clin Invest. 1997;100:1416–21. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu LP, Cupps TR, Whalen G, Fauci AS. Selective effects of cyclophosphamide therapy on activation, proliferation and differentiation of human B cells. J Clin Invest. 1987;79:1082–90. doi: 10.1172/JCI112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]


