Abstract
The role of mitochondrial proteins as antigens to antibodies of anti-M7 sera was analysed by flavin fluorescence, one-and two-dimensional Western blots and blue native gel electrophoresis. Flavin fluorescence of succinate dehydrogenase (SucDH, complex II of the respiratory chain) of rat liver inner mitochondrial membranes correlated with the immunoreactivity of a representative anti-M7 myocarditis serum. Antigens of isolated bovine heart mitochondria reacting with antibodies of myocarditis serum on two-dimensional Western blots were identified by MALDI-TOF and NanoESI mass spectrometry as myosin heavy chain β and as dihydrolipoamide dehydrogenase of the mitochondrial 2-oxoacid dehydrogenase complexes. The SucDH-flavoprotein was not resolved as a discrete protein spot on two-dimensional polyacrylamide gels. However, separation of the rat liver inner mitochondrial membrane complexes by blue native gel electrophoresis followed by Western blotting, and Western blots of purified Escherichia coli SucDH complex revealed that anti-M7 sera contained antibodies directed against the SucDH-flavoprotein subunit.
Keywords: antimitochondrial antibodies, flavoprotein, mitochondrial antigen, myocarditis, succinate dehydrogenase
INTRODUCTION
Sera of patients with myocarditis and idiopathic dilated cardiomyopathy (IDC) have been shown to contain circulating heart reactive antibodies. These antibodies interact with cardiac autoantigens represented by myosin heavy chain ]1[, the structural protein laminin ]2[ and the β1-adrenoreceptor ]3[. They may be of pathogenetic relevance and mice injected with cardiac myosin develop an autoimmune myocarditis, with disease symptoms similar to those following an infection with Coxsackievirus B3 ]1,4[. Antimitochondrial antibodies (AMA) have also been reported in the sera of these patients. Antigens identified as targets of AMAs were the ADP/ATP carrier ]5[ and the branched chain ketoacid dehydrogenase complex ]6[. In a systematic analysis by two-dimensional (2D) immunoblots, followed by amino-terminal sequencing of the antigens recognized by AMAs in sera of patients with IDC, Pohlner et al.]7[ identified as relevant antigens a 75-kDa subunit of the NADH-ubiquinon reductase (complex I of the respiratory chain), the two core proteins P1 and P2 of the ubiquinol-cytochrome c reductase (complex III of the respiratory chain) and two subunits of the pyruvate dehydrogenase complex, namely subunit E1β (pyruvate decarboxylase) and E3 (dihydrolipoamide dehydrogenase). No component of succinate dehydrogenase (SucDH, complex II of the respiratory chain) was identified in this investigation as autoantigen on 2D Western blots. Recently, in a similar investigation of autoreactive antibodies against heart tissue proteins in sera of patients with rheumatic heart disease ]8[, creatine kinase and the mitochondrial enzymes aconitate hydratase and dihydrolipoamide dehydrogenase were identified as antigens of autoantibodies in these sera. Again, no component of the SucDH complex was among the antigens identified on 2D Western blots.
Klein and Berg ]9[ described antimitochondrial antibodies (anti-M7) ]10[ in patients with myocarditis and idiopathic dilated cardiomyopathy (IDCM) directed against an aproximately 68 kDa antigen of the inner mitochondrial membrane, present in both bovine heart mitochondria and rat liver mitochondria. These sera contain antiflavoprotein (αFp) antibodies directed against enzymes with covalently bound flavin of bacterial or mammalian mitochondrial origin ]11,12[. The heart mitochondrial membrane antigen detected on Western blots by these sera probably represents the SucDH flavoprotein (Fp) subunit ]11[, which is the only protein with covalently attached FAD in cardiomyocytes. However, no direct demonstration of the flavoprotein nature of the antigen was performed, leaving the identity of this antigen uncertain. As SucDH-Fp failed to be identified on 2D Western blots as mitochondrial antigen to serum antibodies of heart patients ]7,8[ the question remained open whether the asignment of the inner mitochondrial membrane antigen as SucDH-Fp was valid.
This unsolved situation determined an in-depth investigation of mitochondrial antigens to autoantibodies in anti-M7 sera by flavine fluorescence, 1D and 2D Western blots and blue-native polyacrylamide gel electrophoresis (BN-PAGE). The analysis demonstrated that, besides autoantibodies against myosin heavy chain and dihydrolipoamide dehydrogenase of the 2-oxoacid dehydrogenase complexes, the inner mitochondrial membrane associated SucDH flavoprotein subunit of the respiratory chain complex II represents an autoantigen to antibodies of anti-M7 sera.
MATERIALS AND METHODS
Chemicals and immunochemicals
FAD was obtained from Sigma (Munich, Germany). All other chemicals were of the highest purity available. Monoclonal SucDH-Fp was purchased from Molecular Probes (Leiden, the Netherlands) and antiserum against 6HDNO was raised in rabbits as described previously ]13[. Mouse alkaline phosphatase coupled monoclonal antihuman and antirabbit IgG antibodies were obtained from Sigma (Munich, Germany).
Patient
Serum of a patient with myocarditis was obtained by consent of the patient and according to the guidelines of the ethic commission of the University of Freiburg. The diagnosis of acute myocarditis was based on the presence of a dilated heart with systolic disfunction in the absence of coronary artery or valvular heart disease as documented by heart catheterization, echocardiography, myocardial scintigraphy and coronarography. The freshly collected serum when tested on Western blots gave a characteristic immunoreaction with mitochondrial antigens and was taken as representative for anti-M7 sera ]11[. Serum from a healthy individual was included as control. Sera kept at – 20°C from a second patient with myocarditis and sera of three patients with IDCM have been described as anti-M7 before ]11[. They were included to confirm findings obtained with the freshly colected myocarditis serum.
Isolation of mitochondria and mitochondrial inner membranes
Mitochondria were isolated from bovine heart as described in ]14[. Isolation of inner mitochondrial membranes was performed according to Pallotta et al.]15[ and were a kind gift of Dr Carmen Brizio (University of Bari, Italy). Succinate dehydrogenase complex isolated from Escherichia coli was kindly provided by Dr Garry Cecchini (San Francisco, CA, USA).
Two-dimensional PAGE, BN-PAGE and Western blots
SDS-PAGE and 2D PAGE of 14 × 14 cm was performed according to standard protocols using Immobilon Drystrips pH 3–10 NL, 18 cm (Amersham Pharmacia Biotech, Freiburg, Germany). BN-PAGE was according to ]16[. Proteins were transferred from the gels to nitrocellulose or PVDF filter membranes (Millipore Corporation, Bedford, USA) and the Western blots were developed with myocarditis serum and human control serum at a dilution of 1:500, or with αFp antibodies containing antiserum raised in rabbits with the covalently flavinylated bacterial enzyme 6HDNO, in a dilution of 1:2000.
MALDI-TOF and NanoESI mass spectrometry
The SDS-PAGE separated proteins were excised from the gel, reduced with DDT, alkylated with iodoacetamide and cleaved with sequencing grade trypsin (Promega, Madison, WI, USA) as described in ]17[. All MALDI-TOF analyses were performed using a TofSpec 2E MALDI-TOF (Micromass, Manchester, UK) operated in the delayed extraction and reflector mode according to the directions of the manufacturer. The spectra were calibrated externally and corrected with an internal lock mass using an autodigestion peptide of porcine trypsin. Proteins were identified using the ProteinLynx Global Server software searching the Swissprot 39 database. Alternatively the extracted tryptic peptides were subjected to NanoESI tandem mass spectrometry (MSMS) performed according to ]18[. The mass spectra were acquired on a API 300 mass spectrometer (PE Sciex, Toronto, Ontario, Canada) equipped with a NanoESI source (Protana, Odense, Denmark). Proteins were identified using the WWW version of the peptide search program of M. Mann at http://peptsearch.protana.com/.
RESULTS
Correlation between flavin fluorescence of SucDH-Fp and immunoreaction with antibodies of myocarditis serum
First we analysed on one-dimensional gels the correlation between the fluorescence of the flavin carrying SucDH-Fp and an immunoreaction with myocarditis serum. Besides bovine mitochondria, we used a preparation of purified rat liver inner mitochondrial membrane. If the antibodies of the myocarditis serum contain indeed αFp antibodies, the source of mitochondria should be irrelevant, and only the presence of a protein with bound flavin should be of importance for the occurrence of an antibody–antigen reaction. We employed in this investigation the serum of a patient with acute myocarditis. This serum reacted on Western blots of bovine heart mitochondria with the 68 kDa antigen and was therefore taken as representative for anti-M7 sera.
Rat liver inner mitochondrial membrane proteins and bovine heart mitochondrial proteins were separated by SDS-PAGE on 10% polyacrylamide gels, soaked in 10% acetic acid and inspected on an UV-transilluminator. The bacterial 6-hydroxy-d-nicotine oxidase (6HDNO) protein was used as a control for protein-bound flavin fluorescence ]12[. The fluorescent band was marked and the gel stained with Coomassie blue (Fig. 1a). As can be seen in Fig. 1b, a strong fluorescent band was present in the mitochondrial inner membrane fraction, and a weak fluorescent band, migrating at the same position, could be identified in the bovine heart mitochondrial fraction (Fig. 1b). This fluorescent protein band (Fig. 1a, arrowhead), migrating at a Mr of approximately 68 kDa, represents the Fp subunit of SucDH. The band was carefully excised and eluted from the minced gel slices. Western blots on nitrocellulose membrane were prepared with the eluted protein, with rat inner mitochondrial membrane and with bovine heart mitochondria and developed with myocarditis serum. As an αFp antibody positive serum, the rabbit anti6HDNO serum was employed ]14[. As can be seen from (Fig. 1c, lanes 1–3), both the rabbit α-6HDNO serum and the myocarditis serum recognized a 68-kDa band of the inner mitochondrial membrane. As expected, there were additional protein antigens recognized by antibodies of the myocarditis serum in the bovine heart mitochondrial preparation. They may represent antigens identified previously as targets to antibodies in sera of patients with myocarditis ]1–7[. The eluted fluorescent 68 kDa protein band also gave an immunoreaction with the myocarditis serum, but not with a serum from a healthy control person (Fig. 1c, lanes 4 and 5). Preincubation of the rabbit α-6HDNO serum and the myocarditis serum with FAD suppressed the reaction of the sera with the 68 kDa protein band on Western blots (Fig. 1c, compare lanes 6, 7 and 8, 9, respectively). Apparently the protein bound flavin moiety of the flavoprotein subunit represented part of the antigenic determinant required for recognition by the antibodies of the myocarditis serum.
Fig. 1.
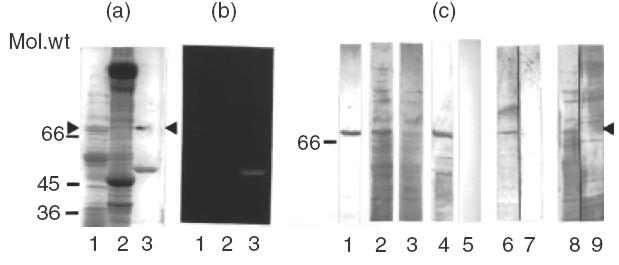
Correlation of SucDH-Fp fluorescence and immunoreaction with myocarditis serum. (a) SDS-PAGE followed by Coomassie blue staining of: lane 1, rat liver inner mitochondrial membrane proteins (10 μg); lane 2, bovine heart mitochondrial proteins (30 μg); lane 3, 6HDNO (5 μg); the upper band in the lane marks the position of the fluorescent protein band shown in (b) (arrowhead). Molecular weight markers are indicated on the left hand site of the panel. (b) Fluorescent protein bands revealed on the gel shown in (a) by a UV-transilluminator following incubation in 10% acetic acid. Lanes same as in (a). (c) Rat liver inner mitochondrial membrane proteins (lanes 1 and 2) and bovine heart mitochondrial proteins (lane 3) were separated by SDS-PAGE, blotted to nitrocellulose membrane and individual lanes of the Western blot were developed with: lane 1, rabbit α-6HDNO antiserum; lane 2, myocarditis serum; lane 3, myocarditis serum; lane 4, the fluorescent protein band shown in (b), lane 1, was excised, eluted from the gel, applied to the same gel used to separate the mitochondrial proteins and the corresponding lane of the Western blot was developed with myocarditis serum; lane 5, Western blot of inner mitochondrial membrane proteins reacted with human control serum; lanes 6, 7, and 8, 9 present Western blots performed with rat liver inner mitochondrial membrane proteins, developed either with rabbit α-6HDNO antiserum before (lane 6) and after preincubation with 100 μm FAD for 1 h at room temperature (lane 7), or with myocarditis serum before (lane 8) and after preincubation with FAD (lane 9). Sera without added FAD were incubated for the same time with FAD at room temperature for 1h.
Separation of bovine mitochondrial antigens by two-dimensional PAGE
Next we tested whether the SucDH-Fp subunit can be identified on 2D gels by its flavin fluorescence. When the proteins of bovine heart mitochondria were separated by two-dimensional PAGE and the gels inspected for the presence of flavin fluorescence, no fluorescent mitochondrial protein spot could be detected. The proteins separated by two-dimensional PAGE were blotted onto PVDF membrane and the Western blot reacted with serum of the myocarditis patient. The serum reacted consistently with the same protein spots on the Western blots. Control serum from a person without heart disease gave no immunoreaction (not shown). One representative example of a Western blot developed with the serum of the myocarditis patient is shown in (Fig. 2). Several antibody–antigen reactions became visible. The corresponding protein spots were excised from the Coomassie blue stained two-dimensional gels and analysed by MALDI-TOF. The SucDH-Fp was not revealed as one of the protein antigens reacting with antibodies of the myocarditis serum. Instead, the MALDI-TOF spectra of the tryptic peptides of the immunoreactive protein spots in the size range of 66 kDa and 45 kDa (Fig. 2, spots 7 and 6), as well as several protein spots in the size range of 36 kDa (Fig. 2, spots 1, 2, 3), could all be identified as carboxy-terminal fragments covering approximately amino acid 1600 to the carboxy-terminal end of myosin heavy chain β. Apparently myosin heavy chain β fragments remained attached to the bovine heart mitochondria during their preparation. Two immunoreactive protein spots (Fig. 2, spots 4 and 5) could not be identified by MALDI-TOF and were analysed further by NanoESI-MSMS. Four sequence tags of spot 4, and three sequence tags of spot 5, identified the peptides with the amino acid sequences, written in the amino acid one-letter code, EANLAASFGK, IDVSIEAASGGK, VCHAHPTLSEAFR, IPNIYAIGDVVAGPMLAHK and EANLAASFGK, IDVSIEAASGGK, VCHAHPTLSEAFR, respectively. They all could be derived from the amino acid sequence of the human dihydrolipoamide dehydrogenase (Swissprot: P09622).
Fig. 2.
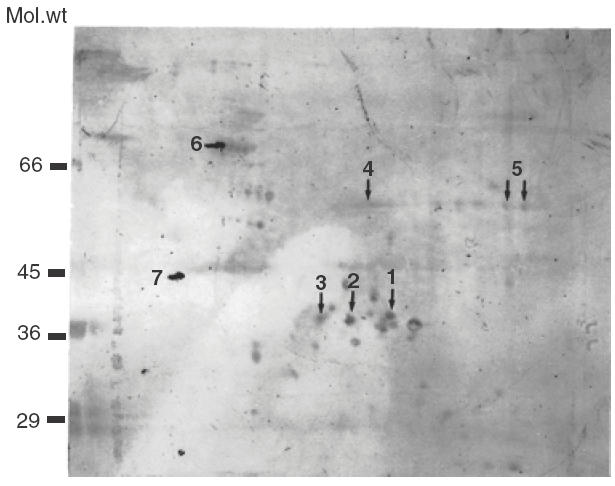
Two-dimensional Western blots; 500 μg bovine heart mitochondrial proteins were subjected to two-dimensional PAGE, blotted onto PVDF membrane and the Western blots developed with mycarditis serum at a dilution of 1:500. The protein spots corresponding to the marked spots on the Western blot were excised from the Coomassie blue stained two-dimensional gel and subjected to analysis by MALDI-TOF and NanoESI Mass spectrometry. Proteins identified were as follows: spots 1, 2, 3, 6 and 7, fragments of myosin heavy chain β; spots 4 and 5, isoforms of dihydrolipoamide dehydrogenase.
Separation of the SucDH complex by BN-PAGE
The respiratory chain complexes may be separated from solubilized inner mitochondrial membranes into individual protein complexes by BN-PAGE ]14[. Complex II migrates as an approximately 130 kDa protein complex. The protein complexes of the inner mitochondrial membrane separated by BN-PAGE (Fig. 3, lane 1) were blotted onto PVDF membrane and the Western blots were developed with mouse monoclonal antibody against the SucDH-Fp, to verify the position of the complex, and with myocarditis serum. Rabbit α-6HDNO antiserum was taken as positive control for αFP antibody containing serum. (Figure 3) shows that both control sera, the monoclonal anti-SucDH-Fp and the αFP antibody containing rabbit serum gave an immunoreaction with the same region of the gel (lanes 2 and 3). The myocarditis serum also reacted with the antigens separated in this part of the gel but in addition, as expected, recognized additional antigens present in the protein complexes of the inner mitochondrial membrane and separated by BN-PAGE (Fig. 3, lane 4). The serum of a control person did not react with the Western blot (Fig. 3, lane 5).
Fig. 3.
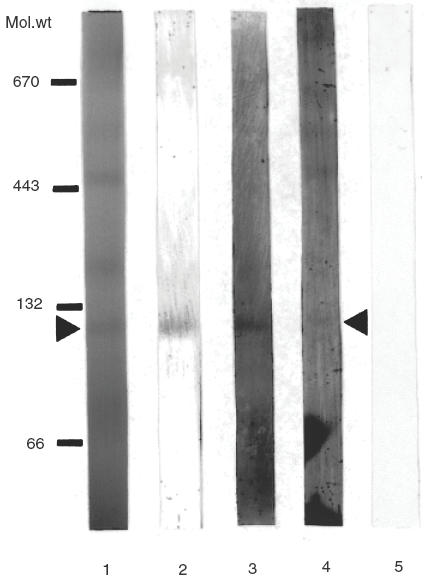
Separation of inner mitochondrial membrane protein complexes by BN-PAGE followed by Western blotting. Rat liver inner mitochondrial membrane protein complexes were solubilized with digitonin and separated on a 6% to 16·5% gradient polyacrylamide gel (lane 1). Following electrophoresis, the gel was blotted to PVDF membrane, and individual lanes were cut and developed with mouse monoclonal antibody against SucDH-Fp (lane 2), with rabbit α-6HDNO antiserum (lane 3), with myocardatis serum (lane 4) and with human control serum (lane 5). Position of molecular weight markers are indicated on the left hand side of the figure (132/66 kDa, bovine serum albumin dimer and monomer, respectively; 443 kDa, horse spleen apoferritin; 670 kDa bovine thyroglobulin).
Western blot of purified E. coli SucDH developed with myocarditis and IDCM sera
The failure to reveal SucDH-Fp as antigen on 2D Western blots of bovine mitochondria and the presence in the mitochondrial preparation of immunoreacting fragments of myosin heavy chain in the size range of 66 kDa raized the possibility that the myocarditis serum and the polyclonal rabbit 6-HDNO antiserum may, in fact, react on the one-dimensional Western blots presented in Fig. 1 with myosin heavy chain fragments. In this case the immunoreaction at 68 kDa may be falsely attributed to SucDH-Fp. To eliminate this possibility we employed in Western blots purified succinate dehydrogenase isolated from E. coli. The flavoprotein subunit of this protein complex contains covalently bound FAD, as does the mammalian enzyme, but E. coli lacks myosin. The result of the Western blots are presented in Fig. 4. Besides the myocarditis serum analysed thus far, a second myocarditis serum and 3 IDCM sera which had been characterized as anti-M7 before ]11[ were tested. The sera reacted with the same band (Fig. 4c, lanes 1, 2 and 3–5). The human control serum gave no immunoreaction (Fig. 4c, lane 6). This band corresponds to the Fp subunit as revealed by its fluorescence (Fig. 4a) and by its position on the Coomassie stained gel (Fig. 4b).
Fig. 4.
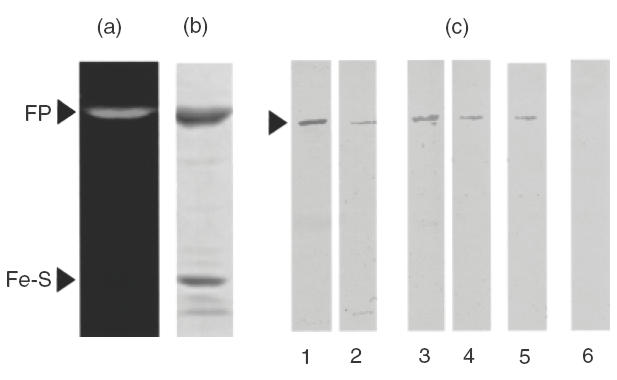
Flavin-fluorescence, Coomassie brillant blue stain and Western blots of purified E. coli SucDH complex; 5 μg of SucDH were separated by SDS-PAGE on 10% polyacrylamide gels and analysed for flavin-fluorescence (a), stained with Coomassie brillant blue (b) or 1 μg blotted onto nylon membranes and the Western blots (c) developed with myocarditis sera (lanes 1 and 2), with IDCM sera (lanes 3–5), or with human control serum (lane 6). Fp, SucDH flavoprotein subunit; Fe-S, SucDH iron-sulphur subunit.
DISCUSSION
Some sera (anti-M7) of patients with myocarditis and IDCM were reported to contain antibodies directed against a mitochondrial inner membrane antigen ]9,10[, and preliminary evidence indicated that this antigen may represent the Fp subunit of the SucDH complex ]11[. The SucDH complex of the inner mitochondrial membrane consists of two integral membrane proteins of 15 kDa and 13·5 kDa and associated with these membrane anchors are a 27-kDa iron-sulphur subunit and the Fp subunit ]19[. The bacterial membrane SucDH complex is structured similarly ]19[. The mature bovine SucDH-Fp subunit has a molecular weight of 68 130 ]20[ and may be identified by its flavin fluorescence ]14[. This work demonstrates that the autoantigen recognized by the αFp antibody described for anti-M7 sera ]11,12[ represents the SucDH-Fp. The failure to resolve the SucDH-Fp as a fluorescent spot by two-dimensional PAGE may be due to an impaired isoelectric focusing of the components of the SucDH complex by the pH gradient of the first dimension. 6HDNO was identified on two-dimensional gels without difficulty by its fluorescence (not shown), indicating that the flavin is not removed from the protein during the isoelectric focusing and electrophoretic run.
Myosin and several components of major mitochondrial complexes involved in energy generation have been involved as autoantigens in myocarditis and IDC ]1,4,6,7[. Indeed, the myocarditis serum contained antibodies directed against myosin heavy chain β. It may be interesting to note that carboxy-terminal fragments of myosin heavy chain were identified in the preparation of bovine heart mitochondria as antigens reacting with antibodies of the myocarditis serum. This finding may reflect the in vivo attachment in cardiomyocytes of the carboxy-terminal part of myosin to mitochondria. In the course of cardiomyocyte destruction due to the inflammatory processes affecting the heart, peptides of myosin and mitochondrial proteins may be released together following phagocytosis of the liberated organelles by antigen presenting cells.
The observation of dihydrolipoamide dehydrogenase of the mitochondrial 2-oxo-acid dehydrogenase complexes as being an antigen to antibodies of patients with IDCM and rheumatic heart diseases ]7,8[ is extended by our findings on two-dimensional immunoblots to myocarditis. Serum antibodies against dihydrolipoamide dehydrogenase have been shown to be present in patients suffering from primary biliary cirrhosis, but can be found in individuals not affected by the disease as well ]21[.
Autoantibodies directed against the SucDH-Fp have been detected recently in sera of patients with ocular myasthenia gravis, general myasthenia gravis and thyroid-associated ophthalmopathy ]22[. Thus SucDH-Fp may represent a common antigen in myocarditis and these diseases. They are characterized by muscle fibre degeneration and αFp antibody may be a general marker of an immune-mediated response directed against muscle antigens. The protein-bound organic FAD moiety with its isoalloxazine ring may represent a potent hapten, leading to a strong immune response.
The inner membrane protein complexes of pyruvate dehydrogenase and the respiratory chain are part of the energy generating machinery of mitochondria. They represent very abundant proteins of cardiomyocytes, in which mitochondria represent a large proportion of the cell mass. They may be released in large amounts upon cell necrosis or induced cell death associated with an inflammatory heart disease and may therefore become preferred antigens eliciting an autoimmune response either by molecular mimicry to epitopes of pathogen antigen or by surpassing the treshold for activation of autoreactive T-cells.
Acknowledgments
This work was funded by a grant from the Deutsche Forschungsgemeinschaft to R. B.
REFERENCES
- 1.Neumann DA, Rose NR, Ansari AA, Herskowitz A. Induction of heart autoantibodies in mice with Coxsackievirus B3-and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994;152:343–50. [PubMed] [Google Scholar]
- 2.Wolff PG, Kühl U, Schultheiss H-P. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am Heart J. 1988;117:1303–9. doi: 10.1016/0002-8703(89)90410-9. [DOI] [PubMed] [Google Scholar]
- 3.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against β-adrenoceptors in human idiopathic dilated cardiomyopathy. Circul Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Ansari AA, Wang YC, Danner DJ, et al. Abnormal expression of histocompatibility and mitochondrial antigens by cardiac tissue from patients with myocarditis and idiopathic dilated cardiomyopathy. Am J Pathol. 1991;139:337–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Schultheiss H-P, Bolte HD. Immunological analysis of auto-antibodies against the adenine nucleotide translocator in idiopathic dilated cardiomyopathy. J Mol Cell Cardiol. 1985;17:603–17. doi: 10.1016/s0022-2828(85)80029-8. [DOI] [PubMed] [Google Scholar]
- 6.Ansari A, Herskowitz A, Danner DJ. Identification of mitochondrial proteins that serve as targets for autoimmunity in human dilated cardiomyopathy. Circulation. 1988;78(Suppl. II):457. [Google Scholar]
- 7.Pohlner K, Portig I, Pankuweit S, Lottspeich F, Maisch B. Identification of mitochondrial antigens recognized by antibodies in sera of patients with idiopathic dilated cardiomyopathy by two-dimensional gel electrophoresis and protein sequencing. Am J Cardiol. 1997;80:1040–5. doi: 10.1016/s0002-9149(97)00600-0. [DOI] [PubMed] [Google Scholar]
- 8.Tontsch D, Pankuweit B, Maisch B. Autoantibodies in the sera of patients with rheumatic heart disease: characterization of myocardial antigens by two-dimensional immunoblotting and N-terminal sequence analysis. Clin Exp Immunol. 2000;121:270–4. doi: 10.1046/j.1365-2249.2000.01283.x. 10.1046/j.1365-2249.2000.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Berg PA. Anti-mitochondrial antibodies (anti-M7) in heart diseases recognize epitopes on bacterial and mammalian sarcosine dehydrogenase. Clin Exp Immunol. 1990;82:289–93. doi: 10.1111/j.1365-2249.1990.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein R, Maisch B, Kochisek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1984;58:283–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Otto A, Stähle I, Klein R, Berg PA, Pankuweit S, Brandsch R. Anti-mitochondrial antibodies in patients with dilated cardiomyopathy (anti-M7) are directed against flavoenzymes with covalently bound FAD. Clin Exp Immunol. 1998;111:541–7. doi: 10.1046/j.1365-2249.1998.00531.x. 10.1046/j.1365-2249.1998.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stähle I, Brizio C, Barile M, Brandsch R. Anti-mitochondrial flavoprotein autoantibodies of patients with myocarditis and dilated cardiomyopathy (anti–M7): interaction with flavin-carrying proteins, effect of vitamin B2 and epitope mapping. Clin Exp Immunol. 1999;115:404–8. doi: 10.1046/j.1365-2249.1999.00832.x. 10.1046/j.1365-2249.1999.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Cold Spring Harbor. NY: Cold Spring Harbor Laboratory Press; 1988. Antibodies. A laboratory manual; pp. 92–135. [Google Scholar]
- 14.Davis KA, Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties and substructure. Biochemistry. 1971;10:2509–16. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- 15.Pallotta ML, Brizio C, Fratianni A, De Virgilio C, Barile M, Pasarella S. Saccharomyces cerevisiae mitochondria can synthesize FMN and FAD from externally added riboflavin and export them into the extramitochondrial phase. FEBS Lett. 1998;428:245–9. doi: 10.1016/s0014-5793(98)00544-4. [DOI] [PubMed] [Google Scholar]
- 16.Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–83. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevchenko A, Wilm M, Vorm O, et al. A strategy for identifying gel-separated proteins in sequence databases by MS alone. Biochem Soc Trans. 1996;24:893–6. doi: 10.1042/bst0240893. [DOI] [PubMed] [Google Scholar]
- 18.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 19.Merli A, Capaldi RA, Ackrell BAC, Kearney EB. Arrangement of complex II (succinate-ubiquinone reductase) in the mitochondrial inner membrane. Am Chem Soc. 1979;18:1393–400. doi: 10.1021/bi00575a001. [DOI] [PubMed] [Google Scholar]
- 20.Birch-Machin MA, Fransworth L, Ackrell BAC, et al. The sequence of the flavoprotein subunit of bovine heart succinate dehydrogenase. J Biol Chem. 1992;267:11553–8. [PubMed] [Google Scholar]
- 21.Maeda T, Loveland BE, Rowley MJ, Mackay IR. Autoantibodies against dihydrolipoamide dehydrogenase, the E3 subunit of the 2-oxoacid dehydrogenase complexes: significance for primary biliary cirrhosis. Hepatology. 1991;14:994–9. [PubMed] [Google Scholar]
- 22.Gunji K, Skolnik C, Bednarczuk T, et al. Eye muscle antibodies in patients with ocular myasthenia gravis: possible mechanism for eye muscle inflammation in acetylcholine-receptor antibody-negative patients. Clin Immunol Immunopathol. 1998;87:276–81. doi: 10.1006/clin.1998.4536. [DOI] [PubMed] [Google Scholar]


