Abstract
Specific blockade by antibodies (Abs) utilized in induction therapy may cause activation-induced cell death (AICD) in lymphocytes of transplant recipients, preactivated via CD95 and tumour necrosis factor-α receptor type 1 (TNFR1), and reduce allograft rejection frequency. Amongst 618 heart transplant (HTX) patients receiving antithymocytes globulin (ATG) therapy, 14 recipients with IVUS-verified freedom of transplant vasculopathy were studied. The control group contained 14 patients awaiting transplantation, classified by the New York Hearth Association heart failure as class IV. From 618 HTX patients 89% were free of rejection grade ISHLT ≥2–3 within 3-month post transplantation and 86% after one year. The death inducing receptors (DIR) such as CD95, CD95L and soluble TNFR1 were significantly increased in HTX recipients versus controls, as demonstrated by FACS, immunoblotting or ELISA (P < 0·001). The presence of increased DIR and in vivo apoptosis in HTX recipients, indicated by annexin-V binding, was further confirmed by the presence of high concentration of histones in the sera of patients. ATG, anti-IL-2R and OKT-3 Abs inhibited cell proliferation in a dose-dependent manner. The induction of apoptosis and/or necrosis was demonstrated in cells cultured with these Abs by annexin-V and 7-aminoactinomycin staining, respectively. Our findings demonstrate that T cells from HTX recipients express high level of CD95, CD95L and soluble TNFR1, and undergo apoptosis and AICD. These cells recognizing donor alloantigens may be selectively eliminated in vivo, and should be responsible for the observed immunological unresponsiveness, indicated by low rejection rates in our patient cohort treated by conventional triple therapy.
Keywords: apoptosis, heart transplant, T lymphocytes, soluble TNFR1, histones
INTRODUCTION
The long-term success of cardiac transplantation, currently limited by the high incidence of transplant-related coronary artery disease, appears to be connected with antecedent episodes of allograft rejection [1,2[, and/or ongoing immune response against donor MHC antigens [3]. The probability of developing a high-grade rejection was markedly decreased six months after heart transplantation (HTX), enabling reduction of immunosuppressive therapy over time [4]. This is accompanied by a progressive diminution in the number of T cell clones recognizing immunodominant donor alloantigen determinants and a gradual shift in the T cell repertoire, leading to recognition of multiple new epitopes [5].
Polyclonal antilymphocyte antibody (ATG) has been proven to be potent immunosuppressive reagents used in organ transplantation since the late 1960s [6]. As comparison to ATG, monoclonal antibody (mAb) OKT3 has been extensively used in organ transplantation with limited success [7]. With the clinical application of mAb against the CD25 in HTX, the importance of induction therapy and its potency to reduce acute allograft rejection rates has advocated its imperative use, concomitant to introduction of triple therapy [8].
Specific and unspecific triggering of naive lymphocytes up-regulate the expression of death-inducing receptors (DIR) and primes cells to activation-induced cell death (AICD) [9]. It has been previously reported that antibodies (Abs) specific for MHC class I molecules [10,11], or recognizing CD2 [12], CD30 [13], CD45 [14[ and CTLA-4 [15] may cause apoptosis in activated T cell, whereas anti-MHC class II and class I Abs can also trigger B cell apoptosis [16]. The major pathway of AICD uses the interaction between the CD95 (Fas receptor; Apo-1) and CD95 ligand (CD95L; FasL) [16,17]. This pathway ensures elimination of activated T cells, and has a crucial role in the maintenance of lymphocyte homeostasis [17].
In the present study we investigated whether the tumour necrosis factor-α receptor type 1 (TNFR1, CD200a), CD95 and CD95L are up-regulated in HTX recipients, and if T cells are susceptible to undergo apoptosis. The capacity of mono-and polyclonal antibody (ATG, anti-IL-2R and OKT3), utilized in induction therapy after allograft transplantation, to inhibit CD3-driven T cell proliferation, and their potency to induce apoptosis/necrosis in T cells from HTX recipients was evaluated.
MATERIALS AND METHODS
Patients and treatment
Retrospectively, 618 adult patients hospitalized at General Hospital of Vienna University between June 1984 and January 2000 were evaluated who presented ISHLT grade II/III rejection within one year. Recipients of previous allografts, left ventricular assist device, positive cross match for T lymphocytes or such who had other therapy regimens, other than described below, were excluded. Immunological studies were performed in 28 patients classified by the New York Hearth association (NYHA) as class IV, and as status I by United Network of Organ Sharing; who either received a cadaver heart (n = 14; designated as HTX group), or remained on medical management awaiting cardiac transplantation (n = 14; further referred to as NYHA class IV control) all, in vivo, experiments were performed in patients who were free of transplant-associated vasculopathy as verified by intravascular ultra-sound (IVUS) at regular 12 month angiogram. Moreover, all patients were free of infection for at least one month and had at time of blood draw C-reactive protein levels below 0·5 mg/dl. The study was approved by the local ethical review board and patients had given informed consent.
As standard immunosuppressive therapy patients received ATG (Pasteur Merieux Connaught, Lyon, France), at a dosage of 2 mg/kg body weights per day for the first 7 days after transplantation in a 4-h course. Cyclosporin A (CyA) was initially applied intravenously (iv), then switched to oral medication and progressively adjusted to 200–250 ng/ml. Azathioprine was administered within 24h after transplantation, and after extubation was given at a dosage of 2 mg/kg bodyweight. Steroids were given iv (500 mg) for allograft prevascularization and then 375 mg daily during the first week. Per oral treatment was initiated with 0·15 mg/kg.
Heart catheterization and IVUS
After intracoronary administration of 0·2 mg nitroglycerine, an IVUS catheter was pushed into the coronaries, and catheter pullback was performed manually. HTX recipients were angiographically evaluated after 1, 5 or 10 years on regular basis or when they developed angina pectoris.
Allograft rejection
Rejection of the allograft was assessed by endomyocardial biopsies, and surveillance was performed after 1, 2, 3, 4 and 7 weeks and 3, 6, 12 months after transplantation. Patients with acute allograft rejection episodes, histological defined as an ISHLT grade 2 or higher, were treated for 3 days with 1000 mg prednisolone. If a steroid-resistant rejection was verified 7 days post diagnosis, OKT-3 mAb (5 mg/day; Coulter, Fullerton, CA, USA) was administered.
Cell immunophenotype and quantification of apoptotic T cells by flow cytometry
Flurochrome-labelled anti-CD4, anti-CD8 and anti-CD95 (PharMingen, San Diego, CA, USA) mAb were used in two-or three-colour immunofluorescence analysis [18].
Peripheral blood mononuclear cells (PBMC) isolated by Ficoll gradient centrifugation were triple stained with mAb directed against annexin-V, 7-aminoactinomycin D (7-AAD), and/or anti-CD4 and CD8 mAb as described previously [18,19]. The samples were analysed with a FACScan 500 flow cytometer (Becton Dickinson, San Jose, CA, USA).
Detection CD95L by Western blot analysis
A total of 5 × 106 PBMC were sonicated in PBS containing protease inhibitor mixture and centrifuged for 30 min at 15 000 × g at 4°C. Protein concentration was measured using Bio-Rad protein assay. To each lane of tris-glycine gels, 7μg of cytosolic extracts were added, gels were then transferred to nitrocellulose membranes and immunoblotting performed using mouse antihuman CD95L mAb (PharMingen). Goat antirabbit IgG labelled with horseradish peroxidase was employed to indicate sites of primary Abs binding. Densitometry was performed using ImageQuant, Molecular Dynamics (Foster City, CA, USA).
Quantification of soluble TNFR1 (sTNFR1) and histones by ELISA
Serum levels of sTNFR1 were measured by ELISA [18]. Soluble histones were quantified by a commercial ELISA using mAb directed against DNA-associated fragments (H1, H2A, H2B, H3, and H4). This allows the specific determination of the cytoplasm-derived mono-and oligonucleosomes in the serum samples. An ELISA plate reader adjusted to an OD of 405 nm measured histones.
Inhibition of cell proliferation by ATG
PBMC (105) were added to 96-well flat-bottomed plates in 200μl complete culture medium. Cells were stimulated with anti-CD3 mAb (10μg/ml) for 48h at 37°C and coculture with various concentrations of IgG, anti-IL-2R (Daclizumab; a humanized Ab against IL-2Rα, purchased from Hoffman-LaRoche, Basel, Switzerland), OKT-3 or ATG. After 48h of culture cells were pulsed for 16h with 3H thymidine (3·7 × 104 Bq/well). Cells were harvested and 3H thymidine uptake was measured in a liquid scintillation counter.
AICD in PBMC from healthy donors and transplant recipients
PBMC (5 × 106) isolated from healthy donors were added to 12-well flat-bottomed plates in 1 ml medium and incubated for 18h with anti-CD3 mAb (10 μg/cc). After discarding supernatant and one wash, PBMC were resuspended in medium supplemented with anti-IL-2R, OKT3 and ATG, and control IgG Abs (50 μg/cc). Cells were cultured for additional 12 h, and triple stained with anti-CD4 mAb, annexin-V and 7-AAD.
In further experiments, a total number of 5 × 106 PBMC, isolated from HTX recipients, were cultured for 24h with anti-CD3 mAb, and with Ab to ATG, OKT3, IL-2R or IgG isotype control Ab (50 μg/cc). Cells were stained with mAb to CD4, annexin-V and 7-AAD and analysed by flow cytometry [19].
RESULTS
Immunosuppressive therapy
The influence of standard immunosuppressive therapy (ATG, CyA, azathioprine and steroids) on the incidence of rejection episodes was investigated retrospectively in a cohort of 618 HTX patients. The calculated freedom of rejection ISHLT ≥2–3 was 89% at 3 months and 86% one year after transplantation (Fig. 1).
Fig. 1.
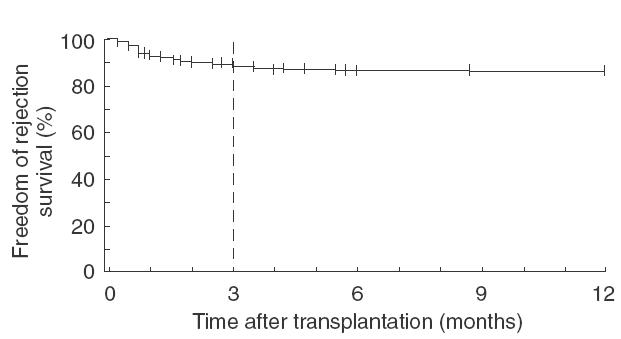
The influence of standard triple immunosuppressive therapy (ATG, CyA and steroids) on the incidence of allograft rejection episodes. In a single centre retrospective cohort study of 618 HTX patients the episodes of rejection are indicated by Kaplan-Meier analysis. The calculated freedom of rejection ISHLT ≥2–3 was 89% at 3 months and 86% one year after transplantation.
CD95 and CD95L expression
As shown in Table 1 the percentage of CD4 and CD8 T cells expressing CD95 molecules was significantly higher in HTX recipients as compared to NYHA class IV controls (P < 0·001).
Table 1.
Differences between PBMC isolated from HTX recipients and NYHA class IV heart failure controls
| n | Control | HTX | |
|---|---|---|---|
| CD95+/CD4+ (%) | (n = 6) | 30·6 ± 6·1 | 64·8 ± 7·4* |
| CD95+/CD8+ (%) | (n = 6) | 28·4 ± 6·3 | 83·6 ± 6·5* |
| Annexin V+/CD4+ (%) | (n = 6) | 6·7 ± 1·2 | 26·6 ± 7·3* |
| Annexin V+/CD8+ (%) | (n = 6) | 5·3 ± 1·3 | 40·3 ± 9·2* |
| Histone (OD405 nm) | (n = 14) | 0·22 ± 0·1 | 0·56 ± 0·1* |
| sTNFR1 (ng/ml) | (n = 14) | 1·1 ± 0·4 | 2·3 ± 0·3* |
PBMC were stained with anti-CD4, anti-CD8 and than with anti-CD95 mAb, and/or with annexin-V and analysed by FACS. Serum level of histones and of sTNFR1was measured by ELISA. The results are expressed as mean ± s.e.m. Statistical significance:
P > 0·001 (calculated by Student’s t-test).
Since CD95L is an apoptosis-inducing member of the TNF family, and production of the CD95L is a calcineurin-dependent process triggered by the TCR/CD3 complex, it was of interest to investigate expression of CD95L in T cells from HTX recipients. Western blotting analysis revealed that T cells from both HTX recipients and NYHA class IV controls have approximately 2–4 fold higher levels of cytoplasmic CD95L, as well as of CD95, in comparison with cells from healthy controls (Fig. 2).
Fig. 2.
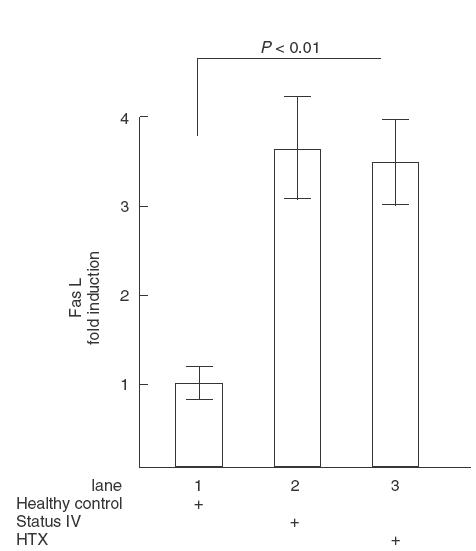
Analysis of CD95 (Fas) and CD95L (FasL) expression by immunobloting. PBMC isolated from healthy donors (lane 1), NYHA class IV heart failure controls (lane 2) and HTX recipients (lane 3) were lysed, blotted on nitrocellulose membrane and stained by with mAb against Fas or FasL as shown in materials and methods. Data of three densitometric measurements are expressed as mean ± s.e.m.
Annexin-V binding and histones expression
T cell triggering via CD95 antigen initiates AICD, which culminates in translocation of phosphatidylserine from the inner leaflet of the plasma membrane to its external leaflet. As can be seen from Table 1, the expression of phosphatidylserine, as defined by annexin-V binding, was significantly increased in both CD4+ and CD8+ T cells from HTX recipients compared with NIHA class IV controls (P < 0·001).
To further confirm these data, circulating histones in the sera of HTX recipients and heart failure controls were measured by ELISA, using mAbs reacting with specific histones. Sera from HTX recipients contained significantly higher levels of histone fragments (P < 0·001) compared to those from NYHA class IV controls (Table 1).
Soluble TNFR1
Activation of lymphocytes and induction of apoptotic pathways is initiated by TNF binding to TNFR, which may be followed by cell activation and TNFR1 shedding. To investigate whether the increased susceptibility of T cells to undergo increased AICD in HTX recipients versus NYHA class IV controls we measured sTNFR1 serum level. The mean serum concentrations of sTNFR1 were significantly higher in HTX patients (P < 0·001) than in NYHA class IV controls (Table 1).
AICD in T lymphocytes of HTX recipients
Since preactivated T cells that express CD95 are susceptible to AICD after stimulation by specific signals via the TCR/CD3 complex, which are then amplified by costimulatory molecules, we investigated whether the observed defects might be related to AICD. A typical example of the flow cytometry profile is shown in (Fig. 3). After 24h cell culture with IgG isotype control, 33·6% of CD3+ T cells from a HTX recipient expressed phosphatidylserine, as indicated by annexin-V binding. Only 3·97% of these cells were 7-AAD+. Stimulation of T lymphocytes with anti-CD3 mAb increased the proportion of 7-AAD+ cells to 8·7%, representing a 119% AICD augmentation. By contrast, there was no difference in the proportion of annexin-V binding cells from NYHA class IV control that underwent cell death after culture with IgG or anti-CD3mAb (1·97 versus 2·13%).
Fig. 3.
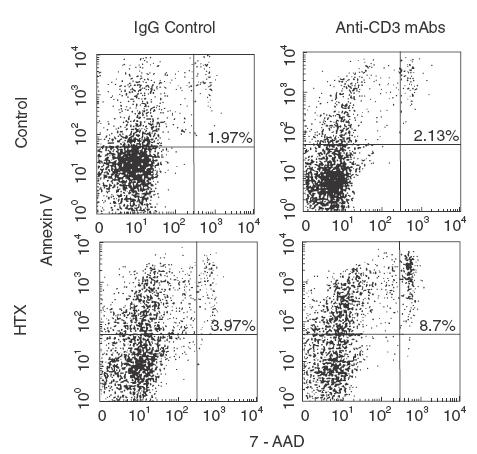
Anti-CD3 antibodies-driven activation-induced CD4+ T cell death. PBMC isolated isolated from HTX recipients and NYHA class IV heart failure controls were cultured for 18h in the presence of IgG or anti-CD3 mAb. Thereafter, cells were harvested, washed and stained with anti-CD4 mAb (PE conjugated) and annexin-V (FITC-labelled) and/or 7-AAD. FACS analysis show the results from one representative experiments. The percentage of CD4+ T cells that bind annexin-V and 7-AAD is indicated in the upper right quadrant.
Inhibition of T cell proliferation by ATG, OKT3 and anti-IL-2R Ab
The capacity of Abs utilized in induction therapy trials to inhibit proliferation of resting T lymphocytes isolated from healthy donors, and costimulated in vitro by anti-CD3 mAbs, was investigated in a conventional blastogenesis assay. A dose-dependent inhibitory capacity, relative to control IgG, was induced by ATG and IL-2R Ab, and partially by OKT3 (Fig. 4).
Fig. 4.
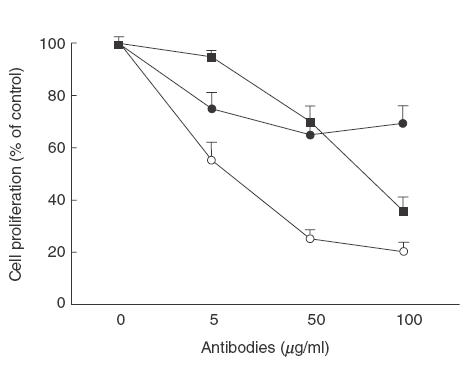
Inhibition of anti-CD3 antibodies-induced T cell proliferation. PBMC isolated from apparently healthy donors (n = 4) were stimulated with anti-CD3 mAb (10μg/ml) for 48 h at 37°C and cocultured with the indicated concentrations of (○) anti-IL-2Rα (Daclizumab; humanized anti-IL-2Rα Ab), (•) OKT-3, (■) ATG or IgG control Abs. After this culture treatment cells were pulsed for 16 h with 3H thymidine (3·7 × 104Bq/well). Cells were harvested and 3H tymidine uptake was measured in a liquid scintillation counter. The proliferative response relative to IgG control (100%) is presented as mean ± s.e.m.
Ab-driven AICD in T cells from healthy controls
Since Abs are able to inhibit T cell proliferation we next investigated whether this phenomena might be related to apoptosis and/or necrosis. The results depicted in (Fig. 5) show a significant increase of annexin-V binding to CD4+ T cells cultured in the presence of ATG, as compared to IgG, OKT3 or IL-2R (P < 0·05). In addition, ATG had the greatest potency to induce AICD in CD4+ T cells, relative to OKT3, anti-IL-2R and IgG control Ab, as indicated by 7-AAD staining (P < 0·001).
Fig. 5.
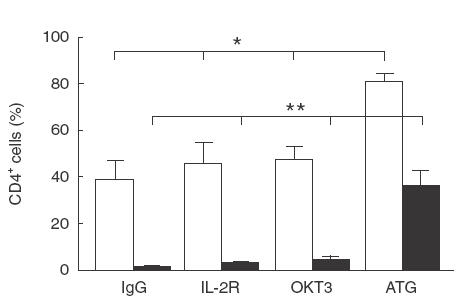
Antibodies-driven AICD in CD4+ T cells from healthy volunteers. PBMC isolated from apparently healthy donors (n = 4) were cultured for 24h with ATG, OKT-3, anti-IL-2Rα or IgG control Abs, and stained with mAb directed to CD4, annexin-V (□) or 7-AAD (■). The results are expressed as mean ± s.e.m. Statistical significance: *P < 0·05; **P < 0·001.
AICD in HTX recipients
In further experiments we compared the sensitivity of T cells from HTX recipients to undergo AICD. The results from FACS analysis, show that anti-IL-2R Ab, anti-OKT3 mAb, and ATG induced a significant fold increase in proportion of CD3+ stained by 7-AAD, relative to the level of IgG control Ab (Fig. 6). Furthermore, ATG was the most potent AICD inducer as compared to OKT3 or anti-IL-2R Ab (P < 0·05).
Fig. 6.
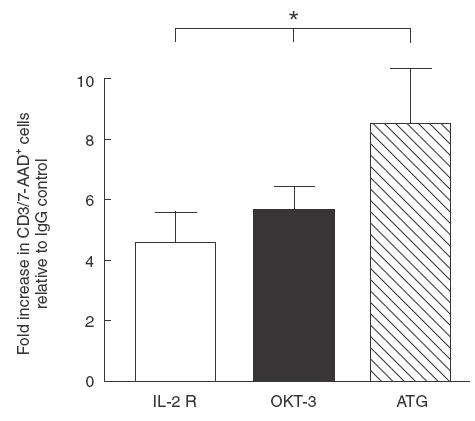
Antibodies-driven AICD in CD3+ T cells from HTX recipients. PBMC isolated from HTX recipients (n = 4) were cultured for 24h with ATG, OKT-3, anti-IL-2Rα or IgG control, and stained with anti-CD3 mAb and 7-AAD. The results are expressed as mean ± s.e.m. Statistical significance: *P < 0·05.
DISCUSSION
The allograft transplantation is associated with increased pronicity of autologous T cells to undergo AICD in vitro mediated by aberrant CD95/TNFR1 activation in vivo. Moreover, mono-and polyclonal Ab utilized as induction therapy, have the ability to activate resting T cells and to induce apoptosis. A major common feature of ATG treatment is the reduction in the number of peripheral blood lymphocytes, which usually persists throughout the administration period, and slowly reverses thereafter. Our results show that ATG has the highest ability to induce apoptosis and AICD in vitro, in comparison to OKT3 or anti-IL-2R Ab, an effect that may partially explain its immunosuppressive activity. Since the probability of developing a high grade rejection is increased during the first six month following transplantation, then progressively diminishes over time, our results suggest that primary clonal deletion after allograft implantation of alloreactive T cells through TNF-, CD95-, CD95L-mediated AICD may be an important mechanism by which a HTX recipient develops progressive tolerance to alloantigens. Concomitant utilization of induction therapy might enforce this process of immunoadaption. ATGs are well tolerated, and therefore offer a good strategy for induction therapy in allotranspantation, despite the occurrence of anti-ATG Ab response and rapid clearance of circulating ATG [20]. IL-2 is an important growth and survival factor, trigger proliferation of activated CD4–, CD8–, CD4+8+, CD4+, CD8+, and γδ subsets of T cells [21]. Furthermore, IL-2 facilitates the induction of cytolytic effector T cells, and induces the proliferation and Ab production by stimulated B cells. In order to exert its biological functions, IL-2 must interact with specific high-affinity membrane receptors [22]. Binding of IL-2 to IL-2Rα (CD25, Tac) trigger intermolecular transphosphorylation and activation of the receptor-associated protein tyrosine kinases, Janus kinase (Jak) 1, which binds to IL-2Rβ (CD122), and Jak3, which binds to IL-2Rγc (CD132) [23,24]. The IL-2Rα is expressed on activated T cells, which are involved in allograft rejection and autoimmune disorders, but not on resting T cells [25]. In our experiments the humanized anti-IL-2Rα Ab Daclizumab inhibited CD3-driven T cell proliferation in a dose-dependent manner and triggered AICD.
In HTX recipients approximately one third of T cells were apoptotic, as indicated by annexin-V binding. It is well known that apoptosis is associated with enhanced DNA accessibility to endonuclease and granzymes-driven fragmentation, that cleaves core histones into small fragments [26]. The high concentration of soluble histones measured in the sera of HTX patients is further indicative of apoptosis. Our results suggest that activated T cells undergo suicide and that CD95-mediated AICD, one of the main mechanisms in maintaining homeostasis, is a hallmark of allograft transplantation. This may be a pathway by which the immune system depletes alloreactive T cell clones in HTX recipients, and develops progressive tolerance to alloantigens, contributing to graft adoption.
This report demonstrates that the autologous immune system is preactivated with increased expression of professional DIR, members of the TNF receptor superfamily. Amongst these structures, the CD95 and TNFRs are well characterized [27]. CD95L and anti-CD95 Ab bind to extracellular part of CD95, and cross-link and oligomerize the CD95 receptor. The cytoplasmic portion of CD95 contain so-called death domain that upon trimerization and conformational changes allow binding of other signalling molecules, and form the death-inducing signalling complex (DISC) [27]. Bcl-2 family of proteins, containing pro-apoptotic members like Bax, Bak and Bad, and antiapoptotic members like Bcl-2 and Bcl-HL, tightly regulates the death machinery [28,29]. Since IL-2 inhibits transcription and expression of cellular FLIP/FLICE-inhibitory protein [30] and enhances transcription of CD95L [30], the IL-2 producing Th1 cells are selectively susceptible to AICD following CD95 engagement. The Th2 subset of CD4+ cells, appear to be resistant to this special type of apoptosis [28], which in transplant recipients may be caused by up-regulation of protective molecules such as Bcl-XL and Bag-1 [31].
TNF, like other cytokines, exerts its biological function by binding to specific, high affinity cell receptors. Two types of TNF receptors, type I (TNFR1; CD120a, TNFR-p55;) and type 2 (TNFR2; CD120b, TNFR-p75) have been identified and characterized [32,33]. Cleaved fragments of both TNFR1 and TNFR2 have been detected in the urine and serum of patients with a variety of disease [34]. sTNFR are considered as parameters of cell-mediated activation, and their measurement may have diagnostic value for monitoring post-transplant allograft and predicting immunological complications such as rejection or infection causing postoperative morbidity. In our study, particularly intriguing was the high level of sTNFR1 in the sera of HTX recipients. This indicates augmented TNF-(gene transcription and cytokine production).
Our results suggest that the allograft implantation may force the immune system to induce a persistent long lasting T cell priming with consecutive state of susceptibility towards AICD. They point to an additional mechanism by which the autologous immune competent cells are aberrantly activated by alloantigens, and demonstrate various efficacies to induce apoptosis and AICD in allotransplant-activated T lymphocytes.
Acknowledgments
Dr HJ Ankersmit designed and coordinated the work and received the young investigator award from the American Society of Transplantation 2001. Dr B Moser performed the majority of the lab work. This work was supported by grant Nr. 8290 from Austrian National Bank.
REFERENCES
- 1.Constanzo-Nordin MR. Cardiac allograft vasculopathy. relationship with acute cellular rejection and histocompatability. J Heart Lung Transplant. 1992;11:S90–103. [PubMed] [Google Scholar]
- 2.Itescu S, Tung TC, Burke EM, et al. An immunological algorithm to predict risk of high-grade rejection in cardiac transplant recipients. Lancet. 1998;352:263–70. doi: 10.1016/S0140-6736(98)09475-6. [DOI] [PubMed] [Google Scholar]
- 3.Suciu-Foca N, Reed E, Marboe C, et al. The role of anti-HLA antibodies in heart transplantation. Transplantation. 1991;51:716–24. doi: 10.1097/00007890-199103000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Rizeq MN, Masek MA, Billingham ME. Acute rejection. significance of elapsed time after transplantation. J Heart Lung Transplant. 1994;13:862–8. [PubMed] [Google Scholar]
- 5.Ciubotariu R, Liu Z, Colovai AI, et al. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosimi AB. Antilymphocyte globulins and monoclonal antibodies. In: Morris PJ, editor. Kidney Transplantation: Principle and Practice. Philadelphia: WB Saunders; 1988. p. 343. [Google Scholar]
- 7.Debure A, Chkoff N, Chatenoud L, et al. One-month prophylactic use of OKT3 in cadaver kidney transplant recipients. Transplantation. 1988;45:546–53. doi: 10.1097/00007890-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Beniaminovitz A, Itescu S, Lietz K, et al. Prevention of rejection in cardiac transplantation by blockade of the interleukin-2 receptor with a monoclonal antibody. N Eng J Med. 2000;342:613–9. doi: 10.1056/NEJM200003023420902. [DOI] [PubMed] [Google Scholar]
- 9.Genestier L, Fournel S, Flacher M, et al. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–8. [PubMed] [Google Scholar]
- 10.Woodle ES, Smith DM, Bluestone JA, et al. Anti-human class I MHC antibodies induce apoptosis by a pathway that is distinct from the Fas antigen-mediated pathway. J Immunol. 1997;158:2156–64. [PubMed] [Google Scholar]
- 11.Skov S, Bregenholt S, Claesson MH. MHC class I ligation of human T cells activates the ZAP-70 and p56lck tyrosine kinases, leads to an alternative phenotype of the TCR/CD3 ξ-chain, and induces apoptosis. J Immunol. 1997;158:3189–96. [PubMed] [Google Scholar]
- 12.Mollereau B, Deckert M, Deas O, et al. CD2-induced apoptosis in activated human peripheral T cells: a Fas-independent pathway that requires early protein tyrosine phosphorilation. J Immunol. 1996;156:3184–90. [PubMed] [Google Scholar]
- 13.Lee SY, Park CG, Choi Y. T cell receptor-dependent cell death of T cell hybridomas mediated by the CD30 cytoplasmic domain in association with tumor necrosis factor receptor-associated factors. J Exp Med. 1996;183:669–74. doi: 10.1084/jem.183.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klaus SJ, Sidorento SP, Clark EA. CD45 ligation induces programmed cell death in T and B lymphocytes. J Immunol. 1996;156:2743–53. [PubMed] [Google Scholar]
- 15.Gribben JG, Freeman GJ, Bousiotis VA, et al. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc Acad Natl Sci USA. 1995;92:811–5. doi: 10.1073/pnas.92.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truman JP, Choqueux C, Tschopp J, et al. HLA class II-mediated death is induced via Fas/Fas ligand interactions in human splenic B lymphocytes. Blood. 1997;89:1996–2007. [PubMed] [Google Scholar]
- 17.Lenardo M, Chan KM, Hornung F, et al. Mature T lymphocytes apoptosis. immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Imunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 18.Ankersmit HJ, Deicher R, Moser B, et al. Impaired T-cell function, increased soluble death-inducing receptors, and activation-induced T-cell death in patients undergoing haemodialysis. Clin Exp Immunol. 2001;125:142–8. doi: 10.1046/j.1365-2249.2001.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ankersmit HJ, Tugulea S, Spanier T, et al. Activation-induced cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:350–5. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 20.Preville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–8. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–10. [PubMed] [Google Scholar]
- 22.Theze J, Alzari PM, Bertoglio J. Interleukin 2 and its receptors: recent advances and new immunological functions. Immunol Today. 1996;17:481–6. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]
- 23.Russell SM, Johnston JA, Noguchi M, et al. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–5. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 24.Behbold F, Erwin-Cohen RA, Wang ME, et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J Immunol. 2001;166:3724–32. doi: 10.4049/jimmunol.166.6.3724. [DOI] [PubMed] [Google Scholar]
- 25.Waldmann TA, O’Shea J. The use of antibodies against the IL-2 receptor in transplantation. Curr Opin Immunol. 1998;10:507–12. doi: 10.1016/s0952-7915(98)80215-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Pasternack MS, Beresford PJ, et al. Induction of rapid histone degradation by the cytotoxic T lymphocyte protease granzyme A. J Biol Chem. 2001;276:3683–90. doi: 10.1074/jbc.M005390200. [DOI] [PubMed] [Google Scholar]
- 27.Krammer PH. CD95 (APO-1/Fas)-mediated apoptosis. live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 28.Ledru E, Lecoeur H, Garcia S, et al. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets. correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol. 1998;160:3194–206. [PubMed] [Google Scholar]
- 29.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–6. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 30.Refaeli Y, Van Parijs L, London CA, et al. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–23. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 31.Ke B, Ritter T, Kato H, et al. Regulatory cells potentiate the efficacy of IL-4 gene transfer by up-regulating Th2-dependent expression of protective molecules in the infectious tolerance pathway in transplant recipients. J Immunol. 2000;164:5739–45. doi: 10.4049/jimmunol.164.11.5739. [DOI] [PubMed] [Google Scholar]
- 32.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 33.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 34.Tracey KJ, Cerami A. Tumor necrosis factor. A Pleiotropic Cytokine Therapeutic Target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]


