Abstract
T helper cell type 1 (Th1) response to gluten has been implicated in the pathogenesis of coeliac disease (CD). To characterize immunological activation and mild inflammations leading to overt CD in potential coeliac patients, jejunal biopsies were obtained from family members of patients with CD or dermatitis herpetiformis (DH). Nine family members and one latent CD, eight CD patients and eight normal controls furnished jejunal biopsy specimens. Immunohistochemical staining of sections for interleukin-1α (IL-1α), IL-2, IL-4, interferon-γ (IFN-γ), tumour necrosis factor α (TNF-α), CD3, γδ-T cell receptor (γδ-TCR), and αβ-TCR was carried out with monoclonal antibodies. Further, expression of IL-4 and IFN-γ messenger RNA was detected by radioactive in situhybridization in these same samples. In lamina propria, CD patients and potential CD patients had higher densities of IL-2 (P = 0·028, P = 0·043), IL-4 (P = 0·021, P = 0·034) and IFN-γ positive cells (P = 0·000, P = 0·009) than did controls. Moreover, CD patients showed a higher density of TNF-α positive cells (P = 0·012, P = 0·001) than the other two groups, and expression of IFN-γ mRNA (P = 0·035) was higher in them than in the other two study groups. Additionally, higher densities of TNF-α and IFN-γ positive cells occurred in potential CD patients with high γδ-TCR+ intraepithelial lymphocytes (IELs). Our findings support the hypothesis that lamina propria T cells and macrophages, through their secretion of cytokines, play a central role in the pathogenesis of coeliac disease. The inflammatory cytokines found in potential CD specimens strongly suggest that these inflammatory markers can be identified long before visible villous changes have occurred.
Keywords: cytokines, immunohistochemistry, mRNA in situ hybridization, potential coeliac disease, T cell subsets
INTRODUCTION
Coeliac disease (CD) is characterized by a gluten-triggered small-bowel mucosal lesion in genetically susceptible individuals; biopsy specimens show villous atrophy with crypt hyperplasia and lymphocytic infiltration [1–3]. Current concepts of the pathogenesis include the activation of lamina propria T-cells and production of antibodies with autoimmune characteristics [3–8]. These autoantibodies predict the development of CD in patients with normal jejunal mucosa [9,10]. In a proportion of individuals with the genetic trait for CD and a similar autoimmune type of reaction, the disease manifests as dermatitis herpetiformis (DH), a chronic blistering skin disease with pathognomic IgA deposits in the dermis [11]. The small-intestinal findings in DH are identical to those found in CD, though usually milder [12,13].
Latent coeliac disease may be seen in patients with DH and normal jejunal structure, as well as in family members of patients with either CD or DH; symptoms are mild or absent [14]. It has also been shown that individuals with normal jejunal architecture, while on a gluten-containing diet, may have latent CD, with overt CD developing at a later date [10,15–17]. As the strict criteria for latent CD are met only rarely, the expression ‘potential CD’ was proposed by Ferguson [14] to describe those for whom the diagnosis of latent or low-grade CD should be considered. Despite normal villous architecture, those with potential CD show typical inflammatory changes in the epithelium: increased density of CD3-positive cells and αβ- and γδ-T cell receptor (TCR)-bearing intraepithelial lymphocytes (IELs) [18–20], and enhanced HLA-DR and -DP expression in epithelial crypts [21,22].
T helper cell type 1 (Th1) cytokines have been implicated in the immunopathogenesis of CD, in particular interferon-γ (IFN-γ) [23–25]. Additionally, macrophage-derived cytokines such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-8, as well as the Th2 associated cytokines IL-4 and IL-5 have been reported in CD [26–28]. Thus, it is conceivable that the mucosa of genetically susceptible individuals for gluten enteropathy may show increased expression of L-2, IFN-γ and TNF-α. However, to our knowledge, no previous work has characterized their cytokine profiles, neither those of family members of patients with CD or DH nor for cases with potential disease.
In this study, we investigated by immunohistochemistry the protein expression of IL-1α, IL-2, IL-4, IFN-γ and TNF-α in jejunal biopsies from 10 potential CD patients. Further, in the same samples we analysed the mRNA expression of IL-4 and IFN-γ by radioactive in situ hybridization. As markers of immune activation we also investigated the lymphocytes, measuring their activation as well as the expression of class II antigens on the epithelium. The cytokine profile evaluated for potential CD patients was compared with the profiles of active CD patients and controls.
MATERIALS AND METHODS
Patients and samples
Small-intestinal biopsy specimens were obtained from seven paediatric patients (mean age 8·8 years, range 2·5–17·3) and one adult (age 31 years) with untreated coeliac disease. Partial or subtotal villous atrophy with crypt hyperplasia was defined as CD (villous crypt ratio <2). At the time of the biopsy, none of the patients was on a gluten-free diet. From one CD patient (Table 1, patient 25), two biopsies were available. In the first one, villous structure was normal but with a high IEL count; this specimen was included in the potential CD study group (Table 1, patient 11). The second biopsy specimen, 11 months later, showed partial villous atrophy. In addition, five specimens from paediatric family members of CD patients (mean age 5·8, range 3·7–8·9) and four specimens from adult family members of DH patients (mean age 55, range 38–75), all with normal jejunal villous structure, were included in the potential CD study group. We selected the specimens for the potential CD study group on the basis of increased density of CD3+ and γδ-TCR+ IELs, but we also included cases in which densities were normal. Normal values were set according to an earlier study [29].
Table 1.
Clinical findings of the patients
| Circulating antibodies | ||||||
|---|---|---|---|---|---|---|
| Patient number | Study group | Sex | Age (years) | IgA-EMA1 | IgA-tTGA2 | Gliad-IgA/IgG3 |
| Healthy controls | ||||||
| 1 | M | 2.6 | NT | NT | <5/50 | |
| 2 | All | F | 4.4 | Neg | Neg | 21/63 |
| 3 | M | 5.0 | Neg | Neg | <5/<5 | |
| 4 | normal | M | 5.7 | NT | NT | <5/>20 |
| 5 | F | 8.2 | NT | NT | <5/36 | |
| 6 | controls | M | 10.1 | Neg | NT | 12/>40 |
| 7 | F | 12.7 | Neg | Neg | <5/72 | |
| 8 | F | 13.7 | NT | NT | 0/>40 | |
| Potential CD patients | ||||||
| 9 | Sister to patient 21 | F | 8.9 | 400 | 14% | 5/5 |
| 10 | Patient’s mother has CD | F | 3.7 | 3200 | 11% | 3/34 |
| 11 | Latent CD, same as 25 | M | 14.5 | 1600 | 161% | 40/24 |
| 12 | Brother to patient 21 | M | 7.1 | Neg | Neg | 12/74 |
| 13 | Patient’s mother has CD | M | 4.0 | Neg | NT | <10/<10 |
| 14 | Patient’s mother has CD | F | 5.1 | 1600 | Neg | 1/10 |
| 15 | Sibling to a DH patient | F | 38 | Neg | NT | <5/<5 |
| 16 | Sibling to a DH patient | M | 53 | Neg | NT | 6/6 |
| 17 | Sibling to a DH patient | F | 54 | Neg | NT | 7/7 |
| 18 | Sibling to a DH patient | F | 75 | Neg | NT | 6/1 |
| CD patients | ||||||
| 19 | CD, sibling to a DH patient | F | 31 | >5 | NT | <5/<5 |
| 20 | CD | F | 2.5 | 200 | 57% | 200/<5 |
| 21 | CD | F | 4.2 | 1600 | 643% | 80/37 |
| 22 | CD | F | 5.7 | 1600 | 393% | 80/>40 |
| 23 | CD | F | 6.6 | 1600 | 71% | 12/6 |
| 24 | CD | M | 10.2 | 50 | Neg | 9/24 |
| 25 | CD, same patient as 11 | M | 15.4 | 800 | 69% | 16/16 |
| 26 | CD | M | 17.3 | 200 | 10% | 9/9 |
Jejunal biopsies on eight control paediatric patients (mean age 7·8 years, range 2·6–13·7) were performed because of growth retardation, gastrointestinal symptoms, positive antigliadin antibodies or any combination of these. The morphology of the jejunum was normal in each case. Clinical data are summarized in Table 1.
A Watson biopsy device was used to take a specimen from the proximal jejunum or a gastroscope from the distal duodenum. The specimens were divided for routine histology and immunohistochemical studies. The portion for immunohistochemistry was embedded in optimal cutting temperature (OCT) compound (Miles Laboratories, Elkhart, IN, USA), snap-frozen in liquid nitrogen, and stored at – 70°C until used. Serial sections, each 8μm, were cut and processed for either immunohistochemistry or in situ hybridization. The sections were coded and evaluated without knowledge of the specimen.
Immunohistochemistry
The avidin–biotin immunoperoxidase system, as described previously [18], was used on native cryostat sections to stain mononuclear cells singly for CD3, γδ- and αβ-TCR; mucosa singly for HLA class II (HLA-DR, HLA-DP) antigens and intercellular adhesion molecule 1 (ICAM-1); and proliferative epithelial cells with Ki-67 [32]. For immunostaining of cytokines prior to incubation with monoclonal antibodies (MoAbs), permeabilization was performed by incubation of the slides in 0·01% PBS-Tween20 for 10 min in RT on a shaking platform before the slides were incubated with the MoAbs, diluted in 1% NS in 0·01% PBS-Tween20 for 1h at + 37°C.
Monoclonal antibodies
The monoclonal primary antibodies (MoAbs) are listed in Table 2. As controls for specific staining, one additional MoAb for IL-4 (clone 82·2, Mabtech) and IFN-γ (clone7-B6-1, Mabtech) were used, and similar staining patterns obtained. Non-immune mouse IgG1 was used as the negative primary antibody control (DAKO) and incubation with 1% normal horse sera on additional sections as the negative control for the reagents in the avidin–biotin immunoperoxidase system.
Table 2.
Monoclonal mouse anti-human antibodies (MoAbs) used in this study
| MoAb | Specific for | Dilution | Company; clone |
|---|---|---|---|
| CD3 | CD3-T cell receptor | 1:200 | Becton-Dickinson, San Jose, CA, USA |
| TCR γδ | γδ-T cell receptor | 1:200 | Endogen, Woburn, MA, USA |
| TCRbF1 | αβ-T cell receptor | 1:50 | Endogen; clone 8A3 |
| ICAM-1 | Intercellular adhesion molecule 1 | 1:1500 | Endogen; clone VF27 |
| HLA-DR | MHC class II | 1:2000 | DAKO; clone CR3/43 |
| HLA-DP | MHC class II | 1:40 | Becton-Dickinson |
| Ki-67 | Nuclear antigens in proliferating cells | 1:100 | DAKO, Glostrup, Denmark |
| IL-1α | Interleukin-1α | 1:100 | Biosource International; clone 20B8, |
| Camarillo, CA, USA | |||
| IL-2 | Interleukin-2 | 1:50 | Biosource International; clone 7A3 |
| IL-4 | Interleukin-4 | 1:50 | Mabtech, IL-4II; clone 12.1 |
| IFN-γ | Interferon-γ | 1:50 | Mabtech; clone 1-D1K, Nacka, Sweden |
| TNF-α | Tumour necrosis factor α | 1:50 | Biosource International; clone 68B6A3 |
Microscopic evaluation
The numbers of CD3, γδ- or αβ-TCR positively stained cells were counted under a light microscope through a calibrated graticule at × 1000 magnification, as described previously [29,33]. In the same specimen, the positive cells in both the epithelium and the lamina propria, comprising at least 30 fields, were counted and expressed as the mean number of positive staining cells/mm of epithelium and as cells/mm2 in the lamina propria, respectively. Positively staining cells for the different cytokines were counted in the lamina propria between the epithelium and the muscularis mucosae and expressed as cells/mm2, as described above.
MoAbs to the constant fragments of HLA-DR and -DP chains were used to stain epithelial cells for HLA class II expression. Epithelial staining with these antibodies was graded from 2 to 6 according to their intensity and cellular distribution (1–3) and area of staining (1–3) [34]. The proportion of the lamina propria which stained positively for ICAM-1 was estimated and graded from 1 to 3 according to intensity of staining, 1 representing faint and 3 strong staining. Proliferating cells were stained with Ki-67, and positive cells were calculated as percentages of the crypt cells, with at least 200 crypt cells counted in each specimen.
Radioactive RNA in situ hybridization
The samples were the same as used for immunohistochemistry, and serial sections from the biopsies were processed for in situ hybridization. Because of the small size of the specimens, we lacked adequate material from patient numbers 1, 12 and 23 for IL-4 and from patient numbers 1, 12, 19, and 23 for IFN-γin situ hybridization.
Frozen tissue samples were cut into 8-μm sections and fixed in 4% paraformaldehyde in PBS. The sections were subjected to in situ hybridization for human IL-4 and IFN-γ riboprobes obtained from the cDNAs described below. Tissue sections were incubated with 1·2 × 106 cpm of [33P]-labelled (1000– 3000 Ci/mmol; Amersham, Life Technologies, Arlington Heights, IL, USA) antisense or sense riboprobe in a total volume of 80 μl following the in situ protocol described in detail previously [35,36].
Human IL-4 and IFN-γ cDNAs kindly provided by Ilkka Julkunen, MD, PhD, were used for preparing the riboprobes. Briefly, a 462-bp human IL-4 cDNA and a 501-bp human IFN-γ cDNA were synthesized by PCR, enclosing the whole coding region for both cytokines, and additionally the signal peptide for IFN-γ[37]. The PCR products were cloned into pGEM®-3ZF vectors (Promega, Madison, WI, USA) and the sequences of the cloned PCR products were verified with an ABI PRISM 377 DNA sequencer (Perkin-Elmer, Applied Biosystems, Foster City, CA, USA). The lengths of the [33P]-labelled riboprobes, both antisense and sense, were checked by SDS PAGE analysis.
Microscopic evaluation of RNA in situ hybridization
Positive mRNA expression for IL-4 and IFN-γ was analysed with a photomicroscope equipped with a camera and a colour monitor. In each case, positive mRNA expression was counted in two different representative areas in the lamina propria, as well as in the background staining adjacent to the tissue over high-power fields (magnification × 400). The relative intensity of positive mRNA signal was determined by division of the positivity in the tissue by the positivity of the background. The lamina propria was assessed from the area immediately below the surface epithelium, excluding the epithelia and areas with lymphatic aggregates. For the specific probes, a minimum of two sections were prepared for each patient investigated.
Statistical analysis
Correlations between the cell densities and the percentages of positive stainings in the three study groups were revealed with one-way analysis of variance, using a non-parametric procedure, the Kruskal–Wallis one-way analysis by ranks. In addition, the Mann–Whitney U-test was used to compare positive cells within the three subject groups; a P-value of <0·05 was considered significant. Calculation was performed on a PC using SPSS software. Cell densities for each group are expressed as median values, with the interquartile range given in brackets. Because of the small size of the study groups, values outside the 25–75%iles were considered abnormal for the group.
Ethical considerations
Specimens from paediatric patients were taken for diagnostic purposes. The St Mary’s Hospital ethics committee approved biopsies taken from the adult siblings of patients with DH. Use of the biopsy specimens in this study was approved by the ethics committee of the Hospital for Children and Adolescents, Helsinki University Central Hospital. Additionally, all the subjects studied gave their informed consent.
RESULTS
Immunohistochemistry
Lymphocytes in epithelium and lamina propria. Densities of CD3, γδ-TCR, and αβ-TCR positive cells were significantly higher in the epithelium of coeliac patients than in potential CD patients (P < 0·05, P < 0·05 and P < 0·01, respectively) and normal controls (P < 0·001 in all cases). As the family members were selected on the basis of increased density of CD3+ and γδ-TCR+ IELs, it was not surprising that the density of CD3+ IELs in potential CD patients was significantly higher than in normal controls (P < 0·01). However, differences between the T-cell receptors were not significant between the two groups. The density of γδ-TCR+ cells in the lamina propria was significantly higher in CD patients than in normal controls (P < 0·05). Eight potential CD patients showed higher CD3+ densities in the epithelium, of which six also showed a high number of γδ-TCR+ cells in the epithelium (Table 3).
Table 3.
Immunohistochemical staining of lymphocytes in the surface epithelium and lamina propria
| Density of positive cells in the surface epithelium (cells/mm) | Density of positive cells in the lamina propria (cells/mm2) | ||||||
|---|---|---|---|---|---|---|---|
| CD3 | TCR γ/δ | TCR α/β | CD3 | TCR γ/δ | TCR α/β | Percentage of dividing crypt cells Ki-67 (%) | |
| Coeliac patients | 63 (31–84) | 35 (15–48) | 47 (33–68) | 713 (608–863) | 191 (148–391) | 951 (582–1152) | 29 (26–32) |
| Normal controls | 9 (5–10) | 1.6 (1.2–3.9) | 8 (5–11) | 596 (466–720) | 61 (51–123) | 625 (573–711) | 11 (6–16) |
| Potential CD group | 21 (12–34) | 7.0 (1.7–13) | 14 (10–30) | 808 (668–864) | 145 (101–248) | 718 (566–757) | 9 (7–18) |
| Potential CD patients | |||||||
| 9 | 75 | 54 | 32 | 1375 | 603 | 710 | 2 |
| 10 | 53 | 14 | 34 | 1289 | 428 | 737 | 9 |
| 11 | 20 | 29 | 40 | 560 | 264 | 764 | 34 |
| 12 | 9 | 1 | 9 | 503 | 200 | 625 | 19 |
| 13 | 8 | 3 | 2 | 788 | 66 | 458 | NT |
| 14 | 21 | 7 | 11 | 655 | 99 | 540 | 7 |
| 15 | 35 | 1 | 24 | 827 | 109 | 949 | 1 |
| 16 | 14 | 10 | 16 | 870 | 181 | 1107 | 15 |
| 17 | 30 | 7 | 11 | 846 | 109 | 547 | 8 |
| 18 | 12 | 0 | 7 | 707 | 60 | 725 | 18 |
Values for study groups given as medians (interquartile ranges). Individual results for potential coeliac patients given after patient’s number. Bold for values outside interquartile range of normal control group. NT, not tested.
Staining with HLA class II antibodies. Strong positive staining of epithelial cells with HLA-DR and HLA-DP antibodies was present, respectively, in two and in three of the potential CD cases (Table 1, patients 9–11), whereas the expression of HLA-DR and HLA-DP was significantly higher in the epithelium of patients with CD (data not shown).
Staining with anti-ICAM-1 (CD54). In potential CD patients and in controls, the staining with anti-ICAM-1 showed faint to moderate positivity, the majority of the positive staining being located on cells in the lamina propria, but some also seen extracellularly. Three specimens from potential CD patients showed strong positive staining. These patients were the same as those showing strong positivity for staining of HLA-DR and HLA-DP. All the coeliac specimens stained strongly with ICAM-1, a difference significant when compared with specimens of potential CD patients and normal controls (P < 0·05 and P < 0·001, data not shown).
Staining with Ki-67. The percentage of proliferative cells in the crypts was significant higher in CD specimens than in those from potential CD patients and controls (P < 0·05 and P < 0·001). The percentages of positive cells in normal controls and in potential CD patients showed no difference, indicating that the turnover rate of epithelial cells in potential CD patients is normal (Table 3).
Cytokines in the lamina propria. The density of IL-1 cells in the lamina propria was similar among CD patients, potential CD patients, and controls; the densities of IL-2 and IL-4 were significantly greater in CD patients and in potential CD patients than in normal controls (P < 0·05 in all cases). The density of IFN-γ cells was significantly higher in CD patients than in potential CD patients (P < 0·05) and controls; their density was also significantly greater in potential CD patients than in controls (P < 0·01). TNF-α positive cells were found in higher density in CD patients than in potential CD patients (P < 0·05) and normal controls (Figs 1 and 2a).
Fig. 1.
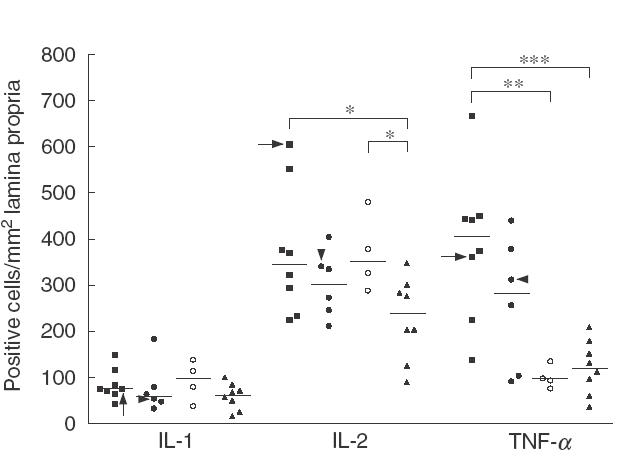
Immunohistochemical staining for interleukin-1 (IL-1), IL-2 and tumour necrosis factor-α (TNF-α) in jejunal biopsies of coeliac patients (■), potential coeliac patients (•○), and healthy controls (▴). Patients in the potential coeliac group divided according to increased (•) or normal (○) density of intraepithelial γδ-T cell receptor positive cells. Individual results shown as positively stained cells per square millimetre of lamina propria. Horizontal lines show median values of each group. Arrows indicate the adult coeliac patient and arrowheads the latent coeliac patient in the potential group. *P < 0·05, **P < 0·01, ***P < 0·001.
Fig. 2.
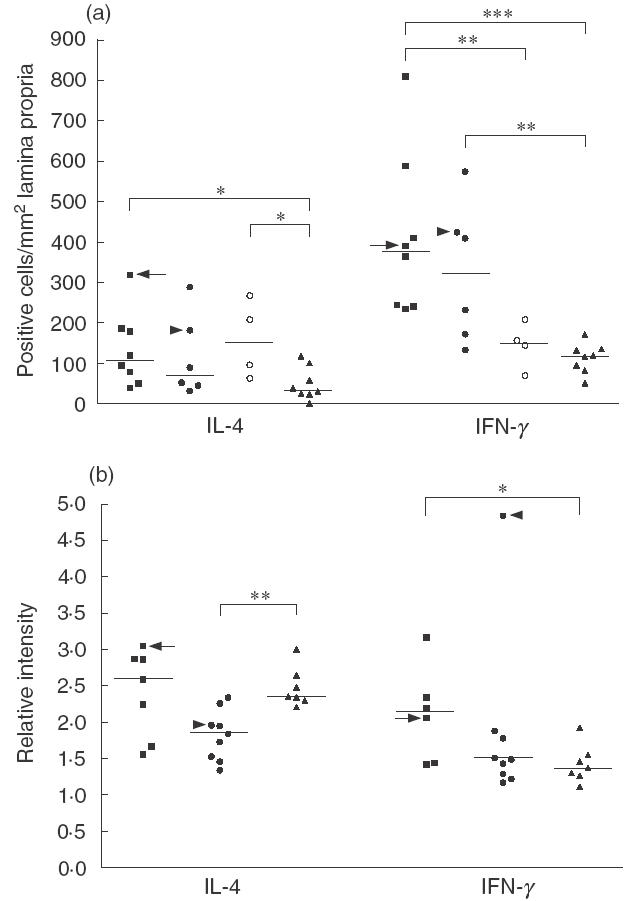
(a) Immunohistochemical staining for interleukin-4 (IL-4) and interferon-γ (IFN-γ) in jejunal biopsies of coeliac patients (■), potential coeliac patients (•○) and healthy controls (▴). Patients in the potential group divided according to increased (•) or normal (○) density of intraepithelial γδ-T cell receptor positive cells. Individual results are shown as positively stained cells per square millimetre of lamina propria. Median values shown by horizontal lines. Arrows indicate the adult coeliac patient and arrowheads the latent coeliac patient. *P < 0·05, **P < 0·01, ***P < 0·001. (b) Expressions of IL-4 and IFN-γ mRNA in coeliac patients (■), potential coeliac patients (•), and healthy controls (▴) detected by radioactive in situ hybridization. Values given as relative intensity compared to background positivity. Horizontal lines show median values of each group. Arrows indicate the adult coeliac patient and arrowheads the latent coeliac patient in the potential group. *P < 0·05, **P < 0·01.
When the patients in the potential CD group were divided according to higher density of intraepithelial γδ-TCR+ cells, high number of γδ-TCR+ IELs seemed to correlate with higher density of IFN-γ and TNF-α positive cells, although these differences were not significant between the subgroups (P < 0·09 for both). However, the density of IFN-γ positive cells was significantly greater in potential CD patients with high γδ-TCR+ IELs than in controls, whereas IFN-γ and TNF-α cells were found in higher densities in CD patients than in potential CD patients with normal IELs. In contrast, the densities of IL-2 and IL-4 positive cells were significantly higher in potential CD patients with normal IELs than in normal controls. No such association was found for IL-1 (Figs 1 and 2a).
Radioactive RNA in situ hybridization
IL-4 mRNA expression. The jejunal mucosa of all the patients contained cells expressing mRNA for IL-4. This positive hybridization signal was located mainly in the lamina propria, with little or no signal in the surface epithelium. In all specimens, intestinal mononuclear cells scattered in the lamina propria expressed IL-4 mRNA, some positive signal also appearing extracellularly in a diffuse fashion. Additionally, in most specimens strongly positive cells resembling macrophages appeared in the lamina propria (arrow in Fig. 3g–h). The expression of IL-4 mRNA was significantly higher in normal controls than in potential coeliac patients, whereas no difference existed between controls and CD patients (Figs 2b and 3d–e, 3g–h, 3j–k). All sections hybridized with the IL-4 sense probe showed only background signals (Fig. 3a–b).
Fig. 3.
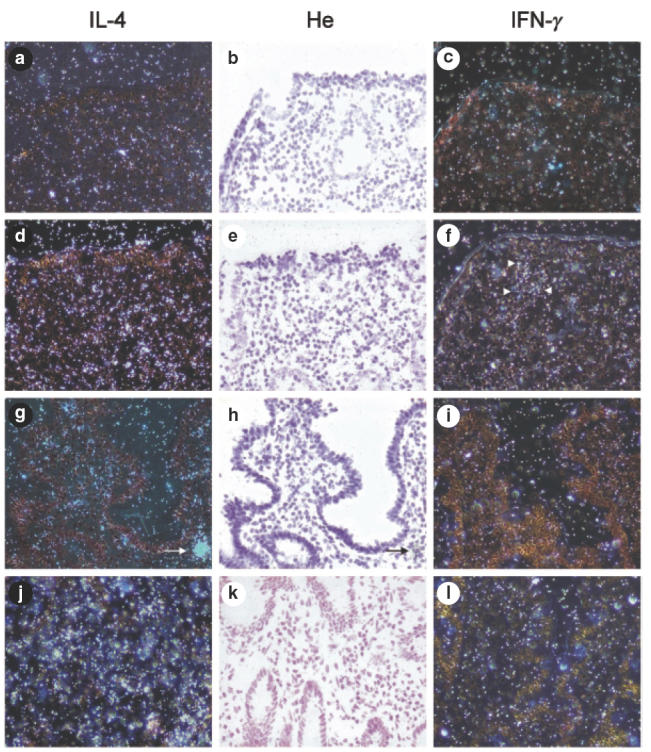
Radioactive in situ hybridization of interleukin-4 (IL-4) and interferon-γ (IFN-γ) mRNA in duodenal biopsies; bright (b, e, h, k) and dark field views (a, c, d, f, g, I, j, l). Hybridization with sense probes in a coeliac specimen (a–c), and with antisense probes in a coeliac specimen (d–f), in a paediatric family member (g–i) and in a healthy control (j–l). IL-4 positivity is seen in lamina propria of coeliac and control specimens, with less positivity in the potential coeliac specimen. High expression of IFN-γ mRNA is scattered in the upper lamina propria, mainly in the coeliac specimen, indicated by arrowheads in (f). Only background signal is seen with sense probe (c). Arrows indicate a strongly IL-4 positive cell, morphologically resembling macrophages (g–h). He = haematoxylin. Original magnification (×200).
IFN-γmRNA expression. In situ hybridization with the antisense probe for IFN-γ showed that positively expressed cells were found mainly in the superficial lamina propria, and occasionally in the deeper parts of the lamina propria. The surface epithelium and IELs showed no or very little expression of IFN-γ. In the CD specimens, expression of IFN-γ mRNA was significantly higher than in normal controls (Figs 2b and 3e–f compared to 3h–i and 3k–l). As for IL-4, the IFN-γ sense probe expressed only background signals, and this expression was identical in all the specimens (Fig. 3b–c).
DISCUSSION
To our knowledge, this study describes for the first time cytokine profiles in the lamina propria of potential coeliac patients. Because we were interested in the development of mild inflammations leading to overt CD, the family members in our study were chosen on the basis of IELs densities, with the majority of cases in the potential CD study group thus expressing high IELs. This does not therefore represent an unselected group of family members of CD or DH patients, among whom the potential form of CD is still fairly uncommon [21].
The higher number of IFN-γ positive cells in the lamina propria of our CD patients than of their family members and of controls is consistent with previous results [23,24,38,39]. T cells in the coeliac mucosa have been for the most part Th1 cells producing IFN-γ[40,41]. In one study, IFN-γ mRNA-producing cells were identified by in situ hybridization in duodenal specimens, indicating that the coeliac lamina propria contains numerous positive cells, with such cells being fewer in controls [23]. However, we have also demonstrated IFN-γ positive cells in potential CD patients as well as in controls. The higher level of IFN-γ mRNA expression and positive cells in our CD patients and in some of the family members suggests their enhanced inflammatory reactions, as shown recently elsewhere [25,42]. TNF-α and IFN-γ have been shown to have cytotoxic effects capable of directly damaging the target tissue, in this case the epithelial cells. It may therefore be postulated that the mucosal changes found in untreated CD patients are caused by the increased local production of IFN-γ alone or in combination with TNF-α. The expression of these cells in the lamina propria of potential coeliac patients with high IELs could suggest an incipient inflammatory process in these specimens that could lead to apparent CD. The presence of very high densities of these cells in our patient who later developed CD supports this hypothesis further.
The higher densities of IL-2 and IL-4 positive cells in the lamina propria of our CD patients and potential CD patients with normal IELs than in our controls, indicate both Th1 and Th2 immune activation in these patient groups; the higher density of these IL-4 positive cells in potential CD patients may reflect the protective effect of this cytokine, down-regulating the inflammatory response. Previous in vitro studies also have suggested the activation of Th0 cells, which produce both IL-2 and IL-4 [25,41]. Maiuri et al.[43] demonstrated, during an in vitro gliadin challenge of cultured intestinal specimens, a rapid release of IL-4 from degranulated mast cells, followed later by production of Th1 type cytokines. These findings emphasize that the expression and secretion of cytokines is not limited to T cells and macrophages; many other cells may be involved within and outside the immune system in the lamina propria. We also observed, in cells morphologically resembling macrophages, strong positivity for IL-4 mRNA.
Similar to our findings, a recent study demonstrated IL-4 mRNA expression in the majority of biopsy samples from paediatric CD patients, as well as in paediatric controls [42]. Additionally, other researchers showed that under physiological conditions a substantial proportion of duodenal lymphocytes secrete IFN-γ and IL-4 spontaneously [44]. Recently, Beckett et al.[26] found IL-4 and IL-10 expressing cells in similar quantities in CD patients and controls, indicating that no primary deficiency in these cytokines existed in the intestine of CD patients. The higher densities of IL-4 positive cells detected by immunohistochemistry in CD patients and potential coeliac patients was inconsistent with the mRNA in situ hybridization. Expression of IL-4 mRNA was higher in controls than in potential coeliac patients with normal IELs, although the difference was not dramatic. This discrepancy is probably caused by the differing techniques used- mRNA levels may be misleading with regard to actual cytokine production and protein expression, as some cytokines are post-transcriptionally regulated [25].
The three potential coeliac patients with the highest densities of γδ-TCR+ IELs (patients 9–11) all showed high densities of IFN-γ and TNF-α positive cells, corresponding to those of CD patients. Furthermore, these three patients also showed positive endomysium (IgA-EMA) and transglutaminase antibodies (IgA-tTGA) and increased expressions of epithelial class II major histocompatibility complex antigens and ICAM-1, as a consequence of immune activation. However, the fourth patient (patient 14) with positive IgA-EMA did not show increased densities of IFN-γ and TNF-α positive cells. Interestingly, this patient was the only one of these four who had no positive IgA-tTGA. We thus infer that positive tTGA antibodies, together with increased density of γδ-TCR+ IELs, agrees fairly well with grade of inflammation in the jejunum. The present concept of the pathogenesis of CD includes creation of new antigenic epitopes between gluten and tTGA that then may initiate a immune response in genetically susceptible people [4,7]. Whether tTGA plays a role in the pathogenesis of CD beyond changing the gluten antigenicity is, as yet, unknown.
In conclusion, we have shown that IL-2, IL-4, IFN-γ and TNF-α are expressed mainly in the lamina propria and are detected in coeliac patients, in potential coeliac patients and in healthy controls, which is in agreement with previous findings. Additionally, a high density of γδ-TCR+ IELs seemed to be associated with more profound inflammatory responses, as indicated by the presence of TNF-α and IFN-γ positive cells in the lamina propria. Our study supports the hypothesis that lamina propria T cells and macrophages, through their secretion of cytokines, play a primary role in the pathogenesis of CD. The inflammatory cytokines found in the latent CD case and in two of the family members strongly suggest that these inflammatory markers can be identified long before visible villous changes occur. Finally, these findings may indicate that an altered immune response towards a Th1 type pathway has commenced.
Acknowledgments
We are indebted to Ilkka Julkunen MD, PhD, National Public Health Institute, Helsinki, for the kind gift of IL-4 and IFN-γ cDNAs and to Prof Markku Heikinheimo, Hospital for Children and Adolescents, Helsinki, for the expertise and help with RNA in situ hybridization. The skilful technical assistance of Mrs Sirkku Kristiansen is acknowledged. This study was financially supported by the Juselius Foundation and the Finnish Medical Association.
REFERENCES
- 1.Trier JS. Celiac sprue. N Engl J Med. 1991;325:1709–19. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- 2.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 3.Mäki M, Collin P. Coeliac disease [see comments] Lancet. 1997;349:1755–9. doi: 10.1016/S0140-6736(96)70237-4. [DOI] [PubMed] [Google Scholar]
- 4.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald TT, Spencer J. The role of activated T cells in transformed intestinal mucosa. Digestion. 1990;46:290–6. doi: 10.1159/000200399. [DOI] [PubMed] [Google Scholar]
- 6.Strober W, Fuss IJ. Gluten-sensitive enteropathy and other immunologically mediated enteropaties. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. 2. San Diego: Academic Press; 1999. pp. 1101–28. [Google Scholar]
- 7.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease [see comments] Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 8.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease [see comments] Gastroenterology. 1998;115:1322–8. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 9.Collin P, Helin H, Mäki M, Hällström O, Karvonen AL. Follow-up of patients positive in reticulin and gliadin antibody tests with normal small-bowel biopsy findings. Scand J Gastroenterol. 1993;28:595–8. doi: 10.3109/00365529309096094. [DOI] [PubMed] [Google Scholar]
- 10.Troncone R. Latent coeliac disease in Italy. The SIGEP Working Group on Latent Coeliac Disease. Italian Society for Paediatric Gastroenterology and Hepatology. Acta Paediatr. 1995;84:1252–7. doi: 10.1111/j.1651-2227.1995.tb13543.x. [DOI] [PubMed] [Google Scholar]
- 11.Fry L, Seah PP, Riches DJ, Hoffbrand AV. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet. 1973;1:288–91. doi: 10.1016/s0140-6736(73)91539-0. [DOI] [PubMed] [Google Scholar]
- 12.Reunala T, Kosnai I, Karpati S, Kuitunen P, Török E, Savilahti E. Dermatitis herpetiformis. jejunal findings and skin response to gluten free diet. Arch Dis Child. 1984;59:517–22. doi: 10.1136/adc.59.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reunala T. Dermatitis herpetiformis. coeliac disease of the skin [editorial] Ann Med. 1998;30:416–8. doi: 10.3109/07853899809002482. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson A, Arranz E, O’Mahony S. Clinical and pathological spectrum of coeliac disease – active, silent, latent, potential [see comments] Gut. 1993;34:150–1. doi: 10.1136/gut.34.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein WM. Latent celiac sprue. Gastroenterology. 1974;66:489–93. [PubMed] [Google Scholar]
- 16.Mäki M, Holm K, Koskimies S, Hällström O, Visakorpi JK. Normal small bowel biopsy followed by coeliac disease. Arch Dis Child. 1990;65:1137–41. doi: 10.1136/adc.65.10.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corazza GR, Andreani ML, Biagi F, Bonvicini F, Bernardi M, Gasbarrini G. Clinical, pathological, and antibody pattern of latent celiac disease: report of three adult cases. Am J Gastroenterol. 1996;91:2203–7. [PubMed] [Google Scholar]
- 18.Savilahti E, Arato A, Verkasalo M. Intestinal gamma/delta receptor-bearing T lymphocytes in celiac disease and inflammatory bowel diseases in children. Constant increase in celiac disease. Pediatr Res. 1990;28:579–81. doi: 10.1203/00006450-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Holm K, Mäki M, Savilahti E, Lipsanen V, Laippala P, Koskimies S. Intraepithelial gamma delta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339:1500–3. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 20.Mäki M, Holm K, Collin P, Savilahti E. Increase in gamma/delta T cell receptor bearing lymphocytes in normal small bowel mucosa in latent coeliac disease. Gut. 1991;32:1412–4. doi: 10.1136/gut.32.11.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holm K, Savilahti E, Koskimies S, Lipsanen V, Mäki M. Immunohistochemical changes in the jejunum in first degree relatives of patients with coeliac disease and the coeliac disease marker DQ genes. HLA class II antigen expression, interleukin-2 receptor positive cells and dividing crypt cells. Gut. 1994;35:55–60. doi: 10.1136/gut.35.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaukinen K, Collin P, Holm K, Karvonen AL, Pikkarainen P, Mäki M. Small-bowel mucosal inflammation in reticulin or gliadin antibody-positive patients without villous atrophy. Scand J Gastroenterol. 1998;33:944–9. doi: 10.1080/003655298750026967. [DOI] [PubMed] [Google Scholar]
- 23.Kontakou M, Sturgess RP, Przemioslo RT, Limb GA, Nelufer JM, Ciclitira PJ. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situhybridisation. Gut. 1994;35:1037–41. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontakou M, Przemioslo RT, Sturgess RP, et al. Cytokine mRNA expression in the mucosa of treated coeliac patients after wheat peptide challenge. Gut. 1995;37:52–7. doi: 10.1136/gut.37.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsen EM, Jahnsen FL, Lundin KE, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 26.Beckett CG, Dell’Olio D, Kontakou M, et al. Analysis of interleukin-4 and interleukin-10 and their association with the lymphocytic infiltrate in the small intestine of patients with coeliac disease. Gut. 1996;39:818–23. doi: 10.1136/gut.39.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Przemioslo RT, Kontakou M, Nobili V, Ciclitira PJ. Raised pro-inflammatory cytokines interleukin 6 and tumour necrosis factor alpha in coeliac disease mucosa detected by immunohistochemistry. Gut. 1994;35:1398–403. doi: 10.1136/gut.35.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kontakou M, Przemioslo RT, Sturgess RP, Limb AG, Ciclitira PJ. Expression of tumour necrosis factor-alpha, interleukin-6, and interleukin-2 mRNA in the jejunum of patients with coeliac disease. Scand J Gastroenterol. 1995;30:456–63. doi: 10.3109/00365529509093307. [DOI] [PubMed] [Google Scholar]
- 29.Savilahti E, Örmälä T, Arato A, et al. Density of gamma/delta+T cells in the jejunal epithelium of patients with coeliac disease and dermatitis herpetiformis is increased with age. Clin Exp Immunol. 1997;109:464–7. doi: 10.1046/j.1365-2249.1997.4811377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern M. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. Working Group on Serologic Screening for Celiac Disease. J Pediatr Gastroenterol Nutr. 2000;31:513–9. doi: 10.1097/00005176-200011000-00012. 10.1097/00005176-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Kolho KL, Savilahti E. IgA endomysium antibodies on human umbilical cord: an excellent diagnostic tool for celiac disease in childhood. J Pediatr Gastroenterol Nutr. 1997;24:563–7. doi: 10.1097/00005176-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 33.Savilahti E, Örmälä T, Saukkonen T, et al. Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) show signs of immune activation. Clin Exp Immunol. 1999;116:70–7. doi: 10.1046/j.1365-2249.1999.00860.x. 10.1046/j.1365-2249.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klemola T, Savilahti E, Arato A, et al. Immunohistochemical findings in jejunal specimens from patients with IgA deficiency. Gut. 1995;37:519–23. doi: 10.1136/gut.37.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson DG. Whole mount in situhybridization of vertebrate embryos. In: Wilkinson DG, editor. In situ hybridization – a practical approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]
- 36.Heikinheimo M, Ermolaeva M, Bielinska M, et al. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–14. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- 37.Sareneva T, Pirhonen J, Cantell K, Kalkkinen N, Julkunen I. Role of N-glycosylation in the synthesis, dimerization and secretion of human interferon-gamma. Biochem J. 1994;303:831–40. doi: 10.1042/bj3030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przemioslo RT, Lundin KE, Sollid LM, Nelufer J, Ciclitira PJ. Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut. 1995;36:874–9. doi: 10.1136/gut.36.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Troncone R, Gianfrani C, Mazzarella G, et al. Majority of gliadin-specific T-cell clones from celiac small intestinal mucosa produce interferon-gamma and interleukin-4. Dig Dis Sci. 1998;43:156–61. doi: 10.1023/a:1018896625699. [DOI] [PubMed] [Google Scholar]
- 40.Strober W, Kelsall B, Fuss I, et al. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–4. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 41.Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995;37:766–76. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahat N, Shapiro S, Karban A, Gerstein R, Kinarty A, Lerner A. Cytokine profile in coeliac disease. Scand J Immunol. 1999;49:441–6. doi: 10.1046/j.1365-3083.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- 43.Maiuri L, Picarelli A, Boirivant M, et al. Definition of the initial immunologic modifications upon in vitro gliadin challenge in the small intestine of celiac patients. Gastroenterology. 1996;110:1368–78. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- 44.Carol M, Lambrechts A, Van Gossum A, Libin M, Goldman M, Mascart-Lemone F. Spontaneous secretion of interferon gamma and interleukin 4 by human intraepithelial and lamina propria gut lymphocytes [see comments] Gut. 1998;42:643–9. doi: 10.1136/gut.42.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]


