Abstract
Dendritic cells (DCs) were derived from human peripheral blood monocytes or cord blood monocytes cultured in the presence of IL-4 and GM-CSF. Adult and cord DCs were observed to have comparable immature phenotypes. However, the increase in surface expression of HLA-DR and CD86 after addition of LPS was significantly attenuated in cord DCs, with CD25 and CD83 expression also markedly reduced. Cord DCs were also unable to produce IL-12p70, failed to down-regulate expression of the chemokine receptor CCR5 and induced lower levels of IFN-γ production from allogeneic naive CD4+ T cells than their adult counterparts. In contrast, the kinetics of the production of TNF-α and IL-10 in response to LPS stimulation was comparable to adult DCs. The reduced ability of cord DCs to attain a fully mature adult phenotype, and to activate naive CD4+ T cells to produce IFN-γ, suggests that they are intrinsically preprogrammed against the generation of Th-1 immune responses.
Keywords: dendritic cell, LPS, maturation, neonatal, Th1/Th2
INTRODUCTION
Infections remain a major cause of morbidity and mortality in early life, due partly to the immaturity of the developing immune system [1]. Quantitative, phenotypic and functional variations have been reported in many compartments of neonatal immunity within the innate and adaptive systems [1]. Immaturity of T cell function may play a part in the susceptibility of neonates to infection and the relatively poor response of neonates and infants to vaccines. T cells from both human cord blood and neonatal mice proliferate poorly in response to antigenic and allogeneic stimulation, and are inefficient producers of cytokines such as IFN-γ and IL-2 [2–4]. They are, however, intrinsically capable of reaching an adult level of function, but only if their apparent increased requirement for co-stimulatory signals are provided [2].
Antigen presentation is central to the induction of many immune responses, yet little is known of the age-related maturation or functional ontogeny of the central antigen presenting cell, the dendritic cell (DC). DCs are professional antigen-presenting cells, which are required for the initiation of an immune response [5]. In their immature form they capture and process antigens, and following activation they display these antigens in the form of MHC-peptide complexes at their surface [6]. Cells with DC morphology and potent antigen-presenting activity can be derived in significant numbers from human monocytes cultured in the presence of IL-4 and GM-CSF [7,8]. Using this methodology, Goriely et al.[9] have described a failure of production of IL-12 by cord blood derived DCs in response to stimulation by LPS, CD40 ligation or polyinosinic–polycytidylic acid, although the kinetics of activation and cytokine production were not tested. These questions are of considerable importance, as the profile of DC activation has been shown to have a profound influence on the outcome of immune responses in terms of Th-1 and Th-2 polarization [10].
In this study we have examined the cytokine production of neonatal DCs stimulated with LPS, and the influence that this has on the activation and polarization of naive CD4+ T cells. We show that neonatal DCs retain the ability to produce IL-10 and TNF-α with similar kinetics to adult cells, and confirm that IL-12 production is almost completely abolished at all time-points. Furthermore, our findings suggest that they are selectively and intrinsically preprogrammed against the generation of Th-1 immune responses
MATERIALS AND METHODS
Samples
Cord blood was venisected from the umbilical cord attached to the placenta, immediately following separation from normal full-term infants after caesarean section at University College Hospital, London. Informed consent was gained from the mothers prior to caesarian section, and approved by the Great Ormond Street Hospital and the Institute of Child Health Research Ethics Committee. Peripheral blood was venisected from healthy adult volunteers. The blood was collected into sterile heparinized tubes, and used within 1h of collection.
Generation of dendritic cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood by centrifugation with Lymphoprep (Nycomed). The PBMCs were removed from the interface, washed twice in RPMI-1640, then resuspended in complete medium comprising of RPMI-1640 with 25 mm HEPES and l-glutamine supplemented with 10% low-endotoxin FCS (Life Technology) and 1% penicillin–streptomycin, and incubated in 6-well plates (Falcon, cat no. 3046) for 2–3 h. The non-adherent cells were gently washed off, pelleted and resuspended in freezing mix comprising of 10% DMSO with 50% FCS in RPMI-1640, and frozen slowly to – 80°C in Nalgene cryoboxes. These non-adherent cells were then used as source of T cells in subsequent experiments. The remaining adherent fraction was cultured in complete medium supplemented with 100 ng/ml rHu GM-CSF (Leucomax, Schering-Plough) and 25 ng/ml rHu IL4 (gift from D. Katz, Immunology Department, University College London).
After 5–6 days’ culture, the immature dendritic cells were gently washed off the wells, layered over lymphoprep and washed twice with RPMI-1640. Purified DCs were gained by immunodepleting the cells with magnetic immunobeads coated with antibodies against CD3 and CD19 (Dynal). To ensure that homogenous immature DCs were gained, LPS contamination was minimized by the use of prepackaged endotoxin-free/low disposable plasticware and baked glassware. The limulus amoebocyte lysate assay (Sigma) was used to detect endotoxin contamination. Immature DCs were then replated at the desired concentration and either activated with 0·5 μg/ml LPS (from Escherichia coli 026:B6, Sigma) for 8 or 24 h, or left unchallenged.
Dendritic cell phenotyping
Immature and LPS activated, cord and adult DCs were phenotyped with directly labelled antibodies against HLA-DR, CD11c, CD25, CD83, CD86 and CCR5 (Becton Dickinson). The DCs were stained with various combinations of these antibodies and incubated for 30 min on ice. The cells were then washed in PBS and resuspended in 1% paraformaldehyde (PFA). Marker expression was analysed on a Beckman Coulter XL flow cytometer with Expo2 software. Relevant isotype negative control antibodies were used (gated arbitrarily as 1% positive) to allow determination of the percentage of positive events. Values are stated as the mean ± 1s.e.m., quoting percentage of positive events or the median fluorescent intensity (MFI), and using the paired t-test for statistical analysis.
Kinetic profile of cytokine production
The immature DCs from both cord and adult were activated with 0·5 μg/ml LPS and replated into six identical wells. The supernatant was removed carefully from one well at each different time point (4 h, 8 h, 12 h, 18 h, 24 h, 48 h), and stored at –80°C. Unactivated DCs were also run in parallel and incubated for 48 h. IL-12p70, IL-10 and TNF-α production was measured in the supernatants by ELISA, using matched paired antibody ELISA kits from eBioscience.
Isolation and DC stimulation of naive CD4+ T cells
Frozen non-adherent PBMCs obtained from DC generation of an allogeneic sample were defrosted quickly and washed in RPMI-1640. Total CD4+ T cells were gained by negative selection from both adult and cord samples, using anti-CD14, anti-CD19 and anti-HLA-DR immunobeads (Dynal). To gain a naive population, the CD4+ T cells were sorted further by negative selection with anti-CD45RO (Pharmingen), using a Beckman Coulter EPICS Altra cell sorting system. Initially the DCs were either activated with 0·5 μg/ml LPS for 8 or 24 h, or left unchallenged. Then allogeneic CD45RO– CD4+ T cells from either adult or cord were added at a ratio of 1DC:10T and cultured for 5 days in complete medium, then analysed by intracellular cytokine staining and ELISpot. For intracellular cytokine staining, after the initial 5 days of culture, the proliferating T cells were expanded further for 3–4 days with IL-2 (Biotest, Germany).
Intracellular IFN-γ staining
The cells were re-stimulated with 25 ng/ml phorbol myristate acid, 2·5 μg/ml ionomycin and 25 μg/ml brefeldin-A (Sigma) for 4 h. The cells were then lysed and fixed with FACS lysis buffer and then permeabilized with FACS permeabilization buffer. The permeabilized cells were then stained with a directly labelled anti-IFN-γ antibodies and incubated for 30 min. The cells were washed again in PBS, then resuspended in 1% paraformaldehyde. All intracellular staining reagents were purchased from Becton Dickinson. Expression was analysed on a Becton Dickinson FACScalibur flow cytometer with CellQuest software, using relevant isotype control antibodies, values quoted as mean ± 1 s.e.m.
IFN-γ ELISpot
The cells were transferred into anti-IFN-γ (clone 1-D1K, Mabtech, Sweden) coated 96-well multi-screen-IP filter plates (Millipore, USA) and incubated overnight. The plates were then visualized using biotinylated-anti-IFN-γ (clone 7-B6-1, Mabtech, Sweden), followed by ExtrAvidin alkaline phosphatase conjugate (Sigma), then developed with alkaline phosphate substrate (Bio-Rad). The number of spots was then counted using a BioSys ELISpot reader, and values were scaled up to represent the number of spots per 100 000 original input T cells.
RESULTS
Cord DCs remained phenotypically immature after LPS activation
The phenotype of adult and cord derived DCs before and after LPS activation are shown in Table 1 and Fig. 1. Immature DCs derived from adult monocytes showed high expression of CD11c and HLA-DR. There was little/no expression of maturation markers CD25 and CD83, with moderate expression of CD86. Non-DC lineage markers including CD3, CD14, CD19, CD20 and CD56 were not expressed. Immature DCs derived from cord blood showed similar marker expression levels, with comparable HLA-DR and CD86 expression.
Table 1.
Surface marker expression in adult and cord dendritic cells (n = 10)
| Adult | Cord | |||
|---|---|---|---|---|
| Immaturea | LPS activatedb | Immaturea | LPS activatedb | |
| CD11c | 95·6%± 2·7 | 99·4%± 0·1 | 93·5%± 4·6 | 94·5%± 3·2 |
| HLA-DR | 89·5%± 3·4 | 96·9%± 1·7 | 86·5%± 8·1 | 75·3%± 6·4 |
| CD25 | 0·7%± 0·2 | 32·3%± 10·9 | 0·5%± 0·2 | 8·3%± 3·6 |
| CD83 | 10·4%± 1·9 | 59·2%± 6·8 | 5·5%± 2·2 | 17·8%± 5·8 |
| CD86 | 77·1%± 5·4 | 98·8%± 0·3 | 90·5%± 3·0 | 93·6%± 1·7 |
| CCR5 | 31·8%± 7·5 | 8·2%± 3·0 | 36·6%± 12·3 | 31·9%± 8·4 |
DCs were re-plated and left unchallenged for 24 h.
DCs were re-plated and activated with 0·5 μg/ml LPS for 24 h.
Fig. 1.
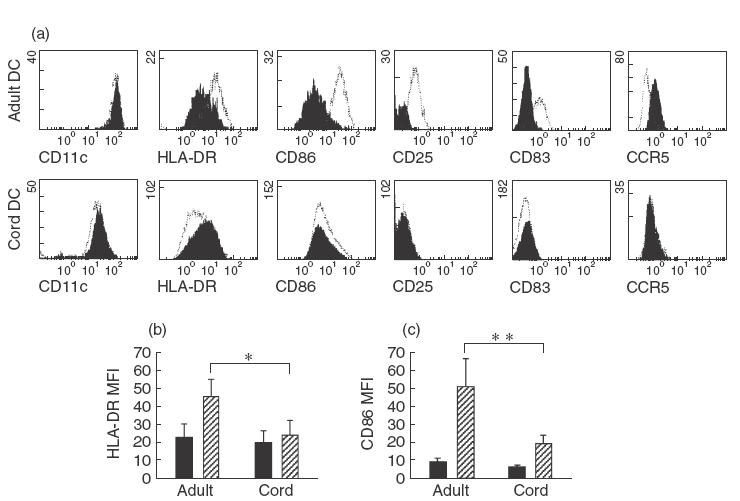
Phenotypic analysis of adult and cord response to LPS. Immature adult and cord DCs showed comparable surface marker expression (a, filled plots). Shifts in marker expression of DCs stimulated with 0·5 μg/ml LPS for 24h were overlayed (a, open plots). Adult DCs dramatically up-regulated HLA-DR (b) and CD86 (c) marker expression MFI; cord DCs, however, had little or no response. The values represent the means of 10 independent experiments, *P =0· 04 and **P =0·007. ■, Immature;  00017;, 24-h LPS.
00017;, 24-h LPS.
Adult DCs were noted to up-regulate HLA-DR and CD86 expression following LPS activation and to adopt a mature phenotype. In contrast cord DCs failed to mature as evidenced by their failure to up-regulate HLA-DR and only minimally increase CD86 expression (Fig. 1a–c). CCR5 expression was down-regulated in adult DCs after LPS stimulation, but remained unchanged in cord DCs (Fig. 1a). Differences were also noted in the expression of CD25 and CD83 with reduced expression in cord compared to adult DCs after LPS stimulation.
With LPS stimulation, cord DCs exhibit similar kinetics of IL-10 and TNFα production to adult DCs
Figure 2 illustrates the kinetics of IL-12p70, IL-10 and TNF-α production. As expected, cord DCs failed to produce IL-12p70, whereas adult DCs demonstrated a characteristic increase in IL-12p70 production after 8–12 h of LPS stimulation. Peak mean IL-12p70 production by adult DCs was 1·58 ng/ml (range 1·28–1·86) and by cord DCs was 0·08 ng/ml (range 0·07–0·11). Interestingly, the kinetics of the production of IL-10 and TNF-α by cord and adult DCs was very similar. IL-10 could be detected after 8 h of LPS stimulation and increased linearly over time, with production sustained up to 48 h. Peak mean IL-10 production by adult DCs was 1·60 ng/ml (range 0·68–2·27), and by cord DCs was 1·41 ng/ml (range 1·06–1·85). TNF-α could be detected after 4 h of LPS stimulation with a peak at 8 h followed by a steady reduction. Peak mean TNF-α production by adult DCs was 12·25 ng/ml (range 6·58–16·06), and by cord DCs was 10·39 ng/ml (range 4·84–14·42).
Fig. 2.
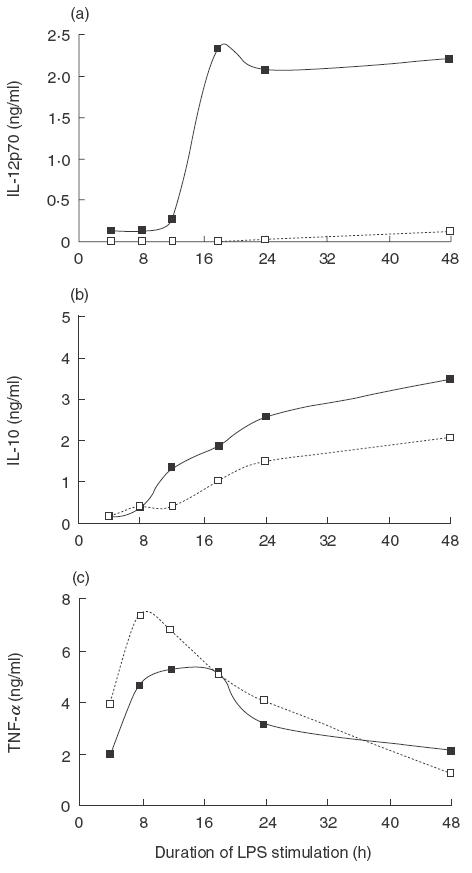
Cytokine profiles of cord and adult DCs stimulated with LPS. The production of cytokines by adult and cord DCs was measured by ELISA, after different durations of LPS stimulation. Representative kinetic profiles from five independent experiments of IL-12p70 (a), IL-10 (b) and TNF-α (c) are shown. □, Cord; ■ adult.
Adult DCs have a greater capacity to stimulate naive allogeneic CD4+ T cells than cord DCs
The ability of adult and cord DCs to stimulate CD4+ T cells to produce IFN-γ is shown in Fig. 3. First, immature adult and cord DCs were used to stimulate allogeneic total CD4+ T cells. Adult DCs were able to stimulate 13·2%± 2·7 of adult CD4+ T cells to produce IFN-γ, while cord DCs had a significantly decreased capacity to stimulate the same T cells, with only 8·1%± 2·4 producing IFN-γ (P = 0·01, Fig. 3a). As expected, fewer cord CD4+ T cells produced IFN-γ than adult T cells when stimulated by adult DCs (4·6%± 1·8). Interestingly, cord DCs were still significantly less efficient in stimulating cord T cells to produce IFN-γ (0·7%± 0·2, P = 0·02, Fig. 3a) than adult DCs.
Fig. 3.
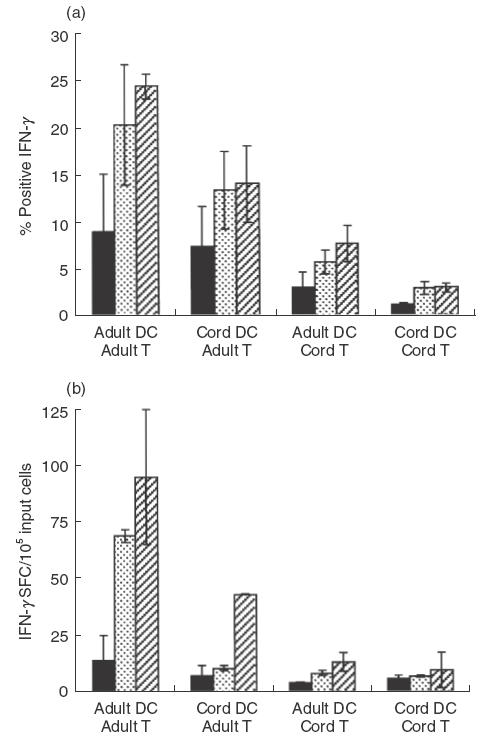
DC stimulation of allogeneic naïve CD4+ T cells to produce IFN-γ. Adult and cord DCs stimulation of naive CD4+CD45RO– cells to produce IFN-γ, using either immature DCs (filled bars) or DCs activated with 0·5 μg/ml LPS for 8h (dotted bars) or 24h (striped bars). IFN-γ production was measured by intracellular cytokine staining (a) and ELISpot (b). For ELISpot, the number of spot-forming cells (SFC) per 1 × 106 cells was determined. The values represent the means of three independent experiments ± 1s.e.m. ■, Immature;  00004;, 8-h LPS;
00004;, 8-h LPS;  00017;, 24-h LPS.
00017;, 24-h LPS.
We next determined whether cord DCs exhibited differences in their ability to activate naïve allogeneic CD4+CD45RO– cells to produce IFN-γ. The stimulatory capacity of both immature, and DCs activated for either 8 or 24h with LPS was examined by intracellular cytokine staining (Fig. 3b) and ELISpot (Fig. 3c). As with total CD4+ T cells, adult immature DCs had a greater capacity than cord DCs to stimulate naive allogeneic adult CD4+CD45RO– cells to produce IFN-γ. After 8h of LPS stimulation, adult DCs stimulated a greater number of naive adult CD4+CD45RO– cells to produce IFN-γ than cord DCs (adult 20·4%± 6·4 and 69·2 spots ± 2·6, cord 13·4%± 4·2 and 10·7 spots ± 1·2). The same pattern was observed with DCs stimulated with LPS for 24 h (adult 24·4%± 1·3 and 95·1 spots ± 29·8, cord 14·1%± 0·2 and 43·1 spots ± 0·1). The same trends were observed for cord DCs when stimulating naive allogeneic cord CD4+CD45RO– cells. Therefore, cord DCs have an intrinsically diminished ability to stimulate allogeneic CD4+ T cells to produce IFN-γ.
DISCUSSION
The neonatal/infant period is marked by an increased susceptibility to infections. Poor responses to certain types of antigen, in particular capsular polysaccharides, creates a window of vulnerability to infections at the time of disappearance of protective maternal antibodies [1]. The increased requirement of neonatal T cells for co-stimulatory signals to reach adult levels of function [2] is reflected in vivo by the requirement of multiple doses of vaccines for infants to achieve antibody responses equivalent to those seen after a single dose in adults [11].
This study demonstrates that cord and adult monocyte-derived DCs behave in distinct ways following stimulation, even though they have comparable expression of surface markers and morphology in their immature state. Adult derived DCs are readily activated by inflammatory stimuli, such as LPS, the major antigenic component of the Gram-negative bacterial cell wall and have been shown previously to mature under such a stimulus [12,13]. LPS-activated adult DCs consequently have a greater capacity to stimulate allogeneic T cells, increasing their cytokine synthesis and proliferation [13]. We have shown that the response of cord DCs to LPS stimulation is consistently abrogated, with little or no change in marker expression characteristic of maturation, and almost complete failure to produce IL-12p70. As a consequence, they exhibit a decreased ability to stimulate naïve allogeneic adult CD4+ T cells to produce IFN-γ, when compared to adult DCs. Cord T cells stimulated in the same system produced lower amounts of IFN-γ compared to their adult counterparts, but cord DCs were again less able to stimulate them than those derived from adult blood. Our findings are therefore consistent with other recent studies, which have shown that cord DCs have decreased functional activity and phenotypic expression [14,15], and are less effective at supporting the proliferation of both adult and cord T cells in response to antigeneic or allogeneic stimulation [14,16,17], but indicate further that they are intrinsically less able to direct Th-1 polarization.
As far as we can determine this failure to mature is not in itself linked to an inability to respond to LPS, as Toll receptor 4, an important receptor for activation of cells by LPS [18], is expressed on cord DCs at equivalent levels (data not shown). As little as 1 ng/ml LPS was sufficient to increase activation markers on adult DCs, with comparable mature phenotypes gained whether 1–1000 ng/ml was used (data not shown), the failure of cord DCs to mature was observed at LPS doses ranging from 10 ng/ml to 1000 ng/ml, highlighting that the abrogated response was not simply due to an increased LPS requirement for stimulation. Furthermore, LPS stimulation of immature cord monocyte derived DCs did result in the production of IL-10 and TNF-α at similar levels and with similar kinetics to adult DCs. However, studies of cultured neonatal monocytes showed decreased TNF-α production in response to LPS stimulation [19], which was not observed with the cord-blood monocyte derived DCs. As has been shown previously, the mechanism for repressed IL-12 production is likely to be due to decreased gene expression of the IL-12p35 subunit [9].
Both Th-1 and Th-2 responses are required for comprehensive immune function. Neonatal mice and humans show a strong bias towards Th-2 polarization [2], which is observed at all phases from the generation of primary effectors to memory responses [20]. However, both mouse and human neonatal T cells retain the ability to mount protective Th-1 responses under specified conditions [21–24]. The ability of neonatal animals to deviate from the Th-1 responses that lead to autoimmunity/rejection and expand Th-2 immune responses may, at least in part, be explained by reduced expression of IL-12 [25]. Furthermore, administration of IL-12 in both mouse and human neonatal systems has the ability to restore/redirect T cell responses towards a Th-1 profile [9,25,26], thereby allowing enhancement of protective efficacy of antiviral vaccination [27], or preventing the establishment of transplantation tolerance [28]. The inability of cord DCs to produce IL-12 may therefore be fundamental to the establishment of aTh-2 bias, perhaps through the action of other dominantly acting Th-2 cytokines such as IL-10.
Autocrine IL-10 can limit the maturation of DCs and their capacity to initiate Th1 responses, DCs treated with neutralizing antibodies to IL-10 produced increased TNF-α and IL-12, and had an increased capacity to activate T cells to a more prominent Th-1 polarization [29]. Addition of IL-10 in conjunction with LPS to adult DCs has also been shown to inhibit the down-regulation of inflammatory chemokine receptors (CCR1, CCR2 and CCR5) by blocking the maturation-induced switch in chemokine receptor expression [30]. Furthermore, these receptors were unable to elicit migration, and may therefore be acting as functional decoys. Unbalanced expression of IL-10 in cord DCs may contribute to the relative immaturity of the cord DCs and their failure of CCR5 down-regulation after LPS stimulation. Interestingly, cord DCs stimulated for 24h in the presence of LPS also exhibit a markedly reduced tendency to form homotypic cell clusters (data not shown), indicating that there are fundamental differences in the migratory and adhesive characteristics of these cells.
Based on the susceptibility of infants and young children to infection, and their inability to respond to certain types of antigen, we hypothesize that antigen presentation is compromised by relative immaturity of DC phenotype and function. The findings reported in this study also suggest that intrinsic and preprogrammed properties of neonatal DCs may contribute significantly to the observed bias of naïve Th polarization away from Th-1.
Acknowledgments
We thank the midwives and theatre staff at University College Hospital, London for their assistance with cord blood collection, and D. Katz for the gift of IL-4. This work was supported by the Child Health Research Appeal Trust of the Institute of Child Health. J.C.B. is supported by a grant from the Wellcome Trust. A.J.T. is a Wellcome Trust Senior Clinical Fellow.
REFERENCES
- 1.Kovarik J, Siegrist CA. Immunity in early life. Immunol Today. 1998;19:150–2. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- 2.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;220:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 3.Harris DT, Schumacher MJ, Locascio J, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci USA. 1992;89:10006–10. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson CB, Lewis DB. Basis and implications of selectively diminished cytokine production in neonatal susceptibility to infection. Rev Infec Dis. 1990;12:S410–S420. doi: 10.1093/clinids/12.supplement_4.s410. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM. The dendritic cell and its role in immunogenicity. Ann Rev Immunol. 1991;8:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12 (p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 10.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and non-polarised cells. Nature Immunol. 2000;1:311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 11.Baraff LJ, Leake RD, Burstyn DG, et al. Immunologic responses to early and routine DTP immunization in infants. Pediatrics. 1984;73:37–42. [PubMed] [Google Scholar]
- 12.Hart DJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 13.Rhasselt V, Buelens C, Willems F, et al. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–25. [PubMed] [Google Scholar]
- 14.Liu E, Tu W, Law HK, Lau YL. Decreased yield, phenotypic expression and function of immature monocyte-derived dendritic cells in cord blood. B J Haematol. 2001;113:240–6. doi: 10.1046/j.1365-2141.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu E, Tu W, Law HK, Lau YL. Changes of CD14 and CD1a expression in response to IL-4 and granulocyte-macrophage colony-stimulating factor are different in cord blood and adult blood monocytes. Ped Res. 2001;50:184–9. doi: 10.1203/00006450-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–43. [PubMed] [Google Scholar]
- 17.Petty RE, Hunt DW. Neonatal dendritic cells. Vaccine. 1998;16:1378–82. doi: 10.1016/s0264-410x(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 18.Wright SD. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–9. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchett SK, Weaver WM, Westall JA, Larsen A, Kronheim S, Wilson CB. Regulation of tumor-necrosis factor/cachectin and IL-1 secretion in human mononuclear phagoctes. J Immunol. 1988;140:3473–81. [PubMed] [Google Scholar]
- 20.Adkins B, Biu Y, Guevara P. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J Immunol. 2001;166:918–25. doi: 10.4049/jimmunol.166.2.918. [DOI] [PubMed] [Google Scholar]
- 21.Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–24. [PubMed] [Google Scholar]
- 22.Adkins B, Biu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164:2347–53. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- 23.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naïve human neonatal CD4+ T cells, analysed at the single-cell level. J Exp Med. 1996;184:473–83. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited. Turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 25.Min B, Legge KL, Pack C, Zaghouani H. Neonatal exposure to a self-peptide-immunoglobulin chimera circumvents the use of adjuvant and confers resistance to autoimmune disease by a novel mechanism involving interleukin 4 lymph node deviation and interferon gamma mediated splenic anergy. J Exp Med. 1998;188:2007–17. doi: 10.1084/jem.188.11.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arulanandam BP, Van Cleave VH, Metzger DW. IL-12 is a potent neonatal vaccine adjuvant. Eur J Immunol. 1999;29:256–64. doi: 10.1002/(SICI)1521-4141(199901)29:01<256::AID-IMMU256>3.0.CO;2-G. 10.1002/(sici)1521-4141(199901)29:01<256::aid-immu256>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 27.Arulanandam BP, Mittler JN, Lee WT, O’Toole M, Metzger DW. Neonatal administration of IL-12 enhances the protective efficacy of antiviral vaccines. J Immunol. 2000;164:3698–704. doi: 10.4049/jimmunol.164.7.3698. [DOI] [PubMed] [Google Scholar]
- 28.Donckier V, Flamand V, Desalle F, et al. IL-12 prevents neonatal induction of transplantation tolerance in mice. Eur J Immunol. 1998;28:1426–30. doi: 10.1002/(SICI)1521-4141(199804)28:04<1426::AID-IMMU1426>3.0.CO;2-P. 10.1002/(sici)1521-4141(199804)28:04<1426::aid-immu1426>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico G, Frascaroli G, Bianchi G, et al. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat Immunol. 2000;1:387–91. doi: 10.1038/80819. [DOI] [PubMed] [Google Scholar]


