Abstract
Infiltration of leucocyte populations into sites of inflammation is a common feature in renal diseases. Glomerular mesangial cells are potent producers of a variety of chemokines, leading to specific attraction of distinct types of inflammatory leucocytes into the glomerulus, but so far there is limited knowledge about the responsiveness of mesangial cells to chemokines. We investigated the expression of chemokine receptors and the responsiveness of primary human mesangial cells (HMC) to the chemokines which they produce, namely monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-8. We found that mRNAs of the chemokine receptors CCR1, which has been shown before, CCR2 and CXCR2 were induced by T-helper cytokine interferon-gamma (IFNγ). In IFNγ-stimulated cells, CCR2 and CXCR2 were detectable by flow cytometry. Following treatment with IFNγ, HMC responded to MCP-1 and IL-8 with an increase of IL-6 mRNA and protein expression, which was in part blocked by pertussis toxin. Moreover, chemokine stimulation of transfected HMC led to an activation of the immunoregulatory transcription factors NFκB and AP-1. Additionally, we found that MCP-1 enhanced the expression of its own mRNA in cells activated to express CCR2, suggesting autocrine feedback mechanisms in MCP-1 regulation. Finally, IFNγ-activated cells migrated towards an MCP-1 gradient in a chemotaxis assay. These results strengthen the assumption that chemokines are not only involved in the recruitment of immune cells to inflamed tissues, but also seem to play a central role in the autocrine regulation of local tissue cells, leading to proceeding inflammation and possibly contributing to healing by mediating cell growth and migration.
Keywords: chemokine, human mesangial cell, nephritis, chemokine receptor, MCP-1
INTRODUCTION
Mesangial cells are mesenchyme-derived cells that are located within the glomerulus of the kidney. These specialized pericytes possess many properties of vascular smooth muscle cells and regulate a number of important renal functions, including glomerular filtration rate, matrix synthesis and integrity maintenance of glomerular structure [1–3]. Pathobiological responses of mesangial cells to inflammatory stimuli often initiate and maintain pathogenic processes within the glomerulus. They seem to participate in the majority of forms of glomerulonephritis (GN), acting as inflammatory cells by generating nitrite and oxygen radicals [4], prostaglandins [1,5] and extracellular matrix components [6], or by up-regulating the expression of surface molecules, especially Fc receptors for IgG [7,8], MHC class II and adhesion proteins [9]. A characteristic feature of GN is the infiltration of leucocytes from the circulation into the kidney tissue, and the subsequent activation of such inflammatory cells at the site of glomerular injury. It has been demonstrated that mesangial cells are a rich source of chemokines attracting different leucocyte populations into the glomerulus [10–13].
Chemokines are a superfamily of small proteins that can be devided into four main branches (CXC, CC, C and CX3C chemokines) according to the position of conserved cysteines [14,15]. These proteins mediate their specific effects upon binding to seven transmembrane domain, G protein-coupled receptors [16]. So far, six receptors for CXC chemokines (CXCR1–6), 11 receptors for CC chemokines (CCR1–11), one CX3C chemokine receptor (CX3CR1) and one C chemokine receptor (XCR1) have been identified [summarized in 17]. Most receptors recognize more than one chemokine, and several chemokines bind to more than one receptor, indicating redundancy of the chemokine system. In addition to their role in mediating leucocyte migration, certain chemokine receptors were found to act as co-receptors for HIV [17].
Along with their well defined role as mediators of transendothelial migration of inflammatory leucocytes, chemokine receptors have been primarily localized on neutrophils, eosinophils, basophils, monocytes/macrophages and lymphocytes [15]. So far there are only a few reports describing the expression of chemokine receptors and their distinct functions in non-haematopoietic tissue cells, including glomerular mesangial cells [18–21]. To extend knowledge of the role of chemokines in glomerular inflammation, we investigated the expression of chemokine receptors and their functions in our well established primary human mesangial cell (HMC) culture system [1,7,8,13].
There is evidence that chemokine receptor expression in leucocytes can be regulated by inflammatory signals such as gamma-interferon (IFNγ) and interleukins (IL) [22,23]. Since glomerular kidney disease is often characterized by the involvement of T lymphocytes [24], we studied the potential of the prominent T-helper lymphokine IFNγ to modulate the expression of chemokine receptors in HMC. Moreover, chemokine-induced inflammatory gene expression and chemotaxis of activated HMC were studied.
MATERIALS AND METHODS
Materials
Recombinant human (rh) IFNγ (2 × 107U/mg) was kindly donated by Dr B. Otto, Fraunhofer Institute, Hannover, Germany. CXCR2-specific primers were a gift from Dr H. Holtmann, Medical School, Hannover, Germany, MCP-1 cDNA was provided by Dr T. Schall, DNAX, Palo Alto, CA, USA, and GAPDH cDNA was donated by Dr B. Hipskind, Medical School, Hannover, Germany. rhMCP-1, rhRANTES, rhIL-8, rhPDGF and Quantikine ELISA assay kits specific for rhIL-6 were from R & D Systems, Wiesbaden, Germany. Oligonucleotide primers for β-tubulin, CCR1 and CCR2 were from MWG-BIOTECH, Ebersberg, Germany. PathDetect cis-Reporting System plasmids pNFκB-Luc or pAP1-Luc were from Stratagene, La Jolla, CA, USA. Pertussis toxin was obtained from BIOMOL, Hamburg, Germany. Restriction enzymes and corresponding buffers used for cDNA probe preparation were either from AGS, Heidelberg, or from Boehringer, Mannheim, Germany. Phosphate-buffered saline (PBS), MCDB-302 medium, insulin (bovine) and transferrin (human) were obtained from Sigma Chemical Co., Deisenhofen, Germany. RPMI-1640, non-essential amino acids (NEA), l-glutamine, sodium pyruvate, mycoplasma-free fetal calf serum (FCS, 20Q19) and trypsin-EDTA were from Gibco BRL, Eggenstein, Germany. Cell culture plastic material was from Nunc, Wiesbaden, Germany. All other materials were obtained commercially at the highest quality available.
Human glomerular mesangial cell preparation, characterization and culture
For the present experimental series, selected tumour-free, healthy tissue of kidneys from two different donors undergoing tumour nephrectomy (who had given their informed consent) was obtained freshly with the help of the Department of Urology, Medical School, Hannover. HMC were prepared as described [4,7]. The different specimens showed no significant differences in growth, proliferation and morphology. Characterization by immunofluorescence staining showed a positive reaction with SMC-myosin and actin, vimentin, fibronectin, desmin and collagen type IV, and a negative reaction with anti-human keratin, factor VIII and MHC class II antigen antisera. There was no morphological evidence of the presence of macrophages (pseudopodia), endothelial or epithelial-like cells (cobblestone), or fibroblasts. Additionally, by means of biochemical characterization, bone marrow-derived resident macrophages and endothelial cells could be effectively disclosed by the following criteria: HMC were MHC class II negative [25], showed a typical profile of prostanoids [1,5] and, in contrast to macrophages, did not express 5-lipoxygenase mRNA or produce leukotrienes [1], and released no reactive oxygen species when unactivated [4,26]. Furthermore, the use of HMC after passage number three excludes macrophage as well as endothelial cell contaminations because these cell types do not survive multiple passaging under the described medium conditions. After the third passage, HMC were grown, as described [4,7,13], in culture medium supporting cell proliferation consisting of RPMI-1640, NEA (1 ml/dl), l-glutamine (2 mm), sodium pyruvate (2 mm), transferrin (5 mg/ml), insulin (125 U/ml) and FCS (10%). For passaging, HMC were detached by trypsin/EDTA (0·125%/0·01%, wt/vol) and split 1:3. Serum-free culture of HMC was performed using MCDB-302 as culture medium without FCS, supplemented with transferrin and insulin, leading to a growth arrest of HMC after 48 h [5,27]. At this time point, the resting medium was removed, cells were washed twice with PBS and were then cultivated for various stimulation periods in resting medium alone (control) or with the addition of (if not otherwise indicated) 1000 U/ml IFNγ, 50 ng/ml IL-8, 50 ng/ml MCP-1 or 50 ng/ml RANTES.
RNA preparation and reverse-transcribed polymerase chain reaction (RT-PCR)
Total cellular RNA was prepared from HMC cultured under resting, serum-free conditions in the absence or presence of the respective stimuli by a modification of Chomczynski’s method [7] using the commercial RNAclean system (AGS, Heidelberg, Germany). To increase the purity of the RNA samples, we extended the basic method described by an additional LiCl2 (4 m) precipitation step [7]. RNA amounts were determined by ultraviolet spectroscopy. As described previously [7], we used a ‘hot start’ RT-PCR protocol according to guidelines for the GeneAmpR RNA PCR Kit (Perkin Elmer Cetus, Norwalk, CT, USA). Primer pairs were selected specific for human CCR1 (sense: 5′-ATGGAAACTCCAAACACCACAGAGG, antisense: 5′-GTCAGAACCCAGCAGAGAGTTCAT; product size: 1069 bp), human CCR2 (sense: 5′-ATGCTGTCCACATCTCGTTCT CGGT, antisense: 5′-CGTTTTATAAACCAGCCGAGACTTC; product size: 1087 bp) [28], human CXCR2 (sense: 5′-AGATG TAGAGGAGAAACTGG, antisense: 5′-ATAAACACCCAAG TAAAAATGG; product size 812 bp), and for a universal β-tubulin cDNA [7]. Before RT-PCR was performed, RNA was treated with 1 unit DNase I (Gibco BRL) per μg RNA for 15 min at room temperature. To establish conditions that allow the comparison of the amount of cDNA produced by RT-PCR (as a semi-quantitative measure of the initial mRNA level), cycle numbers were varied for the respective cDNA. As an internal standard, we amplified β-tubulin mRNA, a ubiquitously-expressed gene. The amounts of the expressed β-tubulin mRNA were unchanged in unstimulated versus stimulated cells. For β-tubulin, cDNA 28, 30, 32 and 34 PCR cycles were applied. CCR1, CCR2 and CXCR2 cDNAs were amplified using 30, 33, 36 and 39 cycles. PCR products were analysed by ethidium bromide-stained agarose gel electrophoresis.
Flow cytometry
HMC were grown under growth-arrested conditions for 48 h and subsequently incubated in the presence or absence of IFNγ (1000 U/ml) for a further 24 h. Cells were washed and detached using trypsin/EDTA (0·125%/0·01%, wt/vol). For membrane receptor recovery, trypsinized cells were incubated in culture medium containing 10% FCS at 37°C and 5% CO2 in a humidified incubator for 1h with frequent agitation. To detect CCR2 surface expression, cells were incubated either with anti-huCCR2 (R & D Systems) and subsequently with goat anti-mouse IgG-FITC (Dianova, Hamburg, Germany), or with biotinylated MCP-1 (R & D Systems) and subsequently with streptavidin-FITC. To verify CCR2 detection by the used CCR2 antibody, PBMC were treated with or without LPS and dexamethasone and used as controls. For CXCR2 detection, HMC were incubated and detached as described above, with subsequent incubation and agitation for receptor resurfacing. Surface CXCR2 was detected using anti-CXCR2 (R & D Systems), followed by incubation with a biotinylated goat anti-mouse antibody. Subsequent steps were incubation with avidin-PE (Vector Laboratories, Burlingame, CA, USA), a polyclonal anti-avidin antibody and a final incubation with avidin-PE. As a positive control to ensure activity of IFNγ, HMC were incubated with anti-huICAM-1 (Boehringer). Negative controls were either unlabelled MCP-1 for detection of CCR2 using biotinylated MCP-1, or irrelevant IgG for CCR2 and CXCR2 detection with specific antibodies. Cells were analysed using a FACScan flow cytometer (Becton-Dickinson, Heidelberg, Germany).
Northern blot analysis
Total cellular RNA from HMC was fractionated on 1% agarose, 6% formaldehyde gels, and transferred to Hybond N nylon membranes (Amersham-Buchler, Braunschweig, Germany) by capillary blotting with 10× SSC (1× SSC: 150 mm NaCl, 15 mm Na-citrate, pH 7). After cross-linking by ultraviolet irradiation, blot membranes were pre-hybridized for at least 2 h at 42°C in 5× SSPE, 5× Denhardt’s solution, 50% formamide, 5% dextransulphate, 0·5% sodium dodecyl sulphate (SDS) and 400 μg/ml denatured salmon sperm DNA. Membranes were hybridized overnight at 42°C in the same solution containing heat-denatured, 32P]-labelled cDNA probes for IL-6, MCP-1, GAPDH or β-tubulin. Radioactive labelling of cDNAs was performed according to standard protocols described elsewhere [29]. The filters were subsequently washed twice in 2× SSC at room temperature, once for 15 min in 1× SSC, 0·1% SDS at room temperature, and once for 15 min in 0·2× SSC, 0·1% SDS at 62°C. Bands were detected by autoradiography using Kodak X-OMAT AR film; exposure time ranged from 3 to 72 h at – 70°C. Semi-quantitative analysis was carried out by densitometric scanning of the autoradiographs with an analytic Gel Doc 1000 Video System (Bio-Rad Laboratories, Hercules, CA, USA).
IL-6 ELISA
Growth-arrested HMC were activated with IFNγ (1000 U/ml) for 18 h. Subsequently, cells were washed and fresh medium with or without pertussis toxin (250 ng/ml) was added. After 6 h, the medium was removed, cells were washed, and fresh medium containing either no chemokine, MCP-1 or IL-8 (50 ng/ml each) was added. After another 24 h incubation, supernatant fluids were harvested and assayed for IL-6.
HMC transfection and luciferase assays
For transient transfection, HMC were seeded in 24-well plates, at a density of 40 000 cells/well, one day prior to transfection. Luciferase reporter plasmids pNFκB-Luc or pAP1-Luc plus a renilla luciferase control vector (total=400 ng DNA/40 000 cells) were transfected using Effectene transfection reagent (Qiagen, Hilden, Germany), following the manufacturer’s guidelines. Forty-eight hours after transfection, cells were lysed and assayed for luciferase activity using the Luciferase Assay System (Promega, Mannheim, Germany). Measurements were performed with a Luminoskan Ascent 96-well luminometer (Thermo Labsystems, Frankfurt/Main, Germany).
Chemotaxis assay
HMC were trypsinized and subsequently incubated in culture medium, containing 10% FCS at 37°C and 5% CO2, in a humidified incubator for 1 h, with frequent agitation to allow receptor recovery. Thereafter, cells were washed twice with PBS and resuspended in serum-free RPMI/1% BSA. Each well of the upper compartment of a microchemotaxis chamber (Neuroprobe, Cabin John, MD, USA) was loaded with 20 000 cells in a total volume of 50 μl. Chemoattractants were loaded in a final volume of 30 μl at indicated concentrations into the lower compartment. The two compartments were separated by a polycarbonate filter with 14 μm pores (Infiltec, Speyer, Germany). The chemotaxis chamber was incubated at 37°C, 100% humidity and 5% CO2 for 3 h. After incubation, the filter was scraped to remove non-migrating cells from the filter surface. Cells were subsequently fixed using formaldehyde and stained with haematoxylin. Numbers of migrating cells were determined microscopically at ×400 magnification.
RESULTS
IFNγ induces chemokine receptor mRNA expression in HMC
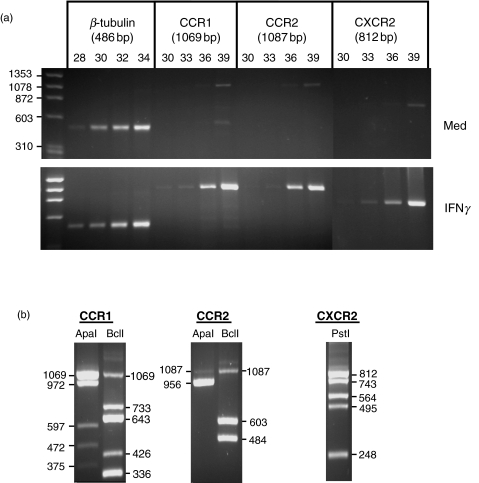
Following RT-PCR analysis, there were almost undetectable cDNA amounts of the chemokine receptors CCR1, CCR2 and CXCR2 in growth-arrested, unstimulated HMC. Activation of HMC with the TH1 lymphokine IFNγ for 18 h resulted in an induction of mRNA expression of CCR1, CCR2 and CXCR2, with bands becoming visible after 36 PCR amplification cycles (Fig. 1a). Upon treatment of HMC with IFNγ for 30 h or longer, there was no increase in mRNA expression of CCR1, CCR2 or CXCR2 compared with controls (not shown), suggesting a transient induction of chemokine receptor gene expression. The identities of the observed chemokine receptor cDNA bands were confirmed by restriction analysis, revealing the expected patterns of restriction fragments (Fig. 1b).
Fig. 1.
Expression of chemokine receptor mRNA expression in HMC. (a) RNA was isolated from growth-arrested cells incubated under medium conditions or stimulated with IFNγ (1000 U/ml) for 18 h; 2 μg total cellular RNA of each preparation were used for reverse transcription and subsequently amplified with oligonucleotide primers specific for β-tubulin, CCR1, CCR2 and CXCR2. Cycle numbers for β-tubulin were 28, 30, 32 and 34. Chemokine receptor cDNAs were amplified using 30, 33, 36 and 39 cycles. PCR products were visualized by ethidium bromide staining in agarose gel electrophoresis. The results shown are representative of five separate experiments. (b) To prove the identity of the observed cDNAs, PCR products were purified and digested with the restriction endonucleases indicated. The resulting fragments were separated by gel electrophoresis.
IFNγ induces CCR2 and CXCR2 membrane expression in HMC
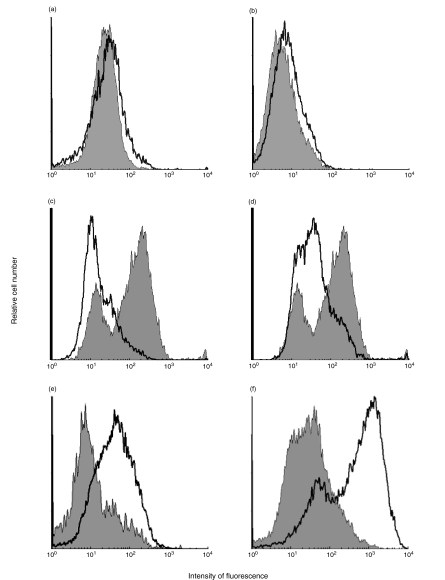
Surface expression of CCR2 and CXCR2 was determined by flow cytometry of serum-starved cells with or without IFNγ treatment. When the cells were incubated with the respective antibodies immediately following detachment from the culture flask surface, no receptor expression was detectable (not shown). However, when trypsinized cells were transferred to 10% FCS in culture medium and incubated for 1h to allow for receptor recovery, surface expression of both CCR2 and CXCR2 could be detected (Fig. 2a,b,e). As an alternative to using an anti-CCR2 monoclonal antibody (Fig. 2a), biotinylated MCP-1, followed by a subsequent incubation with FITC-conjugated streptavidin, was used as the CCR2-binding agent (Fig. 2b). Specificity of the used anti-CCR2 antibody was proven by detecting a marked decrease in CCR2 surface expression of LPS-treated PBMC, which was in part reversed by dexamethasone as described (Fig. 2c,d) [30]. To determine HMC surface expression of CXCR2, a protocol of subsequent avidin-PE and polyclonal anti-avidin was used to enhance CXCR2 detectability (see Materials and Methods). With this method, a marked increase in CXCR2 surface expression was found in IFNγ-treated HMC compared with untreated cells (Fig. 2e). Activity of the IFNγ used in these experiments was proven by a strong increase in ICAM-1 expression in stimulated HMC (Fig. 2f).
Fig. 2.
IFNγ induces HMC surface expression of CCR2 and CXCR2. (a) Increase of CCR2 after stimulation with IFNγ. After 48 h of incubation, cells were detached and incubated as described in Materials and Methods. After receptor recovery, cells were incubated with anti-CCR2, followed by a FITC-conjugated goat anti-mouse antibody. Medium control cells (grey area) were 3·76% positive, whereas stimulated cells (white area) were 11·68% positive for CCR2. (b) Detection of CCR2 using biotinylated MCP-1 as binding agent (see Materials and Methods). Here, CCR2 expression increased from 13·91% in medium control HMC to 23·03% in activated cells. (c) Down-modulation of PBMC CCR2 expression by LPS. To confirm specificity of the CCR2 antibody used, PBMC were treated with or without LPS (10 μg/ml) and assayed for CCR2. Surface CCR2 decreased from 74·03% in medium controls (grey area) to 23·18% in LPS-treated cells (white area). (d) Partial inhibition of LPS-mediated down-regulation of CCR2 by dexamethasone. Simultaneous incubation of PBMC with LPS and dexamtehasone (10−6M) partly blocked CCR2 decrease from 74·03% (medium control, grey area) to 54·14% (LPS plus dexamethasone, white area). (e) Increase of CXCR2 in IFNγ-stimulated HMC. Cells were treated as described above and subsequently incubated with anti-CXCR2, biotinylated goat anti-mouse, avidin-PE, polyclonal anti-avidin and again, avidin-PE. CXCR2-positive cells increased from 11·74% of medium control cells (grey area) to 46·98% of stimulated cells (white area). (f) Increase of ICAM-I expression in IFNγ-activated HMC. To ensure activity of the IFNγ utilized, expression of ICAM-I was detected using anti-huICAM-I. Positive cell numbers increased from 32·31% of medium control cells (grey area) to 90·03% of stimulated cells (grey area). Each experiment was performed at least twice.
Chemokines induce increased expression of IL-6 in IFNγ-activated HMC
To specify functions of HMC chemokine receptors, we analysed the effects of chemokines on IL-6 gene expression in HMC. Therefore, cells were pre-activated with IFNγ for 24 h to stimulate the expression of chemokine receptors. Subsequently, cells were treated with recombinant RANTES, MCP-1 and IL-8 representing ligands for CCR1, CCR2 and CXCR2, respectively. Using northern blot analysis, we found that chemokine stimuli induced an increase in IL-6 mRNA expression only in cells which had been pre-treated with IFNγ (Fig. 3a). These data suggest that IFNγ stimulated the expression of functional chemokine receptors in HMC. To test whether these effects were due to Gi protein-dependent signalling mechanisms, which are general characteristics of chemokine receptor-mediated effects, we investigated the sensitivity of the chemokine-induced IL-6 mRNA increase to pertussis toxin (PTx). We found a complete inhibition of RANTES-stimulated IL-6 mRNA increase in PTx-treated cells, whereas the effects of MCP-1 and IL-8 were partly blocked (Fig. 3b). In protein assays, supernatant fluids of HMC, which were stimulated with IFNγ plus MCP-1 or IL-8, contained elevated amounts of IL-6 (Fig. 3c). Here, the increase of released IL-6 was almost completely blocked by PTx, suggesting Gi protein-mediated mechanisms.
Fig. 3.
Up-regulation of IL-6 expression in HMC by chemokines. (a) Growth-arrested cells were pre-incubated in the absence or presence of IFNγ (1000U/ml) for 24 h and subsequently stimulated with the indicated chemokines (50 ng/ml each) for an additional 24 h. Total cellular RNA was prepared, and 20 μg of each preparation were resolved in agarose–formaldehyde gel electrophoresis and subsequently transferred to nylon membranes. Northern hybridization was performed using 32P-labelled cDNA probes specific for IL-6 and β-tubulin mRNA. Autoradiographs were semi-quantitatively analysed by densitometric scanning and alignment of mRNA bands. All northern analysis results presented show one representative of at least three independent experiments. (b) Inhibition of chemokine-induced increase in IL-6 mRNA expression by pertussis toxin (PTx). RNA from HMC, which were left untreated or activated with IFNγ (1000 U/ml) for 24 h, incubated overnight in the absence or presence of Ptx (250 ng/ml), and subsequently stimulated with the indicated chemokines (50 ng/ml each) for an additional 24 h, was isolated and fractionated in gel electrophoresis (20 μg per lane). Subsequent northern analysis was employed using 32P-labelled cDNA probes specific for IL-6 and GAPDH mRNA. The results of densitometric scanning of autoradiographs are presented in bar graphs, showing IL-6 mRNA amounts relative to β-tubulin or GAPDH mRNA, respectively. (c) IL-6 protein expression. Resting HMC were untreated or activated with IFNγ (1000 U/ml) overnight and subsequently incubated in the presence or absence of PTx (250 ng/ml) for 6 h. After a medium change, chemokines were added, and supernatant fluids were collected and assayed 24 h thereafter. Shown are mean values plus s.e.m. of triplicate samples (P < 0·0005, unpaired t-test).
Chemokines activate transcription factors in HMC
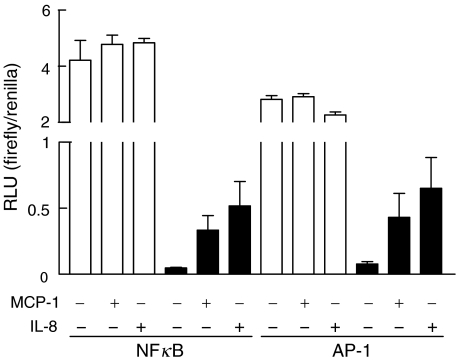
To confirm the capacity of chemokines to stimulate gene expression in activated HMC, reporter studies with transfected HMC were performed, using plasmid vectors containing consensus sequences for the pro-inflammatory transcription factors NFκB and AP-1, directly followed by the luciferase reporter gene. As a primary effect of IFNγ, transcription factor activities were markedly reduced compared with unactivated cells, probably due to the general growth-inhibitory effects of IFNγ (Fig. 4). However, when IFNγ-treated cells were subsequently stimulated with MCP-1 or IL-8, reporter gene expression increased up to eightfold, suggesting a role for these chemokines in pro-inflammatory gene expression.
Fig. 4.
Activation of NFκB and AP-1 by MCP-1 and IL-8. HMC were transfected 24 h after stimulation with IFNγ (1000 U/ml) as described in Materials and Methods. Chemokines (50 ng/ml) were added 8 h after transfection, and cell lysates were prepared and assayed in triplicate for luciferase activity 16 h after chemokine addition. (□) Medium; (▪) IFNγ. Error bars represent s.e.m.
MCP-1 up-regulates the expression of its own mRNA in IFNγ-activated HMC
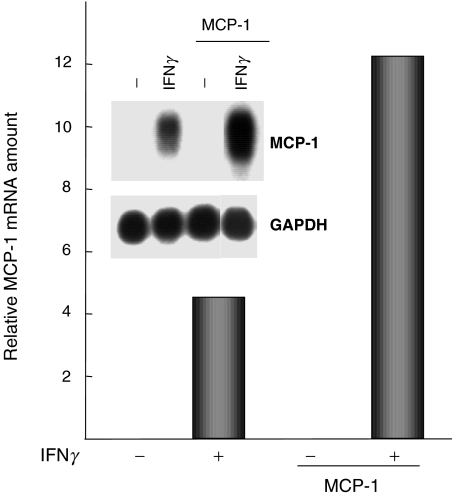
In addition to the chemokine-induced increase in IL-6 mRNA synthesis, we also investigated the autoregulatory potential of chemokines. Growth-arrested HMC were pre-activated for 24 h with IFNγ and subsequently incubated in the absence or presence of recombinant MCP-1, RANTES or IL-8. In northern blot experiments, we found that MCP-1, but not RANTES or IL-8 (not shown), induced a significant up-regulation of the expression of its own mRNA in IFNγ-activated HMC (Fig. 5). Thus, MCP-1 may possess an autocrine potential to regulate its own synthesis in HMC which have been previously activated by T-helper cells.
Fig. 5.
Autoregulation of MCP-1 mRNA expression. HMC were activated with IFNγ (1000 U/ml) for 24 h and subsequently incubated in the absence or presence of MCP-1 (50 ng/ml). RNA was isolated and analysed by northern hybridization using 32P-labelled cDNA probes specific for MCP-1 and GAPDH mRNA. The bar graph shows the relative amounts of MCP-1 mRNA, measured by densitometric scanning of autoradiographs.
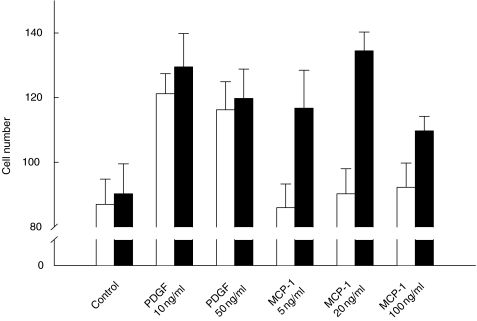
MCP-1 induces chemotaxis in IFNγ-activated HMC
To determine whether chemokine receptor ligands could serve as chemoattractants for HMC, we tested recombinant MCP-1 for its ability to induce HMC migration through the 14 μm pores of a polycarbonate membrane. Since chemotactic responsiveness of HMC to platelet-derived growth factor (PDGF) has been previously reported [31], we used PDGF as a positive control in this assay. Exposure of HMC, which had been pre-activated with IFNγ, to 20 ng/ml of MCP-1 induced the highest number of migrating cells (Fig. 6). By contrast, cells which had been stimulated with MCP-1 without prior activation with IFNγ showed no migratory activity. In a control experiment, we examined the possibility of chemokinesis induced by MCP-1 or PDGF, and found no increase in HMC migration in response to MCP-1 or PDGF compared with the medium control (not shown). Thus, we found that MCP-1 acted as a potent chemoattractant for activated HMC.
Fig. 6.
Chemotactic migration of activated HMC in response to MCP-1. Growth-arrested cells were either untreated or activated with IFNγ (1000 U/ml) for 24 h, detached, recovered as described in Materials and Methods, and loaded into the upper compartment of a microchemotaxis chamber (20 000 cells/well). The chemotactic factors, MCP-1 and platelet-derived growth factor (PDGF), were loaded at the indicated concentrations into the lower compartment, and the two compartments were separated using a 14 μm pore size polycarbonate membrane. After a 3 h incubation at 37°C, migrated cells were fixed and stained. For each data point, three high-power fields were counted. (□) Medium; (▪) IFNγ. The results represent the average numbers plus s.e.m. of four experiments.
DISCUSSION
The results presented in this study show that chemokines, which are produced locally in tissues, can directly affect the inflammatory potential of tissue cells. Using primary human mesangial cells as a model, we could show that such specialized tissue cells not only produce large amounts of different chemokines but, once activated by leucocyte-derived IFNγ, become capable of responding to chemokines. This observation suggests a new role of this particular cytokine family, i.e. tissue-derived chemokines seem to act on their producer cells in an autocrine fashion, which is likely to result from the expression of functional chemokine receptors on such tissue cells. We found that CCR1, CCR2 and CXCR2 mRNAs were expressed only at very low levels under basal conditions. When the cells were activated with the TH1 cytokine IFNγ, receptor mRNA amounts increased. With the exception of CXCR4, which is widely expressed in many tissues [15], there are few reports showing chemokine receptor mRNA expression in mesangial or other specialized tissue cells. Binding of the murine CC chemokine TCA3 to mouse mesangial cells has been demonstrated [18] and recently, the expression of CCR1 mRNA and a chemotactic response to RANTES of primary and immortalized HMC has been reported [19]. Another study demonstrated that CXCR3 was functionally expressed in HMC [20]. Also, murine mesangial cells were shown to respond to MIP-2 and KC, suggesting the expression of an unknown receptor for these chemokines [21]. However, with the exception of these studies, there are limited available data regarding the expression and regulation of chemokine receptor expression in tissue cells and resulting chemokine-mediated effects. In leucocytes such as T cells or monocytes, cytokines like IFNγ or IL-2 have been reported to modulate the expression of chemokine receptors [22,23]. Unlike our observations in mesangial cells, IFNγ selectively down-regulated CCR2 in human monocytes, while CCR1, CCR3, CCR4 and CCR5 were unaffected [23]. In our primary HMC culture system, we found that IFNγ induced CCR2 and CXCR2 mRNA and surface protein expression, and also a chemotactic response to MCP-1. So far, only CXCR3 has been shown to be functionally expressed on mesangial cell surfaces [20]. Here, we report for the first time the detection of CCR2 surface protein using two different detection protocols: (i) a mouse anti-CCR2 antibody as primary and goat anti-mouse FITC-labelled secondary antibody; (ii) biotinylated MCP-1 instead of the primary antibody, and FITC-labelled streptavidin instead of a secondary antibody. It also proved to be of particular importance that the cells were incubated for an additional hour with repeated agitation after detachment from the flask surface, prior to antibody or biotinylated ligand incubation in order to allow receptor recovery. The fact that in this system IFNγ induces both MCP-1, as we and others have previously shown [10,13], and CCR2 expression indicates a prominent role for this particular lymphokine for the regulation of mesangial cell-mediated renal inflammation.
In addition to CCR2, this study demonstrates mesangial cell surface CXCR2 for the first time. This was achieved by amplifying the detectability of CXCR2, using subsequent steps, with a biotinylated secondary antibody, avidin, and an anti-avidin antibody. Thus, the amounts of CCR2 and CXCR2 molecules on HMC surfaces are modest, even under strong stimulatory conditions, although sufficient to elicit ligand-stimulated responses; this could be shown by stimulating HMC with RANTES, MCP-1 and IL-8 as representing ligands for CCR1, CCR2 and CXCR2. Since these chemokines are produced in significant amounts by HMC, receptor expression in HMC might lead to autocrine effects mediated by mesangial cell-derived chemokines. In fact, stimulation of IFNγ-activated HMC with the indicated chemokines resulted in an increased expression of IL-6 mRNA and protein, which was in part blocked by PTx. On the protein level, inhibition of IL-6 expression by PTx was more pronounced than on the level of mRNA. The residual, unblockable amounts of IL-6 protein and mRNA might be due to primary or secondary effects of IFNγ on IL-6 gene expression, which is independent of G proteins.
Among the factors which are expressed in response to activation of the pro-inflammatory transcription factors NFκB and AP-1, there are numerous cytokines, chemokines, growth-factors and adhesion molecules, all of which are key players in inflammation. Our findings that MCP-1 and IL-8 can activate these transcription factors supports our hypothesis that mesangial cells, once activated by inflammatory mediators, can be further driven towards exacerbating an inflammatory response by chemokines, which are present in large amounts in the inflamed glomerulus. Furthermore, by showing that IFNγ-activated cells migrated towards an MCP-1 gradient, we found chemotactic activity of CCR2 in HMC membranes. In contrast, HMC did not migrate in significant numbers in response to IL-8 (not shown). From this we conclude that MCP-1 is both an inflammatory and, in addition to RANTES [19], chemotactic factor for HMC, whereas IL-8 appears to act mainly as a pro-inflammatory mediator rather than by promoting chemotaxis. The relevance of mesangial cell migration in the repair process following mesangiolysis in nephritis has been demonstrated [32]. Thus, the chemotactic activity of MCP-1 on HMC further confirms the significance of chemokine and chemokine receptor expression in mesangial cells in the context of inflammatory glomerular injury.
Since we found that MCP-1 seems to possess the ability of regulating its own mRNA expression via a putative autocrine feedback mechanism in mesangial cells, this chemokine might play a crucial role in the initiation and propagation of inflammatory diseases of the glomerulus. An initial inflammatory stimulus, activating MCP-1 gene expression in HMC, may lead to an acute amplification of mesangial MCP-1 production, resulting in a short-term increase of local inflammation. Since nephritic CCR2 knockout mice exhibited an increased severity of disease compared with control animals [33], an anti-inflammatory function of CCR2 in this model of glomerular disease could be concluded. However, it has been repeatedly demonstrated that MCP-1, as well as mononuclear infiltrates, peaks early in glomerular disease. This suggests that the lack of CCR2 might not be sufficient to prevent the long-term progression of inflammation, because numerous factors and cell types contribute to the manifestation of inflammatory glomerular injury. Thus, the intervention in the early steps of inflammation, namely, the effects of MCP-1, by transiently blocking CCR2 might still be a promising tool for down-modulating inflammatory reactions.
Acknowledgments
We thank Juliane von der Ohe for excellent technical assistance. This study was supported by DFG Ra 525/5–1 and SFB 244/B1.
REFERENCES
- 1.Radeke HH, Resch K. The inflammatory function of renal glomerular mesangial cells and their interaction with the cellular immune system. Clin Invest. 1992;70:825–42. doi: 10.1007/BF00180754. [DOI] [PubMed] [Google Scholar]
- 2.Kashgarian M, Sterzel RB. The pathobiology of the mesangium. Kidney Int. 1992;41:524–9. doi: 10.1038/ki.1992.74. [DOI] [PubMed] [Google Scholar]
- 3.Couser WG. Mediation of immune glomerular injury. J Am Soc Nephrol. 1990;1:13–29. doi: 10.1681/ASN.V1113. [DOI] [PubMed] [Google Scholar]
- 4.Radeke HH, Meier B, Topley N, Floege J, Habermehl GG, Resch K. Interleukin-1α and tumor necrosis factor-α induce oxygen radical production in mesangial cells. Kidney Int. 1990;37:767–75. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- 5.Floege J, Topley N, Wessel K, et al. Monokines and platelet-derived growth factor modulate prostanoid production in growth-arrested human mesangial cells. Kidney Int. 1990;37:859–69. doi: 10.1038/ki.1990.59. [DOI] [PubMed] [Google Scholar]
- 6.Floege J, Radeke HH, Johnson R. Glomerular cells in vitro versus the glomerulus in vivo. Kidney Int. 1994;45:360–8. doi: 10.1038/ki.1994.46. [DOI] [PubMed] [Google Scholar]
- 7.Radeke HH, Gessner JE, Uciechowski P, Mägert H-J, Schmidt RE, Resch K. Intrinsic human glomerular mesangial cells can express receptors for IgG complexes (hFcγRIII-A) and the associated FcɛRIγ-chain. J Immunol. 1994;153:1281–92. [PubMed] [Google Scholar]
- 8.Uciechowski P, Schwarz M, Gessner JE, Schmidt RE, Resch K, Radeke HH. Interferon-γ induces the high affinity Fc receptor I for IgG (CD64) on human glomerular mesangial cells. Eur J Immunol. 1998;28:2928–35. doi: 10.1002/(SICI)1521-4141(199809)28:09<2928::AID-IMMU2928>3.0.CO;2-8. 10.1002/(sici)1521-4141(199809)28:09<2928::aid-immu2928>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Brennan DC, Jevnikar AM, Takei F, Reubin-Kelley VE. Mesangial cell accessory functions: Mediation by intercellular adhesion molecule-1. Kidney Int. 1990;38:1039–46. doi: 10.1038/ki.1990.310. [DOI] [PubMed] [Google Scholar]
- 10.Rovin BH, Yoshimura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992;148:2148–53. [PubMed] [Google Scholar]
- 11.Hora K, Satriano JA, Santiago A, et al. Receptors for IgG complexes activate synthesis of monocyte chemoattractant peptide 1 and colony-stimulating factor 1. Proc Natl Acad Sci USA. 1992;89:1745–9. doi: 10.1073/pnas.89.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf G, Aberle S, Thaiss F, et al. TNF-α induces expression of the chemoattractant cytokine RANTES in cultured mouse mesangial cells. Kidney Int. 1993;44:795–804. doi: 10.1038/ki.1993.314. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz M, Radeke HH, Resch K, Uciechowski P. Lymphocyte-derived cytokines induce sequential expression of monocyte and T cell-specific chemokines in human mesangial cells. Kidney Int. 1997;52:1521–31. doi: 10.1038/ki.1997.482. [DOI] [PubMed] [Google Scholar]
- 14.Schall TJ. The chemokines. In: Thomson AW, editor. The cytokine handbook. London: Academic Press; 1994. pp. 419–60. [Google Scholar]
- 15.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 16.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 17.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Dorf ME. β-Chemokine TCA3 binds to mesangial cells and induces adhesion, chemotaxis, and proliferation. J Immunol. 1996;156:742–8. [PubMed] [Google Scholar]
- 19.Banas B, Luckow B, Möller M, et al. Chemokine and chemokine receptor expression in a novel human mesangial cell line. J Am Soc Nephrol. 1999;10:2314–22. doi: 10.1681/ASN.V10112314. [DOI] [PubMed] [Google Scholar]
- 20.Romagnani P, Beltrame C, Annunziato F, et al. Role of interactions between IP-10/Mig and CXCR3 in proliferative glomerulonephritis. J Am Soc Nephrol. 1999;10:2518–26. doi: 10.1681/ASN.V10122518. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, Lloyd C, Guiterrez-Ramos J-C, Dorf ME. Chemokine amplification in mesangial cells. J Immunol. 1999;163:3985–92. [PubMed] [Google Scholar]
- 22.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–77. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penton-Rol G, Polentarutti N, Luini W, et al. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-γ. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 24.Li HL, Hancock WW, Dowling JP, Atkins RC. Activated (IL-2R+) intraglomerular mononuclear cells in crescentic glomerulonephritis. Kidney Int. 1991;39:793–8. doi: 10.1038/ki.1991.97. [DOI] [PubMed] [Google Scholar]
- 25.Alpers CE, Hudkins KL, Gown AM, Johnson RJ. Enhanced expression of ‘muscle-specific’ actin in glomerulonephritis. Kidney Int. 1992;41:1134–42. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- 26.Radeke HH, Cross AR, Hancock JT, et al. Functional expression of NADPH oxidase components (α and β-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991;266:21025–9. [PubMed] [Google Scholar]
- 27.Floege J, Topley N, Hoppe J, Barret TB, Resch K. Mitogenic effect of platelet-derived growth factor in human glomerular mesangial cells: modulation and/or suppression by inflammatory cytokines. Clin Exp Immunol. 1991;86:334–41. doi: 10.1111/j.1365-2249.1991.tb05819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power CA, Clemetson JM, Clemetson KJ, Wells TNC. Chemokine and chemokine receptor mRNA expression in human platelets. Cytokine. 1995;7:479–82. doi: 10.1006/cyto.1995.0065. [DOI] [PubMed] [Google Scholar]
- 29.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Penton-Rol G, Cota M, Polentarutti N, et al. Up-regulation of CCR2 chemokine receptor expression and increased susceptibility to the multitropic HIV strain 89.6 in monocytes exposed to glucocorticoid hormones. J Immunol. 1999;163:3524–9. [PubMed] [Google Scholar]
- 31.Barnes JL, Hevey KA. Glomerular mesangial cell migration: Response to platelet secretory products. Am J Pathol. 1991;138:859–66. [PMC free article] [PubMed] [Google Scholar]
- 32.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury: a new role of the juxtaglomerular apparatus. J Clin Invest. 1997;100:786–94. doi: 10.1172/JCI119592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bird JE, Giancarli MR, Kurihara T, et al. Increased severity of glomerulonephritis in C-C chemokine receptor 2 knockout mice. Kidney Int. 2000;57:129–36. doi: 10.1046/j.1523-1755.2000.00848.x. [DOI] [PubMed] [Google Scholar]