Abstract
Influenza patients show a high incidence of T lymphocytopenia in the acute phase of the illness. Since CD8+ T cells play an important role in influenza virus infection, we investigated which subset of CD8+ T cells was involved in this lymphocytopenia. CD8+ T cells from eight patients with influenza A were studied for lymphocyte count, surface marker, and intracellular IFN-γ production in the acute (days 1–3) and recovery phases (days 9–12). Total and T lymphocyte counts in the acute phase were approximately three times less than in the recovery phase; however, the CD4/8 ratio was the same in both phases. The cell count reduction in the acute phase was attributed predominantly to the CD28+ CD8+ subset, compared with the CD28− CD8+ subset. The memory/activation marker CD45RO on the CD8+ T cells was assessed. The CD28+ CD45RO− subset, a naive phenotype, was reduced significantly in number in the acute phase compared with the recovery phase. The CD28+ CD45RO+ subset, a memory phenotype, was also reduced in the acute phase, but the reduction was not statistically significant. Intracellular IFN-γ in the CD8+ subset after mitogenic stimulation was measured by flow cytometry; the percentage of CD28+ IFN-γ−/CD8+ subset in the acute phase was significantly less than in the recovery phase. These results indicated that the predominant reduction of peripheral CD8+ T cells in the acute phase of influenza was from naive-type lymphocytes, suggesting that these quantitative and qualitative changes of CD8+ T cells in influenza are important for understanding the immunological pathogenesis.
Keywords: influenza, CD8, CD28, CD45RO, lymphopenia
INTRODUCTION
Influenza is a representative respiratory tract infection in the winter season. Many infected individuals develop typical influenza symptoms after an incubation period of 1–2 days. Most symptoms generally resolve within a week; however, influenza sometimes causes severe complications, such as pneumonia. Decreased peripheral lymphocyte and leucocyte counts in patients with influenza have been described in the Spanish influenza outbreak that occurred early in the 20th century and are considered a marker of poor prognosis [1]. Experimental or natural infection with influenza virus in humans [2–10] and animals [11–15] resulted in lymphocytopenia, which was observed mainly in active phase (days 1–3) and was related to the severity of the influenza symptoms. T cells, but not NK cells, were involved in lymphocytopenia with impaired proliferative response after stimulation with mitogens [2,4,8]. The recently reported avian influenza A virus (H5N1) infection in Hong Kong caused a severe illness or complications with high morbidity and mortality [16]. The severity of illness in this influenza was reported to be associated with a low peripheral white blood cell count or lymphocyte count at admission. It is also known that other viruses can cause lymphocytopenia, although little is known about the mechanism [2,5,17,18]. Thus, the lymphocytopenia seen in the early phase of influenza seems to be related to the pathogenesis of the influenza and its complications.
T cell antigen CD28 is an important cell surface molecule that provides co-stimulatory signals to promote T cell activation and proliferation, concomitant with signals through T cell receptors [19]. In humans, the majority of peripheral CD4+ T cells express CD28; however, a minor subset of CD8+ T cells lacks expression of this molecule [20]. CD28− CD8+ T cells possess more cytolytic activity and elevated levels of IFN-γ than CD28+ CD8+ T cells [20–22], suggesting that circulating CD28−CD8+ T cells are a terminal effector population differentiated functionally from CD28+ CD8+ T cells [23–27]. Recently, several reports have proposed phenotypic distinctions between naive, memory and effector CD8+ T cells in human blood. The phenotypes of naive, memory and effector CD8+ T cells are CD28+ CD45RO− CD45RA+, CD28+ CD45RO+ CD45RA− and CD28− CD45RO− CD45RA+, respectively [23,28]. Small lymphocytes that have matured in the thymus or bone marrow but that have not yet encountered antigen are referred to as naive lymphocytes. Naive CD8+ T cells circulate continuously from the bloodstream to the lymphoid organs where they can contact the peptide antigen from infected microorganisms presented by the dendritic cell. Once naive CD8+ T cells encounter their specific antigen on the dendritic cells, they proliferate and differentiate to effector or memory cells which have several immunological functions, such as lytic activity and IFN-γ production.
CD8+ T cells play a major role in immune response to influenza virus infection, with the effector CD8+ T cells specific for influenza virus antigens moving to the infection site to clear the viruses. In some chronic or subacute human viral infections, CD28 expression on circulating CD8+ T cells changed [22,29]. To investigate which subset of CD8+ T cells is involved in the lymphocytopenia, we show here that the lymphocytopenia observed in the acute phase in patients with influenza occurs equally in CD4+ and CD8+ T cells, while the main reduction is found in the CD28+ 8+ T cell subset. This population is considered the naive type because it does not express CD45RO and does not produce IFN-γ after mitogenic stimulation.
PATIENTS AND METHODS
Patients
During the winter seasons of 1999/2000 and 2000/2001, eight Japanese patients (four men and four women) diagnosed with influenza A were enrolled in this study (Table 1). Mean age ± s.d. was 31·0 ± 10·6. All patients were negative for antibody to human immunodeficiency virus I. Diagnosis was performed by the detection of influenza A antigen from throat swabs using Directigen Flu A (Becton Dickinson, Mountain View, CA, USA). Influenza virus subtyping was performed later by the isolation of influenza virus cultured with Madin–Darby canine kidney cells, or by a fourfold or greater rise in haemagglutination inhibition antibody titre in paired sera. Informed consent was obtained from the all subjects.
Table 1.
Lymphocytopenia without CD4/8 ratio change in the acute phase of influenza
| Lymphocyte count (μl) | T (CD3+) cell count (μl) | CD4/8 ratio * | ||||||
|---|---|---|---|---|---|---|---|---|
| Case (years, sex) | Max body temperature (°C) | Influenza virus subtype | Acute | Recovery | Acute | Recovery | Acute | Recovery |
| 1 (32, M) | 37·6 | A/H3N2 | 1146 | 3131 | 714 | 2276 | 2·78 | 2·99 |
| 2 (30, F) | 38·3 | A/H3N2 | 470 | 1546 | 250 | 821 | 2·01 | 1·36 |
| 3 (23, M) | 39·1 | A/H3N2 | 550 | 1905 | 362 | 1440 | 1·36 | 1·82 |
| 4 (56, M) | 38·9 | A/H3N2 | 1108 | 2606 | 780 | 1965 | 1·85 | 1·96 |
| 5 (29, F) | 39·0 | A/H3N2 | 657 | 1284 | 458 | 1037 | 2·20 | 2·40 |
| 6 (28, F) | 38·8 | A/H3N2 | 583 | 1813 | 357 | 1401 | 2·40 | 2·42 |
| 7 (27, F) | 39·1 | A/H3N2 | 765 | 2654 | 531 | 2038 | 1·04 | 1·32 |
| 8 (23, M) | 38·5 | A/H1N1 | 910 | 1882 | 706 | 1432 | 1·01 | 1·02 |
| Mean ± s.d. | 38·7 ± 0·52 | 774 ± 257† | 2103 ± 629 | 520 ± 196† | 1551 ± 504 | 1·83 ± 0·65 | 1·91 ± 0·67 | |
Peripheral blood stained with anti-CD3, anti-CD4, and anti-CD8 MoAb was analysed by flow cytometry. The ratios were determined by the formula percentage CD3+ 4+ subset/% CD3+ 8+ subset.
P < 0·01 versus recovery.
Monoclonal antibodies
FITC-conjugated mouse antihuman CD45RO MoAb and PE-conjugated mouse antihuman CD28 MoAb were purchased from Coulter (Miami, FL, USA). FITC-conjugated mouse antihuman CD8 MoAb was purchased from Ortho Diagnostic Systems (Raritan, NJ, USA). Phycoerythrin-cyanin 5·1 (PC5)-conjugated mouse antihuman CD3, CD4 and CD8 MoAbs were purchased from Immunotech (Marseille Cedex, France), and FITC- conjugated mouse antihuman IFN-γ MoAb was purchased from Pharmingen (San Diego, CA, USA).
Phenotypical analysis of lymphocytes and cell counts
For the phenotypical analysis of peripheral lymphocytes, triple immunofluorescence staining was performed with the various antibodies described above. Briefly, 50 μl of whole blood was incubated with MoAbs for 30min at 4°C. Incubated blood samples were lysed in 1ml of lysing reagent (Ortho Diagnostic Systems) for 10min at room temperature, washed twice with PBS/BSA (bovine serum albumin) and analysed by flow cytometry, Cytoron Absolute (Ortho Diagnostic Systems) using ImmunoCount 2 software (Ortho Diagnostic Systems).
The absolute number of lymphocytes was counted by the flow cytometer. Dead cells were excluded by propidium iodide (Wako Pure Chemical Industries, Osaka, Japan) staining.
Detection of intracellular IFN-γ
Whole blood was diluted at 1:3 with complete medium (RPMI 1640 supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin and 10% heat-inactivated fetal calf serum), all purchased from Gibco BRL (Rockville, MD, USA). Diluted blood (1ml) was cultured in a 24-well culture plate with 50ng/ml of PMA (Sigma, St Louis, MO, USA) and 1μg/ml of ionomycin (Sigma) in the presence of 1μl/ml of protein-secretion inhibitor GolgiPlug (Pharmingen) containing Brefeldin A, then incubated at 37°C in 5% CO2 for 4h.
The fixation, permeabilization and intracellular cytokine staining of cultured cells were performed with CytoStain Kits (Pharmingen) according to the manufacture’s instructions. Briefly, cultured blood samples were harvested and lysed in 10ml of 1 × pharm lyse solution for 10min at room temperature and washed twice with PBS (Nissui Chemical Industries, Tokyo, Japan) with 0·5% BSA. Cells were stained with both anti-CD8-PC5 and anti-CD28-PE MoAbs and incubated for 15min at 4°C. After staining with MoAbs, cells were fixed and permeabilized by addition of 500μl of cytofix/cytoperm solution and incubated for 20 at 4°C. Cells were washed by addition of 1 ml of 1 × perm/wash solution, incubated for 10 min at room temperature and resuspended with 100 μl of 1 × perm/wash solution. Cells were stained with both anti-IFN-γ MoAbs for 30 min at 4°C. After the wash with PBS/BSA, cells were analysed by three-colour flow cytometric analysis.
Statistics
Parametric analysis (paired Student’s t-test) was used to determine significance of difference. A P-value less than 0·01 was considered significant.
RESULTS
Lymphocytopenia without change of CD4/8 ratio in the acute phase of influenza
The subjects in the present study (Table 1) were diagnosed with influenza A, showing typical influenza symptoms in the acute phase (days 1–3) and no severe complications of influenza in the recovery phase (days 9–12). The influenza subtype was A/H3N2 in all except case 8 (A/H1N1). Total lymphocyte counts in peripheral blood were significantly reduced in the acute phase of influenza (Table 1). Mean lymphocyte counts in the acute and recovery phase were 774/μl and 2103/μl, respectively. Mean T lymphocyte (CD3+ cell) count in the acute phase was also lower, to 520/μl, than in the recovery phase (1551/μl). However, flow cytometric analysis showed that the mean CD4/8 ratio in the acute phase did not change significantly compared with the recovery phase (1·83 versus 1·91).
The reduction of CD28+ CD8+ T cell number in the acute phase of influenza
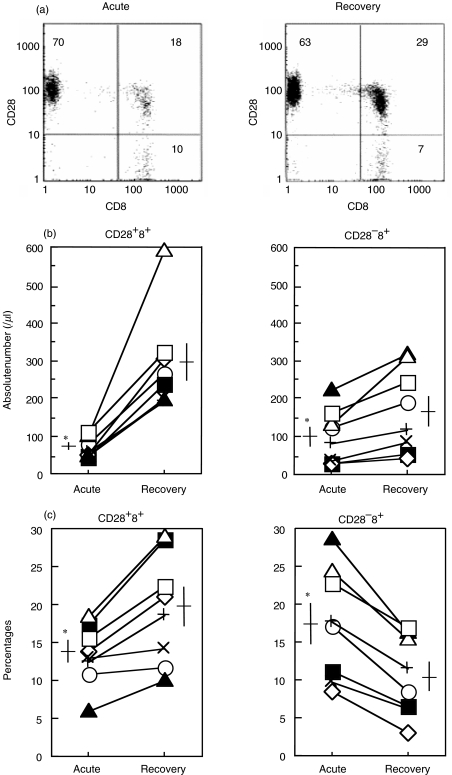
As CD8+ T cells play an important protective role in influenza virus infection we focused on CD28 expression, a differentiation marker, of the peripheral CD8+ T cells from each subject with influenza (Fig. 1a). Flow cytometric analysis showed that a reduction of cell count in the acute phase was seen predominantly in the CD28+ CD8+ population (Fig. 1b). Mean counts in the acute and recovery phases were 66/μl and 287/μl, respectively. The CD28− CD8+ T cell population was also reduced in number, but its reduction was much less than the CD28+ CD8+ population. This reduction was more prominent when the CD28+ CD8+ cells were displayed by the percentages among the CD3+ cells (Fig. 1c). The percentage of the CD28+ CD8+ subset was reduced in the acute phase (mean; 13·3%) and increased in the recovery phase (19·4%). Conversely, the CD28− CD8+ subset was increased in the acute phase (17·5%) and reduced in the recovery phase (10·5%).
Fig. 1.
Peripheral CD28+ CD8+ T cell subset reduction in the acute phase of influenza. Peripheral blood from eight influenza patients in the acute and recovery phases was stained with anti-CD3, CD8 and CD28 MoAb, then analysed by flow cytometry. (a) The representative cytogram pattern gated in CD3+ (T cell) lymphocytes from a patient with influenza (case 2 in Table 1). (b) The absolute number of CD28+ 8+ and CD28− 8+ cells and (c) percentages of CD28+ 8+ and CD28− 8+ cells in the CD3+ subset. In (b) and (c), symbols indicate the patient number from Table 1. Horizontal and vertical bars indicate mean values and standard errors, respectively. *P < 0·01 versus recovery phase. ○, 1; ▪, 2; ◊, 3; ▴, 4; +, 5; ×, 6; ▵, 7; □, 8.
Specific reduction of peripheral CD8+ T cells of the naive phenotype in the acute phase of influenza
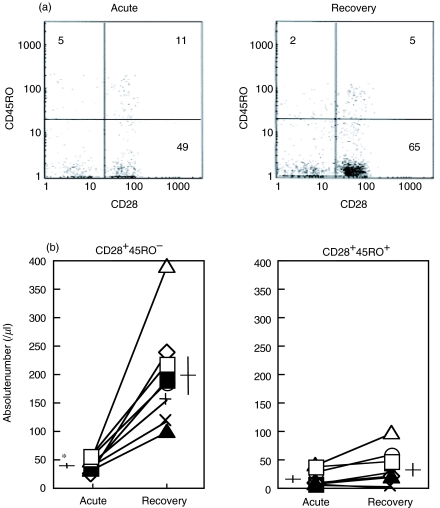
To clarify the maturation process of peripheral CD8+ T cells, we next assessed the activation marker, CD45RO, in the CD28+ 8+ T cells from the influenza patients. Recent studies subdivided the human CD8+ T cells into CD28+ CD45RO−, CD28+ CD45RO+ and CD28− CD45RO− populations, which represent naive, memory and effector CD8+ T cells, respectively [21,29]. Figure 2a shows the representative cytogram pattern gated in CD8high population analysed by flow cytometry in the acute and recovery phases of influenza. The naive phenotype (CD28+ CD45RO−) in CD8+ cells were significantly lower in number in the acute phase of influenza (mean; 41/μl) than in recovery phase (200/μl) (Fig. 2b). The memory phenotype (CD28+ CD45RO+) CD8+ cells were also lower in number in the acute phase (mean; 18/μl) than in recovery phase (34/μl), but this was not statistically significant (Fig. 2b). Values of CD8+ T cell subsets observed in the recovery phase are not different from those obtained on healthy persons in the same range (data not shown).
Fig. 2.
Reduction of the peripheral CD28+ CD45RO− subset in CD8+ T cells in the acute phase of influenza. Peripheral blood from eight influenza patients in the acute and recovery phases was stained with anti-CD8, CD28 and CD45RO MoAb and analysed by flow cytometry. (a) Representative cytogram pattern gated in CD8high lymphocytes from a patient with influenza (case 2 in Table 1). (b) Absolute number of CD28+ 45RO− and CD28+ 45RO+ cells. Symbols indicate the patient number from Table 1. Horizontal and vertical bars indicate mean values and standard errors, respectively. *P < 0·01 versus recovery phase. ○, 1; ▪, 2; ◊, 3; ▴, 4; +, 5; ×, 6; ▵, 7; □, 8.
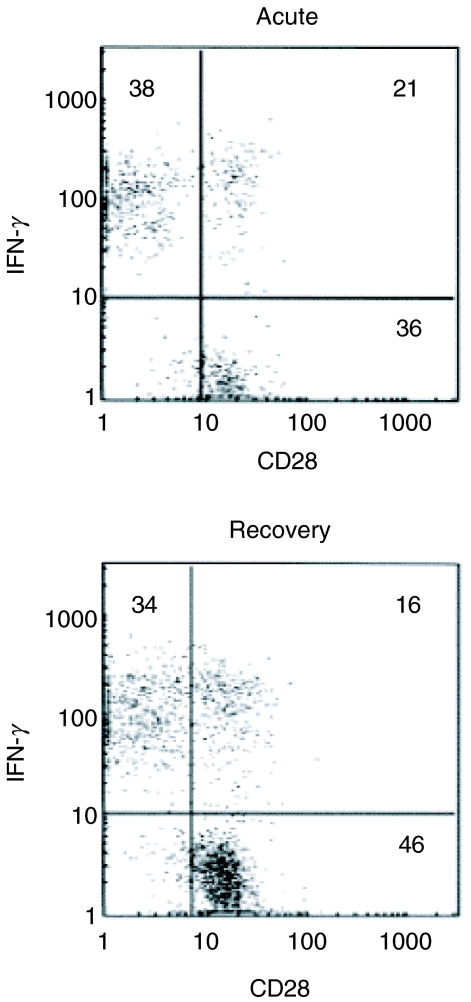
Memory and effector CD8+ T cells are activated quickly and produce cytokines such as IFN-γ and TNF-α after antigenic stimulation. On the other hand, naive CD8+ T cells take several days to be activated and to proliferate [30]. To characterize these CD8+ T cells further, the IFN-γ-producing capacity of CD28+ CD8+ T cells in influenza patients was investigated. In seven of eight patients, intracellular IFN-γ in CD28+ CD8+ cells after stimulation with mitogens was stained and measured by flow cytometry in the acute and recovery phases of influenza. The representative cytogram pattern is shown in Fig. 3. CD28+ IFN- γ−, CD28− IFN-γ+ and CD28+ IFN-γ+ populations were thought to be naive, effector and memory type cells, respectively. The mean percentages of CD28+ IFN-γ− cells in CD8+ cells in the acute phase were significantly lower than in recovery phase (24·1%versus 40·9%) (Table 2). Conversely, the mean percentages of CD28− IFN-γ+ cells in CD8+ cells in the acute phase were higher than in the recovery phase (54·5%versus 38·5%). The percentages of CD28+ IFN-γ+ CD8+ cells was not different between phases (14·7%versus 15·9%). These results indicated that the reduced population of CD8+ T cells in the acute phase of influenza is the naive type.
Fig. 3.
Flow cytometric pattern of intracellular IFN-γ in CD28+ 8+ cells in influenza. Peripheral blood cells from patients with influenza were cultured for 4h with PMA, ionomycin and brefeldin A. Cultured cells were then stained with anti-CD8 and CD28 MoAb. After fixation and permeabilization, intracellular IFN-γ was stained with antihuman IFN-γ MoAb and analysed by flow cytometry. The representative cytogram pattern is shown (case 2).
Table 2.
Reduced frequency of peripheral CD28+ 8+ lymphocytes without the capacity for IFN-γ production in the acute phase of influenza
| CD28+ IFN-γ− (%)* | CD28− IFN-γ+ (%) | CD28+ IFN-γ+ (%) | ||||
|---|---|---|---|---|---|---|
| Case | Acute | Recovery | Acute | Recovery | Acute | Recovery |
| 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 35·6 | 45·6 | 37·9 | 33·9 | 21·1 | 16·3 |
| 3 | 23·7 | 51·5 | 48·7 | 21·3 | 22·7 | 17·6 |
| 4 | 6·8 | 15·3 | 84·5 | 66·5 | 5·3 | 18·1 |
| 5 | 13·1 | 35·5 | 67·6 | 46·7 | 12·7 | 16·8 |
| 6 | 26·3 | 50·7 | 52·6 | 28·6 | 15·7 | 16·1 |
| 7 | 34·5 | 46·2 | 35·2 | 31·4 | 18·3 | 14·2 |
| 8 | 28·5 | 41·2 | 54·9 | 41·2 | 7·2 | 12·0 |
| Mean ± s.d. | 24·1 ± 10·7† | 40·9 ± 12·5 | 54·5 ± 17·1† | 38·5 ± 14·9 | 14·7 ± 6·7 | 15·9 ± 2·1 |
Whole blood was cultured with PMA, ionomycin and protein-secretion inhibitor, then incubated at 37°C for 4 h. After staining with anti-CD8 and anti-CD28 MoAb, cells were fixed, permeabilized and stained with anti-IFN-γ MoAb, then analysed by three-colour flow cytometric analysis. n.d.: not done.
P < 0·01 versus recovery.
DISCUSSION
T lymphocytopenia in influenza has been documented elsewhere, but which T cell subset is involved predominantly is unknown. In the present study, the T lymphocytopenia was observed equally in CD4 and CD8+ cells without a change of CD4/8 ratio, and circulating CD28+ CD8+ T cells were reduced in number compared with CD28− CD8+ T cells during the active phase of influenza. In CD28+ CD8+ T cells, the predominant reduction was observed in the CD45RO− or IFN-γ−non-producing populations, indicating that these CD8+ T cells are the naive type. In all the cases studied, lymphocytopenia was observed in the acute phase (days 1–3) and recovered in the late phase (days 9–12).
In addition to influenza, several human viral infections also induce transient lymphocytopenia [2,5,17,18]. These infectious diseases have the following characteristics; (1) lymphocytopenia occurrs in the early phase of the disease, (2) acute and transient infection with systemic illness such as high fever and (3) more and long-lasting lymphocyte reduction related to the severity of illness. It is uncertain whether a common mechanism underlying the lymphocytopenia among these viral infectious diseases exists.
A relation between lymphocytopenia and the severity of influenza has been widely accepted [1,3,5,10,16]. The severity of influenza depends mainly on its complications. The most common complication is influenza pneumonia, which is seen often in older people, and patients with cardiovascular or pulmonary disease. As a possible mechanism of the development of complicated pneumonia from influenza, it is tempting to speculate that immunosuppression may be caused by lymphocytopenia and suppressed lymphocyte function. A reduction in the number of naive type CD8+ T cells in the periphery in the acute phase of influenza may cause a dysfunction or a delay in acquiring immunity to the pathogens. Thus, lymphocytopenia during the active phase of illness has diagnostic and prognostic significance.
The mechanism of this transient reduction of naive CD8+ T cells (CD45RO− CD28+ IFN-γ−) in peripheral blood is not understood clearly. There are several possible explanations for the reduction of CD28+ CD8+ T cells of the naive phenotype in influenza. First, the distribution of the CD8+ T cell pool in the body may change after infection. Naive T cells circulate continuously from the bloodstream to lymphoid organs, where they contact the thousands of antigens presented by antigen- presenting cells. The migration of peripheral lymphocytes to the lymphoid organ is called homing. It is probable that homing of naive T cells to lymphoid organs after infection causes the reduction of naive CD28+ CD8+ T cells from peripheral blood in the active phase of influenza. In a murine model of influenza, naive and resting memory CD8+ T cells accumulated first in the draining lymph nodes after influenza virus infection [31,32], suggesting the homing of naiive CD8+ T cells to the lymph nodes close to the infection site. Blocking the homing receptor, l-selectin, in mice infected with influenza virus thereafter suppressed the expansion of influenza-specific CD4+ and CD8+ T cells in the draining lymph nodes [31]. These observations suggest that the supply of naive lymphocytes to lymphoid organs by homing is a crucial step in inducing the antivirus effector lymphocytes. Alternatively, since inflammation can induce vascular homing receptors in lung parenchyma, homing of naive lymphocytes to inflammatory sites may also occur in influenza virus infection [33]. In a murine model of influenza, lymphocytes infiltrating the lung included a minor population of the naive phenotype [34].
Secondly, the influenza virus may induce cell death, predominantly in naive CD8+ T cells. Animal models of influenza virus [14] or other virus [35] infection were reported to result in the severe destruction of lymph nodes accompanied by the existence of infectious viruses and lymphocytopenia in peripheral blood, although influenza virus with less virulence did not [13]. Recently, in vivo[36] and in vitro[37] infection with influenza virus induced Fas-dependent apoptosis of lymphocytes [38]. Viruses were cleared through apoptosis-dependent phagocytosis by macrophages in vitro[39], suggesting that the apoptosis triggered by the viruses play an important physiological role in eliminating the viruses. However, direct cell death or apoptosis did not explain the difference between CD28+ and CD28-CD8+ T cell reduction, and there is no evidence that influenza virus can infect CD8+ T cells. Direct cell death may occur in the CD28+ subset rather than in the CD28− subset. Indeed, apoptotic cell death by stimulation in culture was reported to have been seen more often in the CD28+ subset than in the CD28− subset in human [24,25]. Whether CD28− CD8+ T cells are resistant to apoptotic cell death in influenza virus infection in vivo is uncertain. It is quite possible that cell death documented in naive lymphocytes in draining lymph nodes was followed by a reduction of peripheral naive CD8+ T cells during influenza virus infection. Further experiments detecting Fas-expression and DNA fragmentation in circulating CD28+ CD8+ T cells are needed.
Finally, the possibility exists that the down-regulation of CD28 is induced by the influenza virus infection. Several reports have demonstrated that CD28− CD8+ T cells in peripheral blood matured from the CD28+ subset in humans [23,24,27,28]. Human ageing [40] and HIV or EBvirus infection [22,29] have been associated with a decrease of CD28+ CD8+ T cells and an increase of CD28− CD8+ T cells. Some investigators have hypothesized that lymphocytes in aged people and patients with chronic infections were exposed and activated by repeated antigenic stimulation and set in the immune senescent state [23,27]. This state is characterized by an increased number of CD28− CD8+ T cells in the periphery. However, the predominance of peripheral CD28− CD8+ T cells in influenza patients could not be due to immune senescence, because the absolute count of peripheral CD28- CD8+ T cells was decreased slightly in number. For an alternative explanation, the influenza virus may prevent CD28 expression by affecting membrane metabolism.
It is uncertain whether cytokines are related to this lymphocytopenia. We assessed serum IFN-α, IFN-γ, IL-2 and IL-4 by standard ELISA method; however, these cytokines were not detected in all the samples from influeza subjects. A high concentration of cytokines localized in the infection site or draining lymph nodes may influence the reduction of lymphocyte number. Our findings of a reduced population of T lymphocytes in the acute phase of influenza is limited to CD8+ T cells. We were unable to analyse the CD4+ T cells as the great majority of CD4+ T cells expressed CD28 and the positivity of CD28 did not undergo a drastic change during the clinical course of influenza. Further studies are needed to determine populational changes in circulating CD4+ T cells in the acute phase of influenza. Quantitative and qualitative changes of T lymphocytes in influenza are important for understanding the immunological pathogenesis of this disease and providing for successful disease control.
REFERENCES
- 1.Whittingham HE, Sims C. Bacteriology and pathology of influenza. Lancet. 1918;CXCV:865–71. [Google Scholar]
- 2.Casali P, Rice GP, Oldstone MB. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984;159:1322–37. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criswell BS, Couch RB, Greenberg SB, Kimzey SL. The lymphocyte response to influenza in humans. Am Rev Respir Dis. 1979;120:700–4. doi: 10.1164/arrd.1979.120.3.700. [DOI] [PubMed] [Google Scholar]
- 4.Dolin R, Richman DD, Murphy BR, Fauci AS. Cell-mediated immune responses in humans after induced infection with influenza A virus. J Infect Dis. 1977;135:714–9. doi: 10.1093/infdis/135.5.714. [DOI] [PubMed] [Google Scholar]
- 5.Douglas RGJr, Alford RH, Cate TR, Couch RB. The leukocyte response during viral respiratory illness in man. Ann Intern Med. 1966;64:521–30. doi: 10.7326/0003-4819-64-3-521. [DOI] [PubMed] [Google Scholar]
- 6.Faguet GB. The effect of killed influenza virus vaccine on the kinetics of normal human lymphocytes. J Infect Dis. 1981;143:252–8. doi: 10.1093/infdis/143.2.252. [DOI] [PubMed] [Google Scholar]
- 7.Jarstrand C, Wasserman J, Dahl G. Peripheral blood lymphocyte populations in influenza patients. Scand J Infect Dis. 1977;9:1–3. doi: 10.3109/inf.1977.9.issue-1.01. [DOI] [PubMed] [Google Scholar]
- 8.Lewis DE, Gilbert BE, Knight V. Influenza virus infection induces functional alterations in peripheral blood lymphocytes. J Immunol. 1986;137:3777–81. [PubMed] [Google Scholar]
- 9.Scheinberg M, Blacklow NR, Goldstein AL, Parrino TA, Rose FB, Cathcart ES. Influenza. response of T-cell lymphopenia to thymosin. N Engl J Med. 1976;294:1208–11. doi: 10.1056/NEJM197605272942204. [DOI] [PubMed] [Google Scholar]
- 10.Smorodintseff AA, Tushinsky AI, Korovin AA. Investigation of volunteers with the influenza virus. Am J Med Sci. 1937;194:159–70. [Google Scholar]
- 11.Harris S, Henle W. Lymphocytopenia in rabbits following intravenous injection of influenzal virus. J Immunol. 1948;59:9–20. [PubMed] [Google Scholar]
- 12.Kurokawa M, Ishida S, Asakawa S, Iwasa S, Goto N, Kuratsuka K. Toxicities of influenza vaccine: peripheral leukocytic response to live and inactivated influenza viruses in mice. Jpn J Med Sci Biol. 1975;28:37–52. doi: 10.7883/yoken1952.28.37. [DOI] [PubMed] [Google Scholar]
- 13.Van Campen H, Easterday BC, Hinshaw VS. Virulent avian influenza A viruses: their effect on avian lymphocytes and macrophages in vivo and in vitro. J Gen Virol. 1989;70:2887–95. doi: 10.1099/0022-1317-70-11-2887. [DOI] [PubMed] [Google Scholar]
- 14.Van Campen H, Easterday BC, Hinshaw VS. Destruction of lymphocytes by a virulent avian influenza A virus. J Gen Virol. 1989;70:467–72. doi: 10.1099/0022-1317-70-2-467. [DOI] [PubMed] [Google Scholar]
- 15.Woodruff JJ, Woodruff JF. Influenza A virus interaction with murine lymphocytes. I. The influence of influenza virus A/Japan 305 (H2N2) on the pattern of migration of recirculating lymphocytes. J Immunol. 1976;117:852–8. [PubMed] [Google Scholar]
- 16.Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 17.Hillenbrand FKM. The blood picture in rubella: its place in diagnosis. Lancet. 1956;2:66–8. doi: 10.1016/s0140-6736(56)90427-5. [DOI] [PubMed] [Google Scholar]
- 18.Johnson ES, Napoli VM, White WC. Colorado tick fever as a hematologic problem. Am J Clin Pathol. 1960;34:118–24. doi: 10.1093/ajcp/34.2.118. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 20.Azuma M, Phillips JH, Lanier LL. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 21.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vingerhoets JH, Vanham GL, Kestens LL, et al. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin Exp Immunol. 1995;100:425–33. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nociari MM, Telford W, Russo C. Post-thymic development of CD28− CD8+ T cell subset: age-associated expansion and shift from memory to naive phenotype. J Immunol. 1999;162:3327–35. [PubMed] [Google Scholar]
- 24.Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28− cytotoxic effector clones. Int Immunol. 1999;11:229–41. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 25.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34:633–44. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 26.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8 (+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–13. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 27.Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000;121:161–71. doi: 10.1016/s0047-6374(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 28.Hamann D, Kostense S, Wolthers KC, et al. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–33. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 29.Roos MT, van Lier RA, Hamann D, et al. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein–Barr and human immunodeficiency virus infections in humans. J Infect Dis. 2000;182:451–8. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 30.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 31.Tripp RA, Topham DJ, Watson SR, Doherty PC. Bone marrow can function as a lymphoid organ during a primary immune response under conditions of disrupted lymphocyte trafficking. J Immunol. 1997;158:3716–20. [PubMed] [Google Scholar]
- 32.Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol. 1999;163:5535–43. doi: 10.4049/jimmunol.163.10.5535. [DOI] [PubMed] [Google Scholar]
- 33.Berman JS, Beer DJ, Theodore AC, Kornfeld H, Bernardo J, Center DM. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990;142:238–57. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- 34.Tripp RA, Hou S, McMickle A, Houston J, Doherty PC. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–21. [PubMed] [Google Scholar]
- 35.Woodruff JF, Woodruff JJ. Virus-induced alterations of lymphoid tissues. I. Modification of the recirculating pool of small lymphocytes by Newcastle disease virus. Cell Immunol. 1970;1:333–54. doi: 10.1016/0008-8749(70)90053-5. [DOI] [PubMed] [Google Scholar]
- 36.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76:2869–73. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 37.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J General Virol. 1993;74:2347–55. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa T, Fukuda R, Miyawaki T, Ohashi K, Nakanishi Y. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology. 1995;209:288–96. doi: 10.1006/viro.1995.1260. [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–403. doi: 10.1128/jvi.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effros RB, Boucher N, Porter V, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994 1918;29:601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]