Abstract
Eosinophils and neutrophils are two different types of granulocytes evolved from a common haematopoetic precursor in the bone marrow. Eosinophils are mainly involved in parasitic infection and allergic inflammation while neutrophils mainly participate in the defence against bacterial infections. Prolongation of granulocyte life span by inhibition of apoptosis may lead to tissue load of cells, and this has been detected in different inflammatory reactions. The molecular mechanisms and the potential role of the mitochondria in granulocyte apoptosis are poorly understood. In the present study we have characterized further the role of the mitochondria in granulocyte-apoptosis by studying the sequence of mitochondrial permeability transition (MPT) induction, loss of mitochondrial membrane potential (Δψm) and release of cytochrome c. This was made possible by applying tributyltin (TBT), a well-characterized apoptotic stimulus and MPT-inducer. We also studied potential differences in apoptosis-susceptibility between eosinophils and neutrophils. Ten minutes of TBT-exposure resulted in a substantial caspase-3 activity in both eosinophils and neutrophils, followed by phosphatidylserine (PS)-exposure after 30–120 min. Interestingly, caspase-3 activity was not preceded by MPT-induction, loss of Δψm or by cytochrome c-release in either eosinophils or neutrophils. In conclusion, we have demonstrated an extremely rapid induction of caspase-3 activity and apoptosis in human blood granulocytes without prior mitochondrial changes, including loss of mitochondrial membrane potential and release of cytochrome c. Our results open the possibility for a mitochondrial-independent activation of caspase 3 and subsequent apoptosis in granulocytes.
Keywords: caspase 3, cytochrome c, mitochondrial membrane potential, mitochondrial, permeability transition, tributyltin
INTRODUCTION
Eosinophils and neutrophils are two different types of granulocytes, which are evolved from a common haematopoetic precursor in the bone marrow. Both cell types are key effector cells in host defence against infections and they contain and can, upon activation, secrete a large and varied armoury of cytotoxic and degenerative enzymes [1,2]. Eosinophils are most commonly involved in parasitic infections and allergic inflammation, where eosinophilia is a prevalent indication of late-phase allergic inflammation causing tissue damage and asthma severity [3]. Neutrophils, on the other hand, are mainly involved in bacterial infections and other inflammatory reactions [4].
To maintain cellular homeostasis, granulocyte tissue-infiltration is normally followed by elimination of these cells by induction of apoptosis. Different inflammatory mediators, e.g. GM-CSF, complement factor C5a and LPS [5] can modulate the apoptotic process in both eosinophils and neutrophils. Cytokines such as IL-5 and IL-3 have been proposed to prolong the eosinophil life span selectively by inhibiting the apoptotic machinery and thus contribute to tissue loading of eosinophils [6]. Differences in apoptosis-susceptibility between eosinophils and neutrophils have been reported previously during corticoid treatment, which has been employed for many years as a potent anti-inflammatory agent [7,8]. One reported effect of dexamethasone is the rapid and dramatic induction of apoptosis of eosinophils, while the number of neutrophils increased [7,9] indicating differences in the mechanisms regulating the apoptotic machinery between the granulocytes.
In general the molecular mechanisms of granulocyte apoptosis are poorly understood, and only recently were the caspases demonstrated to be involved in eosinophil-apoptosis [10,11]. The caspase family of proteases plays a crucial role in apoptosis [12–14], where they cleave a number of defined substrates at aspartate residues, leading to the irreversible dismantling of the cell. In a number of apoptotic models, the caspases are activated via the mitochondrion by the induction of mitochondrial permeability transition (MPT) and the release of cytochrome c [15,16]. Recently, it was reported that eosinophils contain a small number of mitochondria-like structures, lacking functional respiration, but with a suggested role to participate in the apoptotic process [17].
Tributyltin (TBT) is a potent inducer of caspase activation and apoptosis in human leukaemia T cell lines and human peripheral T lymphocytes [18–22]. The caspase activation is preceded by early mitochondrial changes, including rapid loss of mitochondrial membrane potential (Δψm) and release of cytochrome c. Preincubation with the adenine nucleotide translocator (ANT)-inhibitor, bongkrekic acid (BA), blocked TBT-induced mitochondrial changes and apoptosis in Jurkat cells, suggesting that TBT induces mitochondrial permeability transition (MPT) specifically in these cells [21]. Initially, TBT was studied in the area of bioenergetics for its property of being an inhibitor of the mitochondrial ATP production via a direct interaction with the F0F1-ATP synthase [23–25]. Thus, TBT is an excellent example of a mitochondrial toxin, which can both trigger apoptosis via the mitochondrial pathway and block mitochondrial ATP production.
The present study aimed to characterize further the role of the mitochondrion in granulocyte-apoptosis as well as to investigate potential differences in apoptosis-susceptibility between eosinophils and neutrophils by applying TBT.
MATERIALS AND METHODS
Study material
Blood from healthy donors was collected in citrate tubes (Vacutainer, 5 ml, with 1 ml 0·129 m 9NC, Becton Dickinson, San Jose, CA, USA). The subjects gave their informed consent to participate in the study, which was approved by the Ethics Committee of the Karolinska Hospital, Stockholm, Sweden.
Purification of eosinophils and neutrophils
Blood was purified by Percoll centrifugation. The erythrocytes in the granulocyte pellet were haemolysed with + 4°C isotonic NH4Cl-EDTA lysing solution (154 mm NH4Cl, 10 mm KHCO3, 0·1 mM EDTA, pH 7·2) and incubated for 5 min at +15°C. To obtain purified eosinophils, a magnetic cell separation system MidiMACS (Miltenyi, Biotec, Bergisch, Gladbach, Germany) was used [26]. Cells were washed before being counted in a flow cytometer (see below). The purity of separated eosinophils and neutrophils was >95%.
Purification of lymphocytes
Blood from healthy donors was collected as described above, and purified by Percoll centrifugation. The mononuclear cells from the interface were collected and washed twice with 40 ml PBS and centrifuged for 12 min at 300 g and 400 g, respectively. The mononuclear cells were then resuspended in hepes (10 mm)-buffered RPMI 1640 medium (Gibco Ltd, Paisly, Renfrewshire, UK) supplemented with 40% heat inactivated calf serum in a concentration of 1 million cells per ml. Cells were then incubated in a tissue culture flask for 40 min at + 37°C. Under these conditions the monocytes adhere to the plastic and the lymphocytes can be collected after incubation. Lymphocytes were washed once in 40 ml PBS and centrifuged for 12 min at 300 g before being counted in a flow cytometer (see below). The purity of separated lymphocytes was >95%.
Induction of apoptosis
Purified eosinophils or neutrophils were incubated with HEPES-buffered RPMI 1640 medium (Gibco Ltd) supplemented with 10% heat inactivated calf serum. The death signal was given by 2 μm TBT (Sigma-Aldrich Chemical Co, St Louis, MO, USA) and cells were exposed for 0–4h at + 37°C, 5% CO2.
Caspase 3 activity
Caspase activity in intact eosinophils or neutrophils was measured by using the cell permeable substrate PhiPhiLux-G2D2 (OncoImmunogen, Inc., Gaithersburg, MD, USA) containing the prototypical sequence DEVDG.
A minimum of 100 000 cells/tube were incubated with 10 μm PhiPhiLux and 10% heat inactival calf serum at + 37°C, 5% CO2 for 60 min according to the manufacturer’s instruction. Cells were then analysed in the FL-2 channel of a flow cytometer (see below).
Annexin-V and propidium iodide staining
One way of identifying apoptotic cells is to detect phosphatidylserine (PS), which is exposed on the outer leaflet of the cell membrane in apoptotic cells. Granulocytes were therefore analysed for their exposure of PS, determined by Annexin V staining. Cell permeability was determined by propidium iodide (PI) incorporation. The double staining with Annexin V and PI gives an opportunity to distinguish between apototic and necrotic cells.
A minimum of 100 000 cells were incubated on ice with cold Annexin binding buffer, PI (Beckman Coulter, Inc., Fullerton, CA, USA) and Annexin V (Beckman Coulter) according to the manufacturer’s instructions. The cells were then analysed by flow cytometry (see below).
Mitochondrial membrane potential
To measure the mitochondrial membrane potential (Δψm) in granulocytes, we employed the fluorescent dye tetramethylrhodaminutese (TMRE) (Molecular Probes, Eugene, OR, USA), that accumulates in the inner mitochondrial membrane according to the Δψm. A loss of Δψm can be monitored by flow cytometry as a decrease in FL-2, expressed in a log scale. Briefly, eosinophils or neutrophils (1 million cells/ml) were preincubated with 20 nm TMRE at +37°C, 5% CO2 for 15 min before being exposed to TBT (2 μm). The Δψm was measured every 5 min for 20 min. TBT-treated purified lymphocytes were used as positive control cells, as it has been described previously that TBT reduces the Δψm in these cells [18]. The granulocytes were also tested for their response to the mitochondrial uncoupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (Sigma Chemical Co, St Louis, MO, USA), a well-characterized agent known to dissipate Δψm in many cell types. Eosinophils, neutrophils and lymphocytes were exposed to CCCP (10 μm) and Δψm was monitored every 5 min for 20 min by flow cytometry.
In some experiments, granulocytes were exposed to both TBT (2 μm) and CCCP (10 μm). The Δψm was measured as described above.
Flow cytometric analysis
The different leucocyte preparations were analysed and counted in an EPICS XL flow cytometer (Beckman Coulter). The cells were distinguished by their different light scatter properties; forward scatter (FS) reflects the cell size and side scatter (SS) reflects the complexity/granularity.
The instrument was calibrated daily with standardized 10 mm fluorospheres, Flow-Check (Beckman Coulter). Flow-set (Beckman Coulter), another fluorosphere with controlled fluorescence intensity, was used to standardize the mean fluorescence intensity (MFI) before each experiment. Unstimulated cells (cells incubated with RPMI alone) were used to define the cut off level for positively stained cells.
Morphological analysis of eosinophils
Smears for morphological studies were prepared by cytocentrifugation at 400 rpm for 3 min in a Cytospin 3 Cytocentrifuge (Shandon Scientific Ltd, UK). The cells were stained with May–Grünwald–Giemsa and the morphology was analysed with light microscopy (Nikon Eclipse E400).
Mitotracker Red CMX Ros and cytochrome c double-labelling
Eosinophils, neutrophils and lymphocytes (control cells) were purified and stained with Mitotracker CMX Ros (75 nm) for mitochiondrial localization (Molecular Probes) for 45 min at + 37°C, 5% CO2, washed and followed by exposure to TBT (2 μm) for 0, 10, 30 or 60 min at + 37°C, 5% CO2. The cells were then separated into different test tubes before treated according to a fixation and permeabilization technique, the FOG-method [27]. Cells were then incubated with 100 μl 0·1% BSA-c (Aurion, Seligenstadt, Germany) for 30 min on ice before incubation with an antibody to cytochrome c (mouse IgG1) (Pharmigen, San Diego, CA, USA) or an irrelevant control antibody (mouse IgG1) (Dako A/S, Denmark) for 30 min on ice. Cells were washed again and incubated with a secondary fluorescent antibody Alexa fluor 488 (goat-antimouse) (Molecular Probes) for 30 min on ice. Finally, cells were washed and smears for fluorescent studies were prepared by cytocentrifugation as described above and mounted with flouromount (Dako A/S, Denmark).
Fluorescence studies were performed by confocal laser microscopy (Leica DM IRBE).
Statistics
The results are expressed as median (interquartile range). Statistical evaluation was performed within groups using the non-parametric methods Wilcoxon paired test. Differences were considered statistically significant at P < 0·05.
RESULTS
Caspase activity
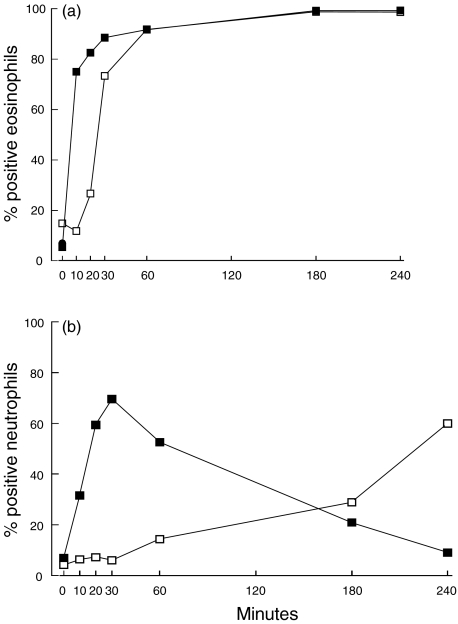
Purified eosinophils were incubated with TBT (2 μm) for up to 240 min and assayed for caspase activity by measuring cleavage of the caspase 3 substrate PhiPhiLux. TBT-exposure induced a time-dependent induction of caspase activity in both eosinophils and neutrophils. After only 10 min of treatment with 2 μm TBT, 75·0% (58·6–91·4) of the eosinophils possess caspase activity, and after 30 min 88·6% (63·9–99·4) were positive (n = 7) (Fig. 1a). The percentage of PhiPhiLux positive eosinophils incubated with RPMI alone was 5·7% 3·7–6·2) across the time-course (n = 17).
Fig. 1.
The percentage of positively stained eosinophils (a) or neutrophils (b) for caspase 3 activity and Annexin binding after incubation with TBT (2 μm) at different time periods. Results are expressed as median value. ▪, Neutrophils PhiPhiLux; □, neutrophils Annexin.
An increased caspase activity was also detected in 31·6% (19·1–68·4) of the neutrophils after 10 min of exposure to 2 μm TBT (n = 3), and in 69·6% (36·8–88·0) after 30 min of incubation (n = 6). (Fig. 1b). Approximately 8·2% (3·1–12·3) of the neutrophils incubated with RPMI alone stained positive with PhiPhiLux across the time-course (n = 7).
Morphological changes associated with apoptosis
To investigate whether the caspase activity occurred concurrently with other apoptotic changes, purified eosinophils were exposed to TBT (2 μm) up to 240 min before being monitored for their exposure of PS, determined by Annexin V staining or May–Grünwald–Giemsa staining for detection of condensed nucleus. Approximately 6·8% (4·9–18·8) of the eosinophils stained positive for Annexin after 10 min of incubation with TBT (n = 4). After 30 min the percentage Annexin-positive eosinophils had increased to 85·3% (53·4–93·9) and after 60 min the positively stained cells had increased further to 92·9% (89·4–95·6) (n = 7) (Fig. 1a). Prolongation of the exposure time for up to 240 min did not result in any additional apoptotic cells; instead the apoptotic cells underwent secondary necrosis (n = 3) (Fig. 1a). The percentage of Annexin-positive cells incubated with RPMI alone was 12·7% (7·4–14·7) across the time-course (n = 15).
After 30 min of exposure to TBT, 4·2% (2·8–5·8) of the neutrophils stained positive for Annexin, and after 60 min 9·2% (2·9–14·1) were positive (n = 6). The percentage of Annexin-positive neutrophils continued to increase to 54·1% (50·4–75·1) after 240 min of incubation (n = 3) (Fig. 1b). Only 3·3% (2·1–4·5) of the neutrophils incubated with RPMI alone stained positive with Annexin across the time-course (n = 9).
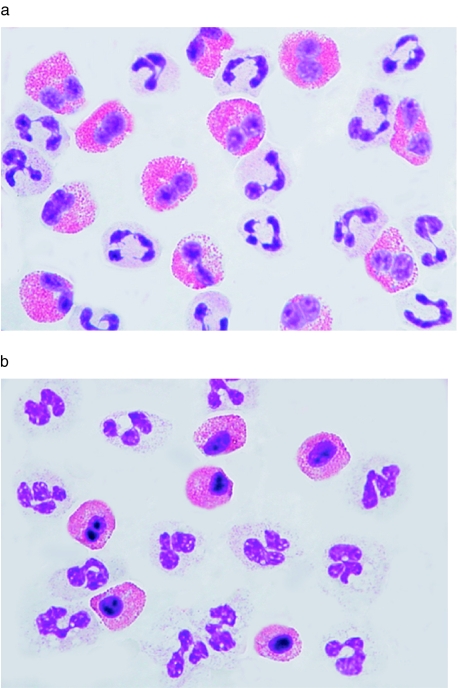
Purified eosinophils and neutrophils incubated either with or without 2 μm TBT for 0–240 min were analysed for morphological changes by May–Grünwald–Giemsa staining. Thirty minutes of incubation with TBT resulted in apoptotic eosinophils with condensed nuclei, a main characteristic for apoptotic eosinophils and neutrophils. No condensed nucleus was detected in neutrophils treated similarly (Fig. 2a,b).
Fig. 2.
The morphology of nontreated eosinophils and neutrophils (a) and eosinophil and neutrophils exposed to TBT (2 μm) for 30 min (b). May–Grünwald–Giemsa stains the acidophilic granules in eosinophils red, while neutrophils, which do not contain acidophilic granules, remain unstained.
Effect of TBT and CCCP on the mitochondrial membrane potential
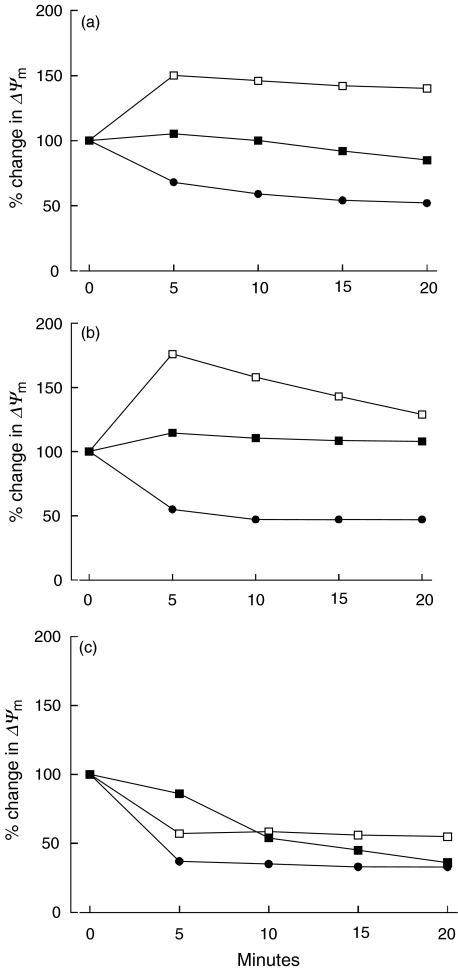
Induction of MPT and an early loss of Δψm are typical of TBT-induced apoptosis in cells with respiring mitochondria. We next examined whether TBT induces MPT and also loss of Δψm in eosinophils with, as reported previously, non-respiring mitochondria [17]. Eosinophils and neutrophils were stained with the Δψ-sensitive dye TMRE and changes in fluorescence were monitored by flow cytometry and presented as change in MFI. Loading the cells with TMRE alone for 15 min was set to 100%. After 20 min of TBT (2 μm) exposure, a slight but non-significant reduction to 92% (84–109) of Δψm was observed in eosinophils (n = 5) (Fig. 3a). The Δψm in neutrophils, on the other hand, remained almost unchanged, although a slight non-significant hyperpolarization 108% (97–121) was detected (n = 5) (Fig. 3b). Purified peripheral lymphocytes were used as a positive control, and we detected a significant (P < 0·04) dissipation of the Δψm after 10 min of exposure (n = 5) (Fig. 3c).
Fig. 3.
The change in mitochondrial membrane potential in eosinophils (a), neutrophils (b) and lymphocytes (c) determined by TMRE staining after exposure to TBT (2 μm), CCCP (10 μm) or TBT (2 μm) + CCCP (10 μm) for different time periods. Results are expressed as mean value. The mitochondrial membrane potential after loading the cells with TMRE alone for 15 min was set to 100%. ▪,TBT; □, CCCP; •, TBT + CCCP.
The mitochondrial uncoupler CCCP dissipates Δψm by increasing membrane permeability to protons without interfering with the respiratory chain or with the mitochondrial ATPase. Eosinophils and neutrophils were exposed to CCCP and changes in TMRE fluorescence was monitored every 5 min up to 20 min. A significant (P < 0·04) hyperpolarization of eosinophil Δψm was observed within 5 min of exposure to CCCP (n = 5) (Fig. 3a). Hyperpolarization of neutrophil mitochondria was also detected, although the change was not statistically significant (n = 5) (Fig. 3b). In lymphocytes, CCCP significantly (P < 0·03) dissipated Δψm within 5 min (n = 5) (Fig. 3c).
Because neither TBT nor CCCP alone reduced Δψm in eosinophils and neutrophils, we next attempted to shut down all possible sources of Δψm by exposing granulocytes to a combination of both substances. Indeed, within 5 min of TBT/CCCP-exposure, a significant (P < 0·04) dissipation of Δψm was observed in both eosinophils and neutrophils (n = 5) (Fig. 3a,b). In lymphocytes, TBT/CCCP-exposure resulted in significantly (P < 0·02) reduced Δψm within 5 min (n = 5) (Fig. 3c).
Detection of cytochrome c
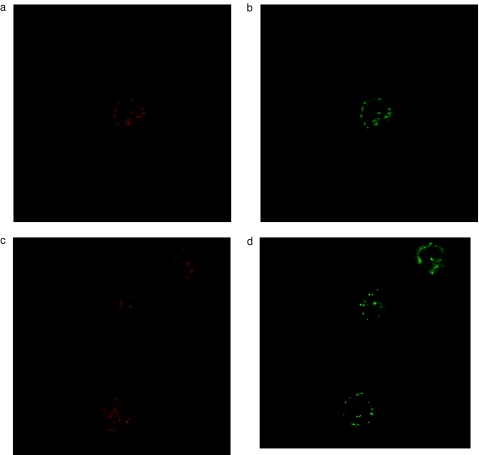
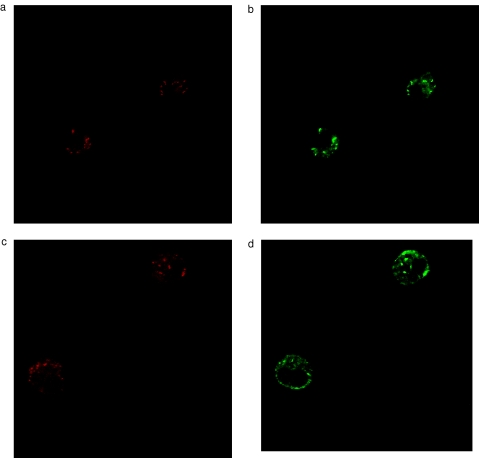
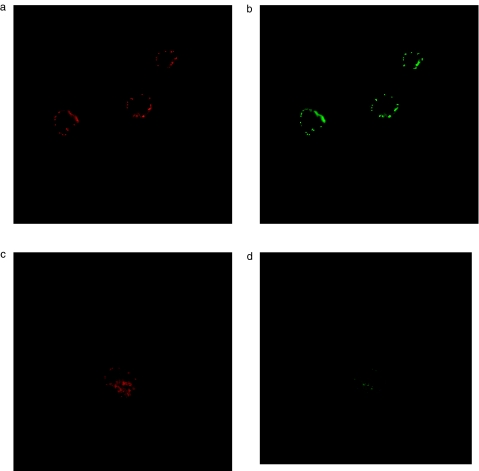
Induction of MPT associated with loss of Δψm has been postulated to be a main mechanism of cytochrome c release in many apoptotic systems. As we did not detect an early loss of Δψm in TBT-treated eosinophils or neutrophils, we next examined the possibility that cytochrome c might be released without associated loss of Δψm. Eosinophils and neutrophils were purified and immunostained with an anticytochrome c antibody followed by examination by confocal laser microscopy. In freshly isolated eosinophil, neutrophils and lymphocytes, cytochrome c exhibited a punctuate distribution, which co-localized with mitochondria stained by Mitotracker CMX Ros (Figs 4a,b, 5a,b and 6a,b). We were not able to detect any release of cytochrome-c from eosinophils or neutrophils within the first 10 min of incubation with 2μm TBT, e.g. prior to caspase activation (n = 4) (Figs 4c,d and 5c,d). Released cytochrome c was demonstrated in eosinophils, but not until after 30 min of TBT-exposure (n = 4) (data not shown).
Fig. 4.
Detection of Mitiotracker CMX Ros (red colour) and cytochrome c (green colour) in eosinophils after treatment with RPMI (a,b) or TBT (2 μm) for 10 min (c,d).
Fig. 5.
Detection of Mitiotracker CMX Ros (red colour) and cytochrome c (green colour) in eosinophils after treatment with RPMI (a,b) or TBT (2 μm) for 10 min (c,d).
Fig. 6.
Detection of Mitiotracker CMX Ros (red colour) and cytochrome c (green colour) in lymphocytes after treatment with RPMI (a,b) or TBT (2 μm) for 30 min (c,d).
In contrast, in purified lymphocytes treated similarly with TBT, cytochrome c was released within 30 min, e.g. prior to caspase activation (n = 3) (Fig. 6c-d) as reported previously [18,20,21].
DISCUSSION
Apoptosis constitutes the most common form of physiological cell death and is an important process to regulate cell turnover and tissue homeostasis. Therefore, it is reasonable to assume that the regulation of granulocyte apoptosis has a profound bearing on the immunological competence at the inflammatory site. In the present study, we used a potent mitochondrial toxin to further characterize the molecular mechanisms of granulocyte apoptosis. Low concentrations of TBT induced caspase-3-like activity and apoptosis in human blood granulocytes within 1–2 h of treatment. In eosinophils, the caspase activation was extremely rapid, with a 14-fold increase after only 10 min of TBT-exposure, whereas similar treatment of neutrophils resulted in a four to fivefold increase. In both cell types, typical apoptotic morphology succeeded the caspase activity. However, the neutrophils appeared less sensitive to TBT-induced apoptotic death. In these cells, typical morphological markers of apoptosis, such as PS exposure and condensed nuclei, were detected after 120 min of exposure, 110 min after the initiation of caspase activity. In the eosinophils, on the other hand, PS exposure was detected at 30 min, 20 min after the initiation of caspase activity. Apparently, there are differences in the kinetic between eosinophils and neutrophils.
Eosinophil-apoptosis constitutes an important step in the resolution of an allergic inflammation. Eosinophils recruited from blood to allergic foci, e.g. the lung, display an activated state, and the milieu of an allergic inflammation offers several anti-apoptotic cytokines [28]. Both Il-5 and GM-CSF have been detected in bronchoalveolar lavage (BAL) fluids from allergic individuals [29], and in vitro-culture of eosinophils in the presence of these cytokines up-regulated of the anti-apopoptotic family member Bcl-xL, thus contributing to the delayed apoptosis [30]. On the other hand, a GM-CSF-induced delay of neutrophil apoptosis was associated with reduced levels of Bax, a pro-apoptotic member of the Bcl-2 family [31].
Different susceptibilities towards apoptosis put forward theories in differences in the regulation of the apoptotic machinery between granulocyte subsets. One early checkpoint of regulation is the mitochondrion. The mitochondrion plays a central role in the initiation of apoptosis by releasing factors, such as cytochrome c, that participates in the formation of the apoptosome complex that activates the caspases. The mechanism of cytochrome c release has been associated with the opening of the mitochondrial permeability transition pore (MPT) followed by dissipation of Δψm.
The potential role of the mitochondrion in eosinophil-apoptosis is, however, obscure. In a recent study, it was demonstrated that eosinophils do contain a low number of mitochondria with a maintained Δψm, but without significant respiration. The Δψm was demonstrated to be generated by hydrolysis (performed by a reversed ATP synthase) of cytosolic-generated ATP rather than from respiration. The main role of the mitochondrion was hypothesized to be to initiate apoptosis [17]. This was demonstrated by inducing apoptosis by oligomycin, which primary effect is to block the ATP synthesis by interaction with the F0F1-ATP synthase. However, oligomycin does not induce MPT primarily and is, in general, regarded as a poor inducer of apoptosis. TBT, on the other hand, target the mitochondria selectively by binding to the F0F1-ATP synthase, triggers MPT and induces apoptosis rapidly in human T lymphocytes [18,21]. Based on these characteristics, TBT was applied to characterize further the role of the mitochondrion in granulocyte apoptosis.
In contrast to lymphocytes, the Δψm remained stable up to 20 min in TBT-exposed eosinophils and neutrophils. A slight decrease in Δψm was observed at 20 min, a time-point at which the caspases have already been activated. Mitochondrial effects in granulocytes were also investigated upon exposure to the mitochondrial uncoupler, CCCP. In eosinophils and neutrophils no loss of Δψm was detected after 20 min of exposure. However, a strong mitochondrial hyperpolarization, initiated at 5 min, was observed. In energized mitochondria CCCP uncouples respiration from the ATP synthesis by translocation of protons from the matrix to the cytosolic side. In the granulocytes, CCCP did not immediately reduced the Δψm, indicating that the effect of CCCP may be strongly compensated by an increased rate of import and hydrolysis of cytosolic ATP in order to maintain the Δψm. Exposure to TBT alone, on the other hand, inhibits ATP hydrolysis, the source of Δψm, but does not reduce Δψm. When cells were exposed to both an agent that dissipated the Δψm (CCCP) and to an agent that inhibits the ATP hydrolysis (TBT), we detected a significant reduction of Δψm in both the eosinophils and neutrophils. These data support the theory that Δψm in eosinophils is derived by the hydrolysis of cytosolic-derived ATP, and not primarily from respiration [17].
The mitochondrial action of TBT in granulocytes definitely seems to differ from its action in lymphocytes, where ANT plays a crucial role in the initiation of apoptosis. The ANT is one of the key proteins involved in MPT and, possibly, in changes leading to the release of mitochondrial proteins. We have shown previously that TBT-induced loss of Δψm, cytochrome c release and apoptosis in lymphocytes is blocked with bongkrekic acid (BA), an agent that inhibits MPT [21,32]. In eosinophil-mitochondria, the ANT is supposed to carry cytosolic ATP to the mitochondrial matrix, as incubation with the ANT-inhibitor BA alone reduced Δψm, suggesting that eosinophils do contain ANT [17]. However, the potential role of the ANT and the induction of classical MPT in eosinophil mitochondria, lacking respiration, still awaits experimental proofs.
To exclude the possibility that cytochrome c might be released by an alternative mechanism than induction of MPT in granulocyte apoptosis, we performed immunohistochemistry of cytochrome c release in TBT-treated eosinophils and neutrophils. In all donors, cytochrome c was localized in the mitochondrion in both neutrophils and eosinophils at the time-point of initiation of caspase activation (10 min), and not until the late stage of the TBT-induced apoptotic process was release of cytochrome observed. The late dissipation of Δψm and release of cytochrome c after TBT-treatment therefore raises the question of whether apoptosis in eosinophils and neutrophils can be induced through a different pathway than via the mitochondrion. However, we cannot role out the possibility that a small amount of mitochondrial cytochrome c (not detectable by immunohistochemistry) may be released into the cytosol by an alternative mechanism than induction of MPT, and that this small amount of cytochrome c may be sufficient to activate the caspases.
In conclusion, we have demonstrated an extremely rapid induction of caspase-3 activity and apoptosis in human blood granulocytes without prior mitochondrial changes. Moreover, eosinophils were more susceptible to TBT-induced apoptosis than neutrophils, indicating differences in the kinetics between these cell types. As apoptosis-stimuli we used TBT, in many systems a classical way to induce apoptosis via the mitrochondrial pathway by the induction of MPT. In our system, granulocyte apoptosis was not preceded by MPT-induction, loss of Δψm and cytochrome c release. Although our results show little or no evidence of mitochondrial involvement in the initiation of TBT-induced apoptosis, this does not role out the possibility of an important role of mitochondria in other apoptotic systems in eosinophils. However, our results have improved knowledge of the comprehensive view of granulocyte apoptosis, thus opening a plausible opportunity for a mitochondrial-independent pathway of induction of caspase 3 activity and subsequent apoptosis in granulocytes. Finally, the knowledge of the role of mitochondria is still obscure, and needs more extensive examination to be elucidated completely.
Acknowledgments
We thank Gonilla Jacobsson Etman for excellent technical assistance with confocal microscopy analysis.
This study was supported by grants from the Hesselman Foundation, the Swedish Asthma and Allergy Association, Konsul Th. C. Bergs Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research and the Karolinska Institutet.
REFERENCES
- 1.Borregaard N, Lollike K, Kjeldsen L, et al. Human neutrophil granules and secretory vesicles. Eur J Hematol. 1993;51:187–98. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 2.Venge P, Byström M, Carlson L, et al. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999;29:1172–86. doi: 10.1046/j.1365-2222.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw AJ. Eosinophils in the 1990s. New perspectives on their role in health and disease. Postgrad Med. 1994;70:635–52. doi: 10.1136/pgmj.70.826.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslett C, Henson PM. The molecular and cellular biology of wound repair. New York, NY: Plenum Press; 1988. Resolution of inflammation; pp. 185–211. [Google Scholar]
- 5.Lee A, Whyt BLC, Haslett C. Prolonged in vitro life-span and functional longevity of neutrophils induced by inflammatory mediators acting through inhibiting apoptosis. J Leukoc Biol. 1993;54:283–8. [PubMed] [Google Scholar]
- 6.Tai PC, Sun L, Spry CFJ. Effects of IL-5, GM-CSF and IL-3 on survival of human blood eosinophils in vitro. Clin Exp Immunol. 1992;85:312–6. doi: 10.1111/j.1365-2249.1991.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effect of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–8. [PubMed] [Google Scholar]
- 8.Goulding NJ, Guyre PM. Glucocorticoids, lipocortins and the immune response. Curr Opin Immunol. 1993;5:108–13. doi: 10.1016/0952-7915(93)90089-b. [DOI] [PubMed] [Google Scholar]
- 9.Schleimer RP. Effects of glucocorticoids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respi Dis. 1990;141:59–69. [PubMed] [Google Scholar]
- 10.Simon HU. New insights into the pathogenesis of asthma. Curr Probl Dermatol. 2000;28:124–8. doi: 10.1159/000060584. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JP, Won CK, Lam CW. Role of caspase in dexamethazone-induced apoptosis and activation of c-Jun NH2-terminal kinas and p38 mitogen-activated protein kinase in human eosinphils. Clin Exp Immunol. 2000;122:20–7. doi: 10.1046/j.1365-2249.2000.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhivotsky B, Burgess DH, Vanags DM, Orrenius S. Involvement of cellular proteolytic machinery in apoptosis. Biochem Biophys Res Commun. 1997;230:481.8. doi: 10.1006/bbrc.1996.6016. [DOI] [PubMed] [Google Scholar]
- 13.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–6. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 14.Dixit VM. Role of ICE-proteases in apoptosis. Adv Exp Med Biol. 1996;406:113–7. doi: 10.1007/978-1-4899-0274-0_11. [DOI] [PubMed] [Google Scholar]
- 15.Kroemer G, Zamzani N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:163–6. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic programme in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 17.Peachman KK, Lyles DS, Bass DA. Mitochondria in eosinophils: functional role in apoptosis but not respiration. Proc Natl Acad Sci USA. 2001;98:1717–22. doi: 10.1073/pnas.98.4.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stridh H, Kimland M, Jones DP, Orrenius S, Hampton MB. Cytochrome c release and caspase activation in hydrogen peroxide and tributyltin-induced apoptosis. FEBS Lett. 1998;429:351–5. doi: 10.1016/s0014-5793(98)00630-9. [DOI] [PubMed] [Google Scholar]
- 19.Stridh H, Orrenius S, Hampton MB. Caspase involvement in the induction of apoptosis by the environmental toxicants tributyltin and triphenyltin. Toxicol Appl Pharmacol. 1999;156:141–6. doi: 10.1006/taap.1999.8633. 10.1006/taap.1999.8633. [DOI] [PubMed] [Google Scholar]
- 20.Stridh H, Gigliotti D, Orrenius S, Cotgrave I. The role of calcium in pre and postmitochondrial events in tributyltin-induced T-cell apoptosis. Bioch Bioph Y Res Com. 1999;266:460–5. doi: 10.1006/bbrc.1999.1821. [DOI] [PubMed] [Google Scholar]
- 21.Stridh H, Fava E, Single B, Nicotera P, Orrenius S, Leist M. Tributyltin-induced apoptosis requires glycolytic ATP production. Chem Res Toxicol. 1999;12:874–82. doi: 10.1021/tx990041c. 10.1021/tx990041c. [DOI] [PubMed] [Google Scholar]
- 22.Stridh H, Cotgrave I, Müller M, Orrenius S, Gigliotti D. Organotin-induced caspase activation and apoptosis in human peripheral T lymphocytes. Chem Res Toxicol. 2001;14:791–8. doi: 10.1021/tx000156c. 10.1021/tx000156c. [DOI] [PubMed] [Google Scholar]
- 23.Aldridge WN, Cremer J. The biochemistry of organotin compounds. Diethyltin dichloride and triethyltin sulphate. Biochem J. 1955;61:406–18. doi: 10.1042/bj0610406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldridge WN, Street BW. Oxidative phosphorylation. Biochemical effects and properties of trialkyltins. Biochem J. 1964;91:287–97. doi: 10.1042/bj0910287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stridh H, Cotgrave I, Müller M, Orrenius S, Gigliotti D. Chloride-hydroxyl exchange across mitochondrial, erythrocyte and artificial membranes mediated by trialkytin and triphenyltin compounds. Eur J Biochem. 1970;14:120–6. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 26.Hansel T, de Vries J, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Meth. 1991;415:105–10. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 27.Halldén G, Andersson U, Hed J, Johansson SGO. A new membrane permeabilization method for the detection of intracellular antigens by flow cytometry. J Immunol Meth. 1989;124:103–9. doi: 10.1016/0022-1759(89)90191-9. [DOI] [PubMed] [Google Scholar]
- 28.Kay AB, Barata L, Meng Q, Durham SR, Ying S. Eosinophils and eosinophil-associated cytokines in allergic inflammation. Allergy Clin Exp Immunol. 1997;113(1–3):196–9. doi: 10.1159/000237545. [DOI] [PubMed] [Google Scholar]
- 29.Virchov JC, Jr, Walker C, Hafner D, et al. T-cells and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med. 1995;151:960–8. doi: 10.1164/ajrccm/151.4.960. [DOI] [PubMed] [Google Scholar]
- 30.Dibbert B, Daigle I, Braun D, Schranz D, et al. Role of Bcl-xL in delayed eosinophil apoptosis mediated by granulocyte-machrophage colony-stimulating factor and interleukin-5. Blood. 1998;92(3):778–83. [PubMed] [Google Scholar]
- 31.Dibbert B, Weber M, Nikkkolaizzik WH, et al. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci USA. 1999;96:13330–5. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenartowicz E, Bernardi P, Azzone GF. Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr. 1991;23:679–88. doi: 10.1007/BF00785817. [DOI] [PubMed] [Google Scholar]