Abstract
Although intestinal epithelial cells (IECs) are known as an important source for IL-6, it is not known whether mechanical forces affect IL-6 production. We investigated how transmural pressure modulates IL-6 synthesis and activation of transcription factors in IECs. Pressure was loaded onto IEC-18 cells by introducing compressed helium gas into the cell culture flask for 1–48 h. IL-6 release into the culture media was determined by cell proliferation bioassay using an IL-6-dependent mouse hybridoma cell line (7TD1). Exposure to pressure (80 mmHg) significantly enhanced IL-6 release into the culture media from IEC-18 cells at 12 h. Under control conditions, IL-6 secretion was directed to the basolateral side, but after exposure to pressure IL-6 secretion was increased in both the apical and basolateral sides. A nuclear factor kappa B (NF-κB) decoy reversed completely the pressure-induced increase of IL-6 secretion by IEC-18 cells. Pressure treatment enhanced IL-6 mRNA expression in IECs within 6 h. Pressure loading significantly enhanced the activation of both NF-κB and NF-IL-6 from 1h in the nuclear protein of IEC-18 cells as assessed by the electrophoretic mobility shift assay using FITC-conjugated specific primers. Increased phosphorylation of I-kappa B was also demonstrated in the cytosol of IEC cells within 1h by Western blot analysis. These results suggest a possible role for pressure loading in immune modulation of the intestinal mucosa by the stimulation of IL-6 release from intestinal epithelial cells.
Keywords: intestinal epithelial cells, IL-6, NF-κB, NF-IL-6, transmural pressure
INTRODUCTION
The small intestinal mucosa serves as an important barrier where continuous remodelling and production of biologically active substances occur in response to intraluminal factors. The intestinal mucosa is continuously exposed to mechanical forces, i.e. transmural pressure, shear stress and stretch forces, which are produced by intestinal motor activities. In recent years, increased production of inflammatory cytokines by mechanical forces has been reported in a variety of cells, i.e. vascular endothelial cells, cardiomyocytes and osteoblasts [1–3]. Intraluminal transmural pressure may be involved in the regulation of surface mucosal remodelling in the intestine, together with various cytokines. However, information is sparse concerning the increased intraluminal pressure induced by intestinal inflammation or how ileus affects the cytokine production from the intestinal epithelial cells.
Interleukin-6 (IL-6) is a multi-functional cytokine that is involved in diverse biological responses of the host. IL-6 was originally identified as a B-cell-stimulating factor, and it is now recognized widely to have multiple biological functions such as induction of acute phase protein synthesis by hepatocytes, induction of cytotoxic T-cell differentiation and activation of haematopoietic stem cells [4]. Intestinal epithelial cells are known to be an important source for IL-6 [5]. McGee et al. demonstrated that proinflammatory cytokines IL-1β and TGF-β could act synergically to induce IL-6 secretion in rat intestinal epithelial cells and cholera toxin also stimulated IL-6 secretion in the cells [6]. Goodrich & McGee also showed that IL-6 secreted from the rat non-transformed IEC-6 cell line on IgA secretion was found to enhance lipopolysaccharide (LPS) stimulated IgA secretion by IgA+ mesenteric lymph node B cells [7], suggesting the significant involvement of intestinal epithelial cell-derived IL-6 in intestinal immune responses. Recently it has been reported that in the rat ileus model plasma IL-6 concentration was elevated, while TNF-α or IL-1 concentration was not increased [8], suggesting the specific enhancement of IL-6 production in response to increased intestinal pressure. Transcription factors, nuclear factor kappa B (NF-κB) and NF-IL-6 are identified as important for mediating expression of IL-6 [9]. NF-κB dimers are sequestered in an inactive cytoplasmic complex by binding to its inhibitory subunit, IκB. Upon stimulation, IκB is phosphorylated, and the phosphorylation is followed by ubiquitination and rapid degradation by a proteasome-dependent pathway [10,11]. Therefore it is important to determine whether activation of these transcription factors occurs in intestinal epithelial cells after transmural pressure loading.
The purposes of this study were to (1) investigate how loading which pressure affects the IL-6 secretion from cultured intestinal epithelial cell lines, and (2) to determine whether activation of transcription factors is involved in altered IL-6 synthesis in the intestinal epithelial cells after pressure loading.
METHODS
Cell cultures and pressure loading
IEC18 cells (a rat intestinal epithelial cell line; American Type Culture Collection, Manassas, VA, USA) were grown in an atmosphere of 5% CO2 at 37°C in a culture medium composed of Dulbecco's modified Eagle medium containing 5% fetal bovine serum, 10 μg/ of insulin, 50 U/ of penicillin, 50 U/ of streptomycin and 4 mm glutamine. Pressure was added to the subconfluent monolayers of IEC18 from passages 8–9. The pressure-loading apparatus was set up as reported previously [12]. Cell plates were placed in an acryl flask by removing the upper panel, and the flask was resealed with a using rubber seal, and then tightly clamped. The top of each flask was sealed with a rubber cap which was pierced by a needle connected to tubing attached to a three-way rotary valve, sphygmomanometer and a pressure valve. Compressed helium gas was pumped in to raise the internal pressure, so that the partial pressures of the gases contained in the flask were kept constant in accordance with Boyle–Charles’ law [13]. The flask was kept at 37°C.
For the polarity assay of IL-6 release, cells (5 × 105 cells) were seeded on the tops of collagen-coated microporous supports (0·4 μm pore size; 4·7 cm2 growth area) in transwell chambers (Costar, Cambridge, MA, USA). The medium volumes in the apical and basal compartments were 1·5 and 2·6 ml, respectively. The culture supernatants in both compartments were collected after 48h pressure loading for determination of IL-6 concentration. The impermeability of the monolayers was confirmed by the appearance of only 5% of the added 3H]-mannitol in the basolateral component 24h after 80 mmHg loading.
Detection of IL-6 in IEC 18 conditioned medium
IL-6 concentration was measured by bioassay using an IL-6-dependent murine hybridoma cell line, 7TD1 cells (Riken Cell Bank, Tsukuba, Ibaragi, Japan). Proliferation of the 7TD1 cells was determined in an MTT (3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide) colouriometric assay (Promega, Madison, WI, USA). The results of MTT assay were compared with a standard generated using recombinant murine IL-6 levels and were expressed in units, where 1 U is defined as the reciprocal of the dilution giving half-maximal proliferation of the 7TD1 cells. The 7TD1 assay was carried out with serial dilution of IEC 18 in the presence or absence of polyclonal goat antimouse IL-6 IgG antibody (R&D Systems, Minneapolis, MN, USA) [14]. To confirm the specificity of biologically active IL-6, supernatants from pressure-stimulated IEC-18 cells were incubated with various concentrations of the polyclonal goat antimouse IL-6 IgG antibody. The IL-6 antibody (10 ng/) neutralized the 7TD1-stimulating activity to the background level, while the same concentration of normal goat IgG had no effect. In our preliminary experiment, the immunoreactive rat IL-6 level of IEC 18 culture supernatant at controls was 85 ± 23 pg/ when determined by ELISA using rat IL-6 ELISA kit (Biosource International, Camarillo, CA, USA).
Detection of IL-6 mRNA expression by polymerase chain reaction
Total RNA was extracted from cultured IEC 18 by a one-step method using RNA zol (Cinna/Biotex, Houston, TX, USA). Reverse transcription was carried out using a first-strand synthesis kit (Stratagene, La Jolla, CA, USA) to obtain cDNA. PCR was performed using the Takara taq kit (recombinant Taq DNA polymerase; Takara Biochemicals, Tokyo, Japan), with rat-specific primers for IL-6 as follows [14]: Sense 5′-GACTGATGTTG TTGACAGCCACTGC-3′; antisense 5′-TAGCCACTCCTTCTC TGTGACTCTAACT-3′.
The cDNA amplification products were predicted to be 508 bp in length. Sequences of sense primer of GAPDH was 5′-TCCCTCAAGATTGTCAGCAA-3′. Antisense primer was 5′-AGATCCACAACGGATACATT-3′. Thirty-five cycles of amplification were carried out in the DNA thermal cycler with denaturing for 60 s at 94°C, annealing for 60 s at 58°C, and extension for 180 s at 72°C. Reaction product was separated by electrophoresis at 100 V in a 1·5% agarose gel and stained with ethidium bromide. Photographic negatives of the gels were calculated densitometrically by using the National Institute of Health Image 1·62.
Inhibition study
Oligodeoxynucleotides (ODNs) were synthesized using an automated synthesizer (Sawady Technology, Tokyo, Japan). The last three bases at the 3′ end of each the ODNs described below were phosphorothioate modified. The following sequence and its complement was used as NF-κB transcription factor decoy (TFD) and mutated NF-κB transcription factor decoy (MUT) [15]. TFD: 5′GGGGACTTTCCGCTGGGGACTTCCAGGGGGA CTTTCC3′; MUT: 5′GTCTACTTTCCGCTGTCTACTTTCCA CGGTCTACTTTCC3′.
The κb and mutated κB binding sites in each double-stranded OND are underlined. IEC-18 cells were preincubated for 24h at 37°C with 2 μm of ODNs and then transmual pressure was applied.
Western blot analysis of IκB-α phosphorylation
IκB-α phosphorylation activity was assessed with a PhosphoPlusR IκB-α Antibody kit (New England BioLabs, Beverly, MA, USA). IEC-18 cells seeded (1 × 106) in flasks were exposured to a pressure of 80 mmHg for 1 and 3 h. Lysates were prepared by SDS sample buffer and the supernatants were loaded onto SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Western blots were developed on an electrotransfered nitrocellulose membrane using a phospho-I κB-antibody (1:1000), followed by incubation with an HRP (horseradish peroxidase)-conjugated antirabbit secondary antibody (1:2000). After the membrane was washed three times with 15 ml of TBST, incubated with 10 mm 1 × LumiGLO for 1 min at room temperature, the bands were measured by exposure to X-ray film.
Activation of nuclear factor NFκ-B and NF-IL-6
DNA binding activities of NFκ-B and NF-IL-6 were determined by elecrophoretic mobility shift assay (EMSA) using an FITC-labelled NF-κB oligonucleotide (5′-AGTTGAGGGGACTTTC CCAGG-3′) [16] or a NF-IL-6 oligo-nucleotide (5′-TGGGTAT TATGCAATTGGAAG-3′) as the probe [17]. IEC-18 cells (1 × 106) were exposed to pressure at 80 mmHg for 1–2 h. NF-κB oligonucleotide (1 ng) was incubated for 45 min at room temperature with each of the 20 μg nuclear protein extracts. The samples were electrophoresed through non-denaturing 5% polyacrylamide gels for 1 h. For the competition experiment, an unlabelled oligonucleotide (50:1) was used. The fluorescence intensity was quantified by a fluorescence laser scanning system equipped with a computer-assisted image analyser (FluorImager 575; Molecular Dynamics, Sunnyvale, CA, USA).
Statistical analysis
All results were expressed as means ± s.e.m. Differences among groups were evaluated by one-way analysis of variance (anova) and Fisher's post hoc test. Statistical significance was set at P < 0·05.
RESULTS
IL-6 secretion from IEC-18 cells by transmural pressures
Figure 1a shows the effect of transmural pressure on IL-6 secretion from IEC-18 cells. IL-6 secretion was increased significantly to about 2·5–3-fold by a presence of 80 mmHg pressure compared with controls (0 mmHg) at 12h and this increase continued for 48 h. Pressurization for 24h increased the IL-6 secretion at 60–80 mmHg (Fig. 1b) significantly. Maximal release was obtained at 80 mmHg, in which IL-6 secretion reached to 3·3 times (334 ± 68%, P < 0·05) than the control level. Examination by light microscopy and the trypan blue exclusion test at the end of the experiments showed that the cells were morphologically intact, and detached cells were negligible from 0 to 120 mmHg.
Fig. 1.
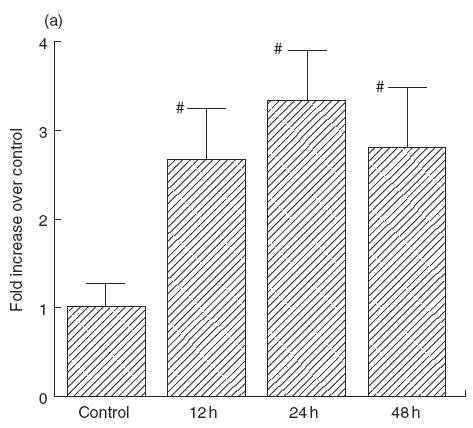
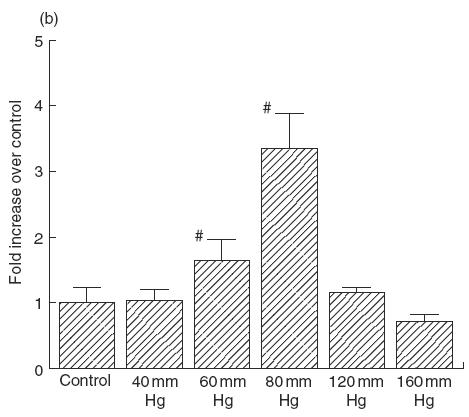
(a) Effect of transmural pressure on IL-6 secretion by IEC-18 cells. Time-course change of IL-6 secretion after pressurization at 80 mmHg (control: 0 h). Mean ± s.e.m. of six experiments. #P < 0·05 versus controls. (b) Effect of transmural pressure (40–160 mmHg) on IL-6 secretion by IEC-18 cells for 24 h. Mean ± s.e.m. of six experiments. #P < 0·05 versus controls (0 mmHg).
To determine the involvement of NF-κB in IL-6 secretion, a 7TD1 bioassay was performed under the presence of a NF-κB decoy. The NF-κB decoy completely reversed the pressure-induced increase in IL-6 secretion by IEC-18 cells after 80 mmHg loading for 24 h; however, mutated NF-κB decoy did not affect IL-6 secretion, as shown in Fig. 2. Figure 3 illustrates the effect of transmural pressure on the polarity of IL-6 secretion. In the control state (0 mmHg), IL-6 secretion was dominant in the basolateral component. Transmural pressure (80 mmHg) significantly up-regulated IL-6 secretion at 24h in both the apical and basolateral compartments, although the degree of increase was more remarkable in the apical compartment, resulting in an increased apical/basolateral IL-6 ratio.
Fig. 2.
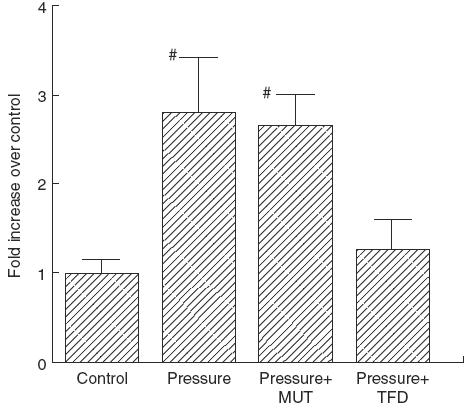
Effect of mutated NF-κB transcription factor decoy (MUT) (2 μm) and NF-κB transcription factor decoy (TFD) (2 μm) on pressure-induced increase in IL-6 secretion. Control: 0 mmHg, Pressure: pressure loading at 80 mmHg for 24 h. Mean ± s.e.m. of six experiments. #P < 0·05 versus controls.
Fig. 3.
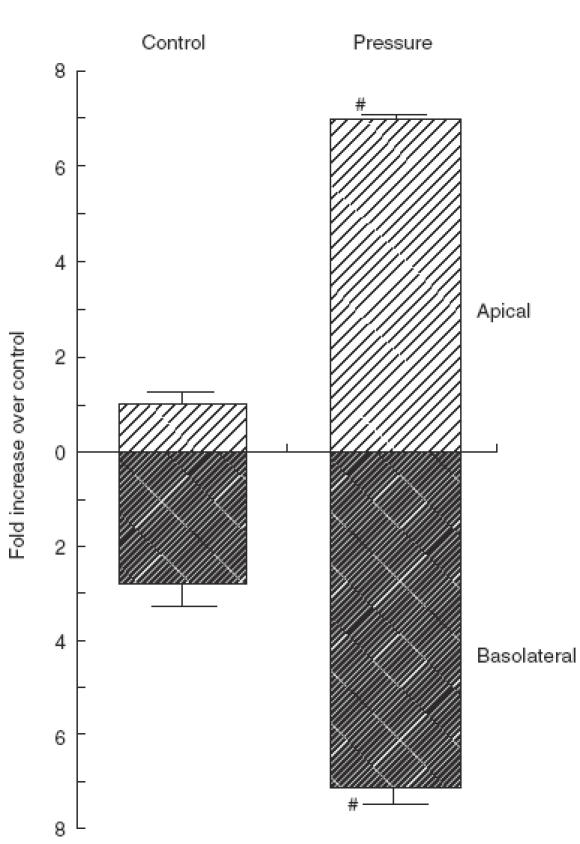
Effect of transmural pressure on the polarity of IL-6 secretion. The culture supernatants in both compartments (apical and basolateral) were collected after 24h pressure loading at 80 mmHg for IL-6 determination. Mean ± s.e.m. of six experiments. #P < 0·05 versus controls (0 mmHg).
mRNA expression of IL-6 genes
To assess whether increased IL-6 secretion was paralleled by increased mRNA levels, the IL-6 mRNA levels were assessed by semiquantitative RT-PCR analysis. As determined by the RT-PCR, IEC-18 cells especially expressed IL-6 mRNA at 508 bp (Fig. 4). Expression of IL-6 mRNA by IEC-18 cells was increased 6–12h after exposure to pressure at 80 mmHg compared with that of non-pressurized cells.
Fig. 4.
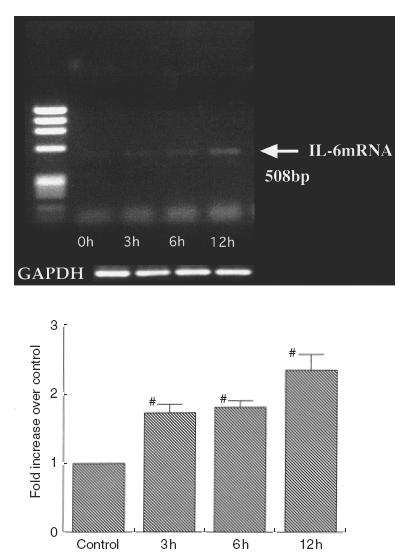
Expression of IL-6 mRNA in IEC-18 cells after exposure to pressure at 80 mmHg as determined by RT-PCR. IEC-18 cells expressed IL-6 mRNA at 508 bp. IL-6 mRNA abundance was normalized to control GADPH mRNA and expressed as relative increase to control at the bottom. Representative results from four experiments are shown.
Activation of nuclear factor NF-κB and NF-IL-6
Figure 5 shows an EMSA assay of NF-κB and NF-IL-6 binding activities after exposure to pressure at 80 mmHg. Nuclear reacts from control cells contained little activated NF-κB (Fig. 5a, left panel, lane 1). An increase in activated NF-κB was evident 1 and 2h after exposure to a pressure at 80 mmHg (lanes 2 and 3, respectively), with greater activation at 1h than 2 h. The specificity of NF-κB binding was confirmed with the successful competition by cold oligonucleotides (Fig. 5a, right panel). The activation of NF-IL-6 after pressure loading is shown in Fig. 5b, left panel. NF-IL-6 activation appeared to increase at 1h and increased further at 2 h. The NF-IL-6 binding was also mostly inhibited by cold oligonucleotides (Fig. 5b, right panel).
Fig. 5.
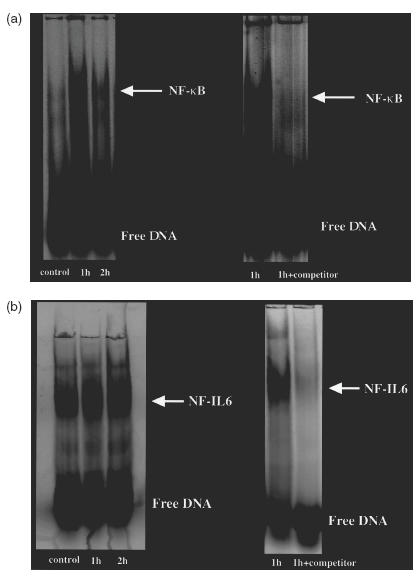
EMSA assay of NF-κB and NF-IL-6 activation after exposure to pressure in IEC-18 cells. (a) NF-κB DNA binding activity as determined by elecrophoretic mobility shift assay (EMSA) using a FITC-labelled consensus NF-κB oligonucleotide as a probe. Left: control IEC-18 cells extracts (lane 1), and 1 and 2h after exposure to a pressure of 80 mmHg (lanes 2 and 3). Right: the specificity of NF-κB binding was confirmed with the competition by cold oligonucleotides. (b) EMSA assay of NF-IL-6 DNA binding activity after exposure to pressure in IEC-18 cells. Left: control IEC-18 cells extracts (lane 1), and 1 and 2h after the exposure of pressure at 80 mmHg (lanes 2 and 3). Right: the specificity of NF-IL-6 binding was confirmed by cold oligonucleotides.
Western blot analysis of IκB-α phosphorylation
The IκB-α phosphorylation was determined by Western blot analysis using a phospho-IκB (Ser32) antibody (Fig. 6). The phosphorylation levels induced by pressure at 80 mmHg were significantly higher than those of controls at 1h and a significant increase was maintained at 2 h.
Fig. 6.

Western blot analysis of IκB-α phosphorylation. The IκB-α phosphorylation was determined by Western blot analysis using a phospho-IκB (Ser32) antibody. The loading of transmural pressure at 80 mmHg induced increases in phosphorylation of IκB-α after 1 and 2h compared with controls (0 h).
DISCUSSION
In this study we have demonstrated a new mechanism that intestinal epithelial cells are able to react to physical stress, such as pressure by the release of biologically active IL-6, that may play a role in the initial responses of the host to enteric pathogens and bacterium [5,18]. It should be noted that IL-6 release was stimulated only when the pressure ranged from 60 to 80 mmHg. Because studies in which the intraluminal pressure of the small intestine was recorded in patients with irritable bowel syndrome showed that the intraluminal pressure in the jejunum can reach more than 50 mmHg [19], we speculate that IL-6 release could be frequently induced not only by enteric pathogens, but also in response to intraluminal pressure of small intestine induced by food or peristalsis. In other words, under physiological condition mechanical stress may also play a significant role for maintaining mucosal defence by releasing of immunological mediators. IL-6 secretion from the intestinal epithelial cells is known to be augmented significantly in an inflammatory response, especially in the presence of TNF-α and IL-1β[20]. Because intestinal epithelial cells also secrete IL-1 and TNF-α in addition to chemotactic cytokines, the question arises as to whether pressure loading regulates IL-6 secretion through IL-1 and TNF-α release. However, in our preliminary study IL-1β and TNF-α were not detected in the cultured supernatant of intestinal epithelial cells after pressure loading, suggesting that pressure-induced IL-6 release may be directly due to the effect of the pressure.
Recently Feving et al. reported that strangulation obstruction induced experimentally by elevating the pressure around the ileum caused an increased release of IL-6 in the intestinal venous blood, but did not cause early changes in plasma levels of endotoxin, TNF or IL-1 [8], and that this IL-6 release was not due to the reduction of arterial blood flow, suggesting that IL-6 could be released from the intestine immediately in response to critical illness, including physical stresses. It is suggested that IL-6 may be one of the earliest responses following adherence and invasion of an enteric bacterium, such as Salmonella typhi[18]. IL-6 is also known as an important determinant in the protection of epithelial cells from intracellular multiplication of Listeria monocytogenes[21]. However, analysis of the expression level for IL-6 mRNA in our study showed the relatively later phase of increase in IL-6 mRNA at 6 h. In osteoblast-like cell line, cyclic mechanical stretch increased the expression of bone growth factors, TGFβ, IGF-1 and βFGF, followed by the later increase in IL-6 mRNA at 16–24h [1], suggesting the direct effect of mechanical strain on osteoblasts, which may be the driving factors of bone growth during destruction. We consider that IL-6 induction by mechanical stress in intestinal epithelial cells could also be involved in the remodelling phase of the mucosa in addition to the acute phase reaction against organisms.
Polarized secretion of IL-6 has been reported in various epithelial cells [22,23]. Using rat IEC-6, Mascarenhas et al. demonstrated that these cells were capable of secreting IL-6 preferentially to the basal surface when stimulated basally with IL-1β, but TNF-α resulted in a equal level of IL-6 secretion to the apical and basal surfaces. 24]. In general, secreted proteins that are not specifically targeted to the apical surfaces of polarized epithelial cells appear to be secreted predominantly at the basolateral surfaces of those cells [25]. Our present results demonstrated that the transmural pressure up-regulated IL-6 secretion in both the apical and basolateral component with preferential increase in the apical compartment, suggesting that the enterocyte production of IL-6 is regulated differentially at the apical and basolateral membranes depending upon different kinds of stimulation.
Our present results revealed that activation of transcription factors, NF-κB and NF-IL-6 occurs in intestinal epithelial cells after transmural pressure loading. The NF-κB or NF-IL-6 transcription factor plays an important role in the regulation of transcription of several interleukins and adhesion molecules. For example, transcriptional activation of the IL-6 and IL-8 genes is controlled by both κB and NF-IL-6 enhancer elements [9]. DNA binding of NFκB is known to be induced by LPS or TNF-α, and in the case of NF-IL-6 its mRNA expression is induced directly by LPS or TNF-α stimulation [26]. Parikh et al. demonstrated that IL-1β-induced IL-6 production by the Caco-2 cells was associated with the activation of NF-κB as determined by EMSA and that the inhibition of IL-6 production by the NF-κB inhibitors indicates that the IL-6 production is regulated by NF-κB [27]. In this study we demonstrated that pressure-induced IL-6 production is also mediated by activation of NFκB by inhibition with sequence-specific DNA-binding proteins. The NF-κB decoy competes for protein binding with the authentic binding elements, interfering with gene regulation, without affecting the production of the target protein [15, 28]. We also demonstrated that there was a pressure-induced phosphorylation of IkappaB, which may be followed by activation of NF-κB with degradation of IkappaBs. Although further investigations are required to compare the cytokine-induced and pressure-induced intracellular signals, similar pathways involving NF-κB activation may be important for IL-6 secretion in intestinal epithelial cells. In this study we have presented evidence that intestinal epithelial cells produce significant levels of IL-6 in response to mechanical pressure, although their exact physiological and pathophsyiological roles in intestinal immune response remain to be elucidated.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture of Japan and by grants from Keio University, School of Medicine, and from National Defense Medical College.
REFERENCES
- 1.Cillo JE, Jr, Gassner R, Koepsel RR, et al. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: implications for distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:147–54. doi: 10.1067/moe.2000.107531. [DOI] [PubMed] [Google Scholar]
- 2.Okada M, Matsumori A, Ono K, et al. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:894–901. doi: 10.1161/01.atv.18.6.894. [DOI] [PubMed] [Google Scholar]
- 3.Pan J, Fukuda K, Saito M, et al. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84:1127–36. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto T. The biology of interleukin 6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 5.McGee DW, Elson CO, McGee JR. Enhancing effect of cholera toxin on interleukin-6 secretion by IEC-6 intestinal epithelial cells: mode of action and augmenting effect of inflammatory cytokines. Infect Immun. 1993;61:4637–44. doi: 10.1128/iai.61.11.4637-4644.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGee DW, Beagley KW, Aicher WK, et al. Transforming growth factor-β and IL-1β act in synergy to enhance IL-6 secretion by the intestinal epithelial cell line, IEC-6. J Immunol. 1993;151:970–8. [PubMed] [Google Scholar]
- 7.Goodrich ME, McGee DW. Effect of intestinal epithelial cell cytokines on mucosal B-cell IgA secretion: enhancing effect of epithelial-derived IL-6 but not TGF-β on IgA+ B cells. Immunol Lett. 1999;67:11–4. doi: 10.1016/s0165-2478(98)00112-6. [DOI] [PubMed] [Google Scholar]
- 8.Fevang J, Ovrebo K, Svanes K, et al. Endotoxin and cytokine release in strangulation obstruction and in partial occlusion of the mesenteric artery in pigs. Eur Surg Res. 1999;31:26–38. doi: 10.1159/000008618. [DOI] [PubMed] [Google Scholar]
- 9.Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–7. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palombella VJ, Rando OJ, Godberg A, et al. The ubiquitin–proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–85. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 11.Brown K, Gerstberger S, Carlson L, et al. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–8. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 12.Hirokawa M, Miura S, Shigematsu T, et al. Pressure stimulates proliferation and DNA synthesis in rat intestinal epithelial cells. Life Sci. 1997;61:667–72. doi: 10.1016/s0024-3205(97)00531-6. [DOI] [PubMed] [Google Scholar]
- 13.Hishikawa K, Nakaki T, Marumo T, et al. Pressure promotes DNA synthesis in rat cultured vascular smooth muscle cells. J Clin Invest. 1994;93:1975–80. doi: 10.1172/JCI117189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osawa H, Yamabe H, Inuma H, et al. TGF-β upregulates interleukin 6 production by rat glomerular epithelial cells in vitro. Nephrol Dial Transplant. 1995;10:1592–7. [PubMed] [Google Scholar]
- 15.Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauert J. Use of phosphorothionate-modified oligodeoxynucleotides to inhibit NF-κB expression and lymphocyte function. Clin Immunol Immunopathol. 1998;86:170–9. doi: 10.1006/clin.1997.4486. [DOI] [PubMed] [Google Scholar]
- 16.Peng HB, Libby P, Liao JK. Induction and stabilization of IκBα by nitric oxide mediates inhibition of NF-κB. J Biol Chem. 1995;270:14214–9. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 17.Sorli CH, Zhang H-J, Armstrong MB, Rajotte RV, Maclouf J, Robertson RP. Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc Natl Acad Sci USA. 1998;95:1788–93. doi: 10.1073/pnas.95.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein DL, O'Neill BL, Metcalf ES. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun. 1997;65:395–404. doi: 10.1128/iai.65.2.395-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–93. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 20.McGee DW, Bamberg T, Vitkus SJ, McGhee JR. A synergistic relationship between TNF-α, IL-1β, and TGF-β1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995;86:6–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Ouadrhiri Y, Sibille Y, Tulkens PM. Modulation of intracellular growth of Listeria monocytogenes in human enterocyte Caco-2 cells by interferon-gamma and interleukin-6: role of nitric oxide and cooperation with antibiotics. J Infect Dis. 1999;180:1195–204. doi: 10.1086/314983. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs AL, Sehgal PB, Julian J, et al. Secretion and hormonal regulation of interleukin-6 production by mouse uterine stromal and polarized epithelial cells cultured in vitro. Endocrinology. 1992;131:1037–46. doi: 10.1210/endo.131.3.1505448. [DOI] [PubMed] [Google Scholar]
- 23.Holtkamp GM, Rossem MV, Vos AFD, Peek BWR, Kijlstra A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clinical & Experimental Immunology. 1998;112:34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascarenhas JO, Goodrich ME, Eichelberger H, et al. Polarized secretion of IL-6 by IEC-6 intestinal epithelial cells: differential effects of IL-1β and TNF-α. Immunol Invest. 1996;25:333–40. doi: 10.3109/08820139609059315. [DOI] [PubMed] [Google Scholar]
- 25.Rindler M, Traber MG. A specific sorting signal is not required for the polarized secretion for newly synthesized proteins from cultured intestinal epithelial cells. J Cell Biol. 1988;107:471–9. doi: 10.1083/jcb.107.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akira S, Isshiki H, Sugita T, et al. A nuclear factor for IL-6 expression (NF-IL-6) is a member of a C/EBR family. EMBO J. 1990;9:1897–906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh AA, Salzman AL, Kane CD, et al. IL-6 production in human intestinal epithelial cells following stimulation with IL-1 beta is associated with activation of the transcription factor NF-kappa B. J Surg Res. 1997;69:139–44. doi: 10.1006/jsre.1997.5061. [DOI] [PubMed] [Google Scholar]
- 28.Morishita R, Sugimoto T, Aoki M, et al. In vivo transfection of cis element ‘decoy’ against nuclear factor-κB binding site prevents myocardial infarction. Nat Med. 1997;3:894–9. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]


