Abstract
The aim of this work was to investigate the effect of cadmium, lead and arsenic on the apoptosis of human immune cells. Peripheral blood mononuclear cells (MNC) were incubated with increasing concentrations of these metals and then cellular apoptosis was determined by flow cytometry and by DNA electrophoresis. We found that arsenic induced a significant level of apoptosis at 15 μm after 48h of incubation. Cadmium had a similar effect, but at higher concentrations (65 μm). In addition, cadmium exerted a cytotoxic effect on MNC that seemed to be independent of the induction of apoptosis. In contrast, concentrations of lead as high as 500 μm were nontoxic and did not induce a significant degree of apoptosis. Additional experiments showed that arsenic at concentrations as low as 1·0 μm had a significant pro-apoptotic effect when cells were cultured in the presence of this pollutant for more than 72. Non-T cells were more susceptible than T lymphocytes to the effect of arsenic and cadmium. Interestingly, MNC from children chronically exposed to arsenic showed a high basal rate of apoptosis and a diminished in vitro sensibility to this metalloid. Our results indicate that both arsenic and cadmium are able to induce apoptosis of lymphoid cells, and suggest that this phenomenon may contribute to their immunotoxic effect in vivo.
Keywords: immunodeficiency, lymphocytes, non-T cells, programmed cell death, toxicity
INTRODUCTION
Apoptosis or programmed cell death plays a key role in the development and homeostasis of the immune system [1,2]. Apoptosis is triggered by a variety of physiological and pathological stimuli that induce different cells to participate in its own death process [1,2]. The morphological changes that characterize this process include loss of microvilli and cell–cell junctions, cytoplasm shrinkage and condensation of nuclear chromatin [1,2]. The biochemical changes that occur during apoptotic cell death include the translocation of phosphatidylserine to the outer layer of cell membrane, the increase of intracellular Ca2+ and a characteristic DNA cleavage pattern [1–4].
Although the mechanisms involved in apoptosis may differ in distinct cell types, this phenomenon can be divided into three distinct phases: in the initiation phase, apoptosis is induced through cell membrane receptors (mainly those of the TNF receptor family) by growth factor withdrawal or DNA damage, among others. The second or effector phase involves mitochondrial alterations as well as different intracellular signals (Ca2+, ceramide, AMPc, oxygen metabolites) that result in the activation of caspases, a phenomenon that is partially under the control of bcl-2 gene family members [5–7]. Finally, during degradation or third phase, the activation of catabolic enzymes originates the characteristic structural features of apoptosis [6].
Apoptosis occurs under a wide range of physiological and pathological conditions, such as normal cell turnover, withdrawal of growth factors, immune-mediated cytotoxicity, and deletion of autoreactive lymphocytes [2,8]. Although apoptosis is very important in the homeostasis of the immune system, this type of cell death can be also associated with disease. An abnormal resistance to the induction of apoptosis has been observed in different neoplastic or autoimmune conditions. In contrast, an enhanced apoptosis has been found in disorders such as AIDS, aplastic anaemia and neurodegenerative disorders [8]. In addition, a growing number of agents that induce apoptosis of lymphoid cells, such as chemotherapeutic drugs, irradiation, ethanol, mercury, p-dioxin, etc. have been described [9–12]. Abnormalities in apoptosis of immune cells can lead to immunoregulatory defects, which may result in immunodeficiency, autoimmune disease or malignancy [8].
Lead (Pb), cadmium (Cd) and arsenic (As) are extremely toxic environmental pollutants. It is well known that exposure to these metals causes damage in different tissues, including the immune system [13–15]. Pb is an ubiquitous toxic metal with different sources of environmental exposure [13]. The activation of cellular functions due to its calcium mimicking effect and inhibition of the activity of different proteins through its binding to sulphydryl (SH) groups of amino acids have been described as the main mechanisms of toxicity of this metal [13,16]. There is evidence that Pb is able to affect the immune system. Both, acute and chronic exposures to lead diminish the resistance to Listeria monocytogenes[17]. In addition, it has been reported that lead inhibits nitric oxide (NO) production that increases TNF-α secretion, together with up-regulation of TNF-α receptor expression on activated mononuclear cells [18,19]. Furthermore, there is evidence that lead increases the number of antibody-producing cells [20], and that favours the activation of Th2 lymphocytes [21].
Cadmium is also able to mimic calcium and to bind to SH groups of proteins [14,16]. Exposure to cadmium occurs mainly via tobacco and occupational industries. Cadmium affects both the cellular and humoral immune responses and seems to have a clear carcinogenic effect in humans [14]. Its immunotoxicity also has been suggested by impaired resistance to L. monocytogenes as well as by the inhibition of antigen-specific T cell responses and the induction of abnormalities in antibody production [22,23].
Arsenic, a human carcinogen, is a worldwide contaminant that is found in soil, water and air. Arsenic is an uncoupler of mitochondrial oxidative phosphorylation that induces generation of reactive oxygen species [24]. Different studies have indicated that arsenic is immunotoxic [15]. Lymphocytes from individuals chronically exposed to this metalloid show a longer generation time compared to controls [25]. Another study reported that arsenic interferes with the antigen-presenting function of splenic macrophages [26]. Finally, arsenic is able to alter the response of IgM and IgG antibody-forming cells to sheep erythrocytes, and the proliferative response of lymphocytes to phytohaemagglutinin [27,28].
The mechanisms responsible for the immunotoxicity of As, Cd and Pb have not been elucidated fully. It has been reported that lead and cadmium cause the destruction of the cell membrane of human lymphocytes and monocytes [29]. In addition, cadmium and arsenic are able to induce apoptosis of hamster ovary cells and rat testicular tissue [24,30]. However, the possible effect of these metals on the apoptosis of immune cells has not been reported. The aim of this work was to assess the effect of As, Cd and Pb on the induction of programmed cell death of human peripheral blood mononuclear cells (MNC). We have found that As and Cd, but not Pb, induce apoptosis of immune cells, mainly B lymphocytes and monocytes. Interestingly, this effect of As was also detected in vivo, in children chronically exposed to this contaminant.
MATERIALS AND METHODS
Experimental design
Peripheral blood MNC were obtained from healthy donors. Cells from each subject were exposed for different periods of time to increasing concentrations of sodium arsenite (0–100 μm), cadmium chloride (0–300 μm) or lead acetate (0–500 μm), and then apoptotic cells were quantified by different techniques. All experiments were carried out in quadruplicate. Differences among treatments were determined by the indicated tests. In addition, the preferential effect of metals on MNC subsets was studied. Finally, children with and without chronic exposure to arsenic were also studied: MNC were obtained and the presence of apoptotic cells was assessed at 0, 12 and 36, with or without the addition of As.
Subjects
MNC were obtained from blood samples of 15 healthy adults. As indicated in the Results and figure legends, not all experiments were performed in every one of them. Seven children (4–6 years old) living in a town in which a gold mine is in the urban zone were also studied. These children lived near to the raw mineral breaker and were chronically exposed to arsenic, the main by-product of the gold extraction process. Their urinary levels of As ranged from 94 to 240 μg/g of creatinine (mean = 143·9 μg/g of creatinine). Five children who lived in a nearby town and who were not exposed to As were studied as controls. Their arsenic urinary levels ranged from 17 to 34 μg/g of creatinine (mean = 24·8 μg/g of creatinine). In all cases, written consent was signed by the parents before the study.
Isolation of mononuclear cells
MNC were obtained from heparinized blood by Ficoll-Hypaque (Sigma Chemical Co., St Louis, MO, USA) density gradient centrifugation. After washing with phosphate-buffered saline solution (PBS), MNC were suspended at 1 × 106 cells/ml in RPMI-1640 culture medium (Diagnostics Inc., Mequon, WI, USA), supplemented with 10% fetal bovine serum (FBS, Sigma), 2 mml-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. T and non-T cells were obtained by rosetting with sheep red blood cells (SRBC) as described [31]. Cell viability was determined by trypan blue dye exclusion and was always >95%.
Detection of apoptosis
Cellular apoptosis was detected by four different methods, as follows.
DNA content assay
This assay was performed as described [32]. Briefly, MNC (1 × 106/ml) were incubated with metals, as indicated above, washed with PBS and resuspended in 2 ml lysis buffer (1 mg/ml sodium citrate, 40 μg/ml RNase, 50 μg/ml propidium iodide and Triton X-100, all reagents from Sigma). Then, cell samples were incubated at 4°C for 30 min in the dark before analysis on an EPICS Profile II flow cytometer (Coulter, Hialeah, FL, USA); a minimum of 20000 cells per sample were analysed. Apoptotic, hypodiploid cells were registered and results were expressed as the percentage of apoptotic cells.
Annexin V binding
After their exposure to metals, 1 × 106 cells were washed in PBS, resuspended in binding buffer (10 mm Hepes/NaOH pH 7·4, 140 mm NaCl, 2·5 mm CaCl2), and stained with FITC-conjugated annexin V (Pharmingen, Becton Dickinson Co., San Diego, CA, USA). Then, cells were incubated for 15 min in the dark at room temperature, washed with binding buffer and analysed by flow cytometry. Results were expressed as the percent of annexin V-binding cells.
DNA fragmentation
Cells were treated as described above, and then were rinsed with PBS, resuspended in 1 ml of lysis buffer (Tris-Cl 10 mm pH 7·6, EDTA 10 mm pH 8, NaCl 50 mm, SDS 20% and proteinase K 100 μg/ml), and incubated overnight at 42°C. DNA was precipitated with isopropanol and rinsed three times in 70% ethanol and dried. DNA samples were electrophoresed in an 1% agarose gel with 0·5 μg/ml ethidium bromide at 60 mV for 90 min. DNA was visualized by ultraviolet transillumination and photographed with a Polaroid MP4 camera system.
DNA breaks fluorescence labelling
The APO-DIRECT™ staining kit (Phoenix Flow Systems, San Diego, CA, USA) was used according to the manufacturer's instructions. This end-labelling method (TUNEL) employs a terminal deoxynucleotidyl transferase (TdT) and FITC-dUTP to detect the DNA breaks that are produced by endonucleases during apoptosis. In some experiments, TUNEL and annexin V staining were combined with labelling with different monoclonal antibodies specific for MNC subsets (CD3, CD19, CD14). Cells were analysed in a FACScan flow cytometer (Becton Dickinson, San Diego, CA, USA) and results were expressed as the percent of positive apoptotic cells.
Arsenic quantification
Urinary As levels were determined as described previously [33]. Briefly, urine samples were digested and As content determined by the hydride evolution technique using a Perkin-Elmer 3110 atomic absorption spectrometer. This method detects total As levels. Results were expressed as μg of As/g of urinary creatinine. Arsenic urinary levels are a reliable marker of recent arsenic exposure [15].
RESULTS
To determine whether Cd, As or Pb were able to induce apoptosis of MNC in vitro, cells were cultured in the presence of increasing concentrations of CdCl2, NaAs02 or Pb(COO)2 for 48, and apoptosis was then detected by propidium iodide staining and flow cytometry analysis. As shown in Fig. 1a, As induced a significant level of apoptosis of MNC at 15 μm, whereas Cd had a similar effect at higher concentrations (85–200 μm, Fig. 1a). However, a great individual difference was evident in the susceptibility of MNC from different donors to the effect of these substances (Fig. 1a,b), mainly for As (range 16·5–68·3% apoptosis, at 15 μm for 48, n = 10, DNA content analysis). For some MNC samples As and Cd concentrations as low as 5 and 50 μm, respectively, induced a significant level of apoptosis (Fig. 1b). In contrast, Pb did not induce apoptosis of MNC from any individual, even at very high concentrations (500 μm, Fig. 1b, and data not shown). Kinetics experiments performed incubating MNC with 15 μm As, 100 μm Cd or medium alone (control) showed that after 16 h of exposure to arsenic or cadmium, approximately 13% of MNC were apoptotic, and that this percentage increased to 22·7 and 16·2% at 24, respectively (Fig. 2a). Arsenic showed a higher induction of apoptosis at almost all times of incubation compared to Cd, and reached approximately 70% at 72h of cell culture. Additional experiments showed that low doses of arsenic (1·0 μm) were also able to induce apoptosis in an important fraction of MNC when cells were incubated for longer periods of time (Fig. 2b). Interestingly, the number of viable cells in the cultures incubated in the presence of Cd (100 μm) diminished from 2 × 106 to less than 1 × 106 after 48 h of incubation (Fig. 2c). In the case of As, it was also detected an important decrease of viable cells (from 2 × 106 to 0·5 × 106), but the percentage of apoptotic cells in these cultures was very high, approximately 50% (Fig. 2d). These results suggested that Cd exerted an additional cytotoxic effect on MNC through a mechanism different to the induction of apoptosis.
Fig. 1.
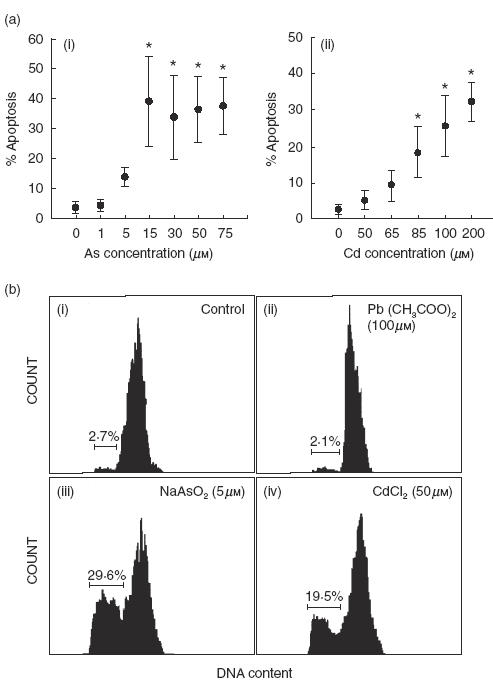
In vitro effect of arsenic and cadmium on the apoptosis of normal human MNC. (a) Dose–response assays. Peripheral blood MNC were incubated in the presence of increasing concentrations of sodium arsenite (i) or cadmium chloride (ii) for 48h and then the percentage of apoptotic cells was determined by propidium iodide staining and flow cytometry analysis, as described in Materials and methods. Data correspond to the arithmetic mean and s.d. of the percentage of apoptotic cells of four independent experiments. Asterisks indicate P < 0·05 compared to the negative control. (b) Peripheral blood MNC obtained from a healthy donor were cultured in the absence (i), or presence of 100 μm lead (ii), 5 μm arsenic (iii), or 50 μm cadmium (iv) for 48 h. Then, MNC apoptosis was determined as in (a). The percentage of hypodiploid, apoptotic cells is indicated. Results correspond to one healthy individual out of 10 studied.
Fig. 2.
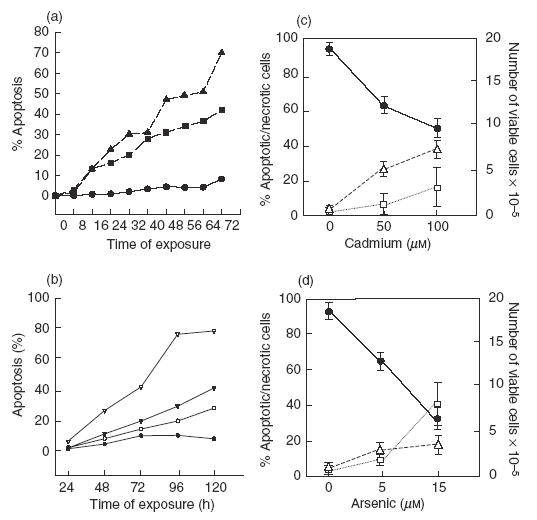
In vitro effect of As and Cd on the apoptosis and necrosis of normal human MNC. (a, b) Kinetics analysis of the effect of As and Cd on the apoptosis of MNC. Cells were exposed to NaAsO2, CdCl2 or culture medium alone for the indicated periods of time and then the percentage of cells undergoing apoptosis was determined by propidium iodide staining, as described in Material and methods. Results correspond to one representative experiment out of three performed. (a) ▴, As (15 μm); ▪, Cd (100 μm); •, medium alone. (b) ▿, As (5·0 μm); ▾, As (2·5 μm); ○, As (1·0 μm); •, medium alone. (c, d) Effect of As and Cd on MNC viability. Peripheral blood MNC were incubated in the presence of the indicated concentrations of Cd (c) and As (d) for 48 h and then the number of viable and necrotic cells was determined by trypan blue staining and direct microscope counting. In cell cultures run in parallel, the percentage of apoptotic cells was determined as in (a). Results correspond to the arithmetic mean and s.d. of five independent experiments. (c, d) •, Viable cells; □, apoptotic cells; ▵, necrotic cells.
The induction of apoptosis by As and Cd was confirmed using alternative techniques for apoptosis detection. Figure 3a shows the results of an experiment in which apoptosis was detected by annexin V-FITC staining. In this case, a very high percentage of cells became annexin V-positive (29·7% and 92·8% for Cd and As, respectively) after 24h of cell culture. In addition, the flow cytometry analysis of cells stained for DNA breaks by TdT/FITC-dUTP end-labelling confirmed that As and Cd were able to induce apoptosis (Fig. 3b). Interestingly, these techniques showed a higher sensitivity than DNA content analysis for apoptosis detection (compare Figs 1a and 3c). On the other hand, when MNC were treated with As or Cd, the characteristic pattern of internucleosomal DNA fragmentation (ladder) was detected after 24 h of incubation with both metals, whereas control cells did not show evidence of DNA damage (data not shown). As expected, when MNC were preincubated with 1 mm ZnS04 (an endonuclease inhibitor) for 1 h before metal addition (100 μm Cd or 15 μm As), a significant inhibition of programmed cell death was observed (data not shown).
Fig. 3.
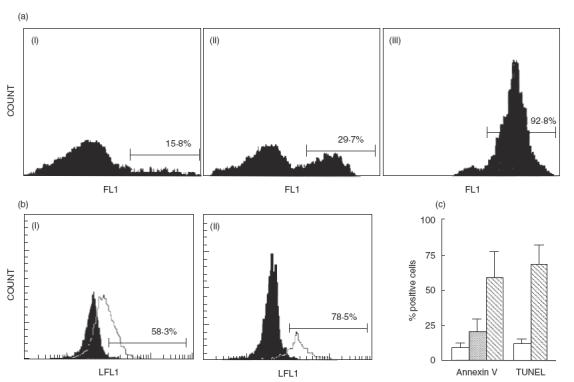
Detection of apoptosis induced by As and Cd by annexin V binding and TUNEL assays. (a) MNC cultured in absence (i) or presence of 100 μm cadmium (ii) or 15 μm arsenic (iii) for 24h were stained with the phosphatidylserine-binding protein annexin V-FITC, and analysed by flow cytometry. Results of one experiment out of five performed are shown. Numbers correspond to the percentage of positive cells. (b) Arsenic induces DNA breaks in MNC. Peripheral blood MNC cultured in the presence or not of 15 μm As for 24h were stained for DNA breaks by TdT/FITC-dUTP end-labelling (TUNEL), as described in Material and methods. Solid histograms correspond to cells incubated in medium alone and empty histograms to cells incubated with As. Lymphocytes (i) and monocytes (ii) were analysed separately, according to their side and forward scatter characteristics. Results of one representative experiment out of four performed are shown. (c) Results of all experiments described in (a) (annexin V binding) and (b) (TUNEL) are shown. Data correspond to the arithmetic mean and s.d. of five (annexin V) and four (TUNEL) independent experiments. Values of TUNEL correspond to the percentage of apoptosis of the whole MNC population. Empty bars = medium alone; grey bar = Cd 100 μm; hatched bars = As 15 μm.
We then investigated whether the immunotoxic effect of Cd and As was preferentially exerted on a MNC cell subset. T and non-T cells were isolated by rosetting with SRBC, and then the apoptotic effect of As and Cd on these cells was tested. As shown in Fig. 4a, non-T cells were more susceptible to the induction of apoptosis compared to T lymphocytes. This effect was more evident in cells treated with As. Double-labelling experiments using anti-CD3, anti-CD14 and anti-CD19 MoAbs plus annexin V-FITC or TUNEL staining confirmed that non-T cells had a higher susceptibility to the effect of As compared to T lymphocytes (Fig. 4b,c).
Fig. 4.
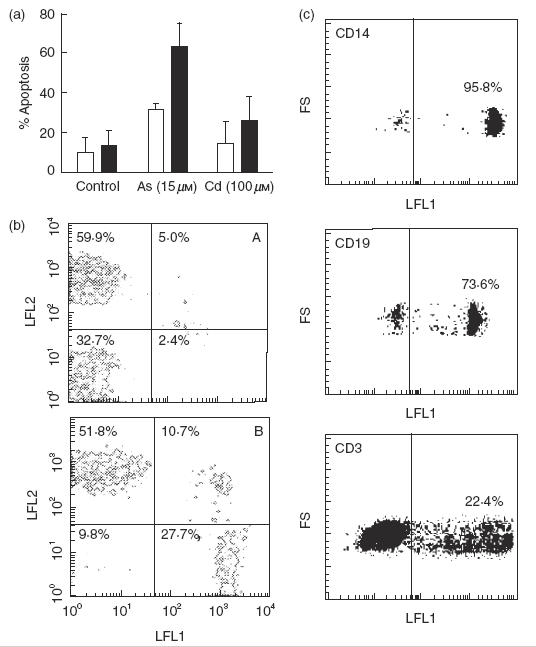
Non-T cells have a high susceptibility to the induction of apoptosis by arsenic and cadmium. (a) T and non-T cells were cultured in the presence or absence of 15 μm arsenic or 100 μm cadmium for 24, and then the percentage of apoptotic cells was determined by flow cytometry after staining with propidium iodide. Results correspond to the arithmetic mean and s.d. of the percentage of apoptosis of four independent experiments. □, T cells; ▪, non-T cells. (b) Peripheral blood MNC were incubated in medium alone (A) or in the presence of 15 μm As for 24h (B), and then cells were double-stained for CD3 (LFL2) and annexin V (LFL1). Numbers correspond to the percentage of cells in each quadrant. Results of one representative experiment out of three performed are shown. (c) MNC incubated with 15 μm As for 18 h were stained by TdT/FITC-dUTP end-labelling (TUNEL) and then labelled with the indicated PE-conjugated antibodies. Cells positive for each antibody were electronically gated and analysed in the histograms that are shown. Data of one representative experiment out of three performed are shown. Numbers correspond to the percentage of apoptotic cells in each MNC subset.
The important pro-apoptotic effect of As found in vitro prompted us to investigate whether this phenomenon could be observed in individuals exposed to this metalloid. Figure 5a shows that fresh isolated MNC from children chronically exposed to As, and with high urinary levels of this contaminant, exhibited a significantly enhanced basal percentage of hypodiploid cells (P = 0·001, Mann–Whitney U-test). However, a non-significant correlation between the basal percentage of apoptotic cells and the concentration of urinary As (rs = 0·56, P > 0·05, Fig. 5b) was found in these exposed children. Interestingly, when MNC from these children were cultured in the presence of As during 12–36, the percentage of apoptotic cells was significantly lower compared with cells from non-exposed children (Fig. 5c, p = 0·037, Mann–Whitney U-test).
Fig. 5.
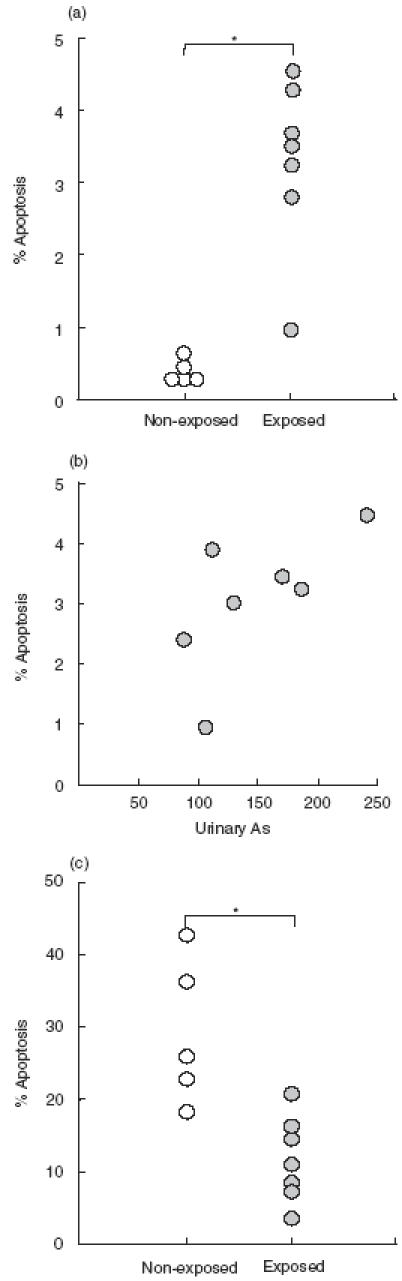
Individuals exposed to arsenic show enhanced in vivo apoptosis of MNC and diminished sensitivity to As in vitro. (a) Peripheral blood MNC were isolated from seven children with chronic exposure to and high urinary levels of As (filled circles), and five healthy non-exposed matched controls (open circles). The fresh isolated cells were immediately stained with PI and analysed for hypodiploid cells by flow cytometry. Asterisk indicates P = 0·001. (b) Lack of significant correlation between the percent of apoptotic MNC and As urinary levels in the seven children shown in (a). rs, Spearman correlation coefficient. rs = 0·56; P > 0·05. (c) MNC from As-exposed and non-exposed children were incubated for 36h in the presence of 15 μm As. Then, apoptotic cells were detected as in (a). Asterisk indicates P = 0·037.
DISCUSSION
It is well known that apoptosis plays a key role in the homeostasis of the immune system, and in recent years it has become clear that programmed cell death is also involved in many pathological conditions, either as the result of its enhancement or inhibition [8]. Thus, abnormalities in programmed cell death are involved in different immunological diseases, including AIDS [8,34,35]. In this regard, it seems evident that the uncontrolled elimination of immune cells may account for immunosupression or immune dysregulation, depending on the lymphoid cell subset affected. Recently, the number of toxic agents with an apoptotic-inducing effect on immune cells has increased [9–12], and these substances seem to be responsible for immunodeficiency.
Metals such as arsenic, cadmium and lead are important polluting agents, and in many places around the world humans are exposed to high concentrations of these metals. It has been described widely that both the acute or chronic exposure to Cd, As or Pb are associated with damage at different levels [13–15]. However, the effects of lead, cadmium and arsenic on immune cells have received little attention. Our results demonstrate that arsenic is able to induce apoptosis of an important fraction of MNC. Although the degree of apoptosis was variable among the individuals tested, cells from all donors exhibited an important apoptotic response to As, with a significant effect at 15 μm after 48h of incubation. These data are in agreement with another study in which 40 μm arsenite was able to induce apoptosis of chinese hamster ovary cells [24]. On the other hand, using a different experimental model (rat thymocytes), it was found that exposure to low concentrations of NaAsO2 (0·01–1·0 μm) resulted in apoptotic changes after 6h of incubation [36]. In this regard, we detected that when MNC were cultured for longer periods of time (≥3 days) very low doses of As were able to induce apoptosis of a significant proportion of MNC. Therefore, arsenic seems to be an efficient stimulus of apoptosis in different cells, including human normal lymphoid cells.
In the case of Cd, we found that higher concentrations than As were necessary to induce apoptosis of MNC. This finding is consistent with a report showing that in rats, the toxic effect of arsenic is higher compared with cadmium [37]. However, our data indicate that Cd exerts an important toxic effect on MNC that is not mediated through the induction of apoptosis. It would be interesting to elucidate the additional mechanism of toxicity of Cd on lymphoid cells. On the other hand, our results are in agreement with previous reports in which the administration of CdCl2 to rodents induced apoptosis in liver and testicular cells [38,39]. However, other studies using isolated nuclei from mammalian cells, indicate that Cd has a dual effect, inhibiting the endonuclease activity triggered by calcium, but inducing this enzymatic activity in the absence of calcium, probably by replacing it [40,41]. It is worth mentioning that our experiments with Cd were performed in the presence of Ca2+, and that under such experimental conditions we observed induction of apoptosis in all MNC samples tested.
We found that lead was unable to induce apoptosis of MNC even at very high concentrations (500 μm). This finding is in agreement with a previous study on the effect of Hg, Ag, Cu, Pb and Zn on human lymphocytes, in which a lower toxicity of lead was found compared with the other metals [29]. Nevertheless, it has been reported that lead is able to promote the apoptosis of other cells as cerebellar neurones [42], and that this phenomenon occurs at low concentrations (1 μm). Thus, although lead seems to be able to induce apoptosis, this effect is not observed in normal human MNC. It will be interesting to explore additional mechanisms of lead toxicity on lymphoid cells. On the other hand, the lack of induction of apoptosis by lead makes evident that the effect of As and Cd is specific and that the induction of apoptosis of lymphoid cells is not an indiscriminate phenomenon triggered by any metal.
The stimuli involved in the induction of apoptosis are very variable depending on cell type. Since MNC is a heterogeneous cell population, it is feasible that some of these cells are more susceptible to the toxic effect of As and Cd. In this regard, the shape and the plateau of the dose–response curves of these metals (Fig. 2) suggest that there are two cell subsets with high and low sensitivity to the induction of apoptosis by these metals. Therefore, we decided to determine whether or not the effect of arsenic and cadmium was differentially exerted on T and non-T cells. Interestingly, we found that the latter cells were more susceptible to the induction of apoptosis by both metals. Additional double-labelling experiments (CD3-CD14-CD19/annexin V or TUNEL) further indicated that As mainly exerts its pro-apoptotic effect on B lymphocytes and monocytes. A preferential toxicity on a lymphoid cell subset has also been observed in the case of ethanol, which induces mainly apoptosis of B cells compared to T cells [10]. On the other hand, we have found previously that stimulation through CD50 mainly triggers the programmed cell death of a T cell subset [43].
It is worth mentioning that the concentrations of As that induced apoptosis of MNC in vitro were similar to those observed in vivo, in individuals exposed to this pollutant (0·5–7·5 μm, see [44]). Therefore, it is feasible that the in vitro effect of Cd and As on MNC observed by us indeed occurs in vivo in the individuals with chronic exposure to these metals. In fact, the high basal level of MNC apoptosis found in children chronically exposed to and with high urinary levels of As suggest strongly that this metal exerts its pro-apoptotic effect on MNC in vivo. This point is supported further by the low in vitro sensitivity of the MNC from children exposed to As, suggesting that the susceptible cells have been partially eliminated in vivo in these individuals. However, we did not find a significant correlation between the urinary level of As and the percentage of basal apoptosis in the exposed children. It is possible that this phenomenon is due to the small number of children studied (n = 7) as well as to the individual differences in the susceptibility to the effect of As observed in this study. On the other hand, the toxic effect of Cd and As could be exerted on other immune cells such as Langerhans cells, which have a key role in the induction of immune response. In this regard, it has been reported that there is a low number of epidermal Langerhans cells in the skin neoplasias induced by As [45]. It will be interesting to assess in vivo, in individuals with chronic exposure to As or Cd, the presence of apoptotic cells in skin biopsies.
The preferential effect of As and Cd on B cells and monocytes could account for the immunotoxic effects of these metals on the humoral immune response, or the functions carried out by monocytes/macrophages such as NO production [18]. However, it has also been reported that both As and Cd have a deleterious effect on the T cell-mediated immune response [22,27], a phenomenon that, at first glance, is not in agreement with our results. It is feasible that the defects in the cellular immune response induced by As and Cd are related to deficiencies in antigen presentation, a function exerted mainly by B cells and mononuclear phagocytic cells, including Langerhans cells. An alternative possibility, as stated above, is that Cd and As have mechanisms of immunotoxicity additional to their pro-apoptotic effect.
Our results demonstrate, for the first time, that As and Cd, but not Pb, are able to induce apoptosis of human normal MNC. These findings are in agreement with the use of arsenic in the treatment of acute promyelocytic leukaemia [46].
REFERENCES
- 1.Cohen JJ. Apoptosis: physiologic cell death. J Lab Clin Med. 1994;124:761–5. [PubMed] [Google Scholar]
- 2.Cohen JJ, Duke RC. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–93. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 3.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortner CD, Oldenburg NBE, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–32. doi: 10.1016/s0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- 5.Pushkareva M, Obeid LM, Hannun YA. Ceramide: endogenous regulator of apoptosis and growth suppression. Immunol Today. 1995;16:294–7. doi: 10.1016/0167-5699(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–87. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- 7.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–9. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 9.Alison MR, Sarraf CE. Apoptosis. regulation and relevance to toxicology. Hum Exp Toxicol. 1995;14:355–62. doi: 10.1177/096032719501400302. [DOI] [PubMed] [Google Scholar]
- 10.Slukvin II, Jerrells TR. Different pathways of in vitro ethanol-induced apoptosis in thymocytes and splenic T and B lymphocytes. Immunopharmacology. 1995;31:43–57. doi: 10.1016/0162-3109(95)00032-4. [DOI] [PubMed] [Google Scholar]
- 11.Shenker BJ, Datar S, Mansfield K, Shapiro IM. Induction of apoptosis in human T-cells by organomercuric compounds: a flow cytometric analysis. Toxicol Appl Pharmacol. 1997;143:397–406. doi: 10.1006/taap.1997.8111. [DOI] [PubMed] [Google Scholar]
- 12.McConkey DJ, Hartzell P, Duddy SK, Hakansson H, Orrenius S. 2,3,7,8-tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988;242:256–9. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- 13.ATSDR. Toxicological profile for lead. Atlanta, GA: Agency for Toxic Substances and Disease Register, US Department of Health & Human Services; 1998. [Google Scholar]
- 14.ATSDR. Toxicological profile for cadmium. Atlanta, GA: Agency for Toxic Substances and Disease Register, US Department of Health & Human Services; 1998. [Google Scholar]
- 15.ATSDR. Toxicological profile for arsenic. Atlanta, GA: Agency for Toxic Substances and Disease Register, US Department of Health & Human Services; 1999. [Google Scholar]
- 16.Klaassen CD, Eaton DL. Principles of toxicology. In: Amdur C, Doull R, Klaasen CD, editors. Casarett and Doull's toxicology. New York: McGraw-Hill; 1993. pp. 26–30. [Google Scholar]
- 17.Kishikawa H, Sang R, Lawrence DA. Interleukin-12 promotes enhanced resistance to Listeria monocytogenes infection of lead-exposed mice. Toxicol Appl Pharmacol. 1997;147:180–9. doi: 10.1006/taap.1997.8308. [DOI] [PubMed] [Google Scholar]
- 18.Tian T, Lawrence DA. Metal-induced modulation of nitric oxide production in vitro by murine macrophages: lead, nickel, and cobalt utilise different mechanisms. Toxicol Appl Pharmacol. 1996;141:540–7. doi: 10.1006/taap.1996.0320. [DOI] [PubMed] [Google Scholar]
- 19.Guo TL, Mudzinski SP, Lawrence DA. The heavy metal lead modulates the expression of both TNF-alpha and TNF-alpha receptors in lipopolysaccharide-activated human peripheral blood mononuclear cells. J Leukoc Biol. 1996;59:932–9. doi: 10.1002/jlb.59.6.932. [DOI] [PubMed] [Google Scholar]
- 20.Borella P, Giardino A. Lead and cadmium at very low doses affect in vitro immune response of human lymphocytes. Environ Res. 1991;5:165–77. doi: 10.1016/s0013-9351(05)80173-2. [DOI] [PubMed] [Google Scholar]
- 21.Heo Y, Parsons PJ, Lawrence DA. Lead differentially modifies cytokine production in vitro and in vivo. Toxicol Appl Pharmacol. 1996;138:149–57. doi: 10.1006/taap.1996.0108. [DOI] [PubMed] [Google Scholar]
- 22.Simonet M, Berche P, Fauchere JL, Veron M. Impaired resistance to Listeria monocytogenes in mice chronically exposed to cadmium. Immunology. 1984;53:155–63. [PMC free article] [PubMed] [Google Scholar]
- 23.Hurtenbach U, Oberbarnscheidt J, Gleichmann E. Modulation of murine T and B cell reactivity after short-term cadmium exposure in vivo. Arch Toxicol. 1988;62:22–8. doi: 10.1007/BF00316252. [DOI] [PubMed] [Google Scholar]
- 24.Wang TS, Kuo CF, Jan KY, Huang H. Arsenite induces apoptosis in chinese hamster ovary cells by generation of reactive oxygen species. J Cell Physiol. 1996;169:256–68. doi: 10.1002/(SICI)1097-4652(199611)169:2<256::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Ostrosky-Wegman P, Gonsebatt ME, Montero R, et al. Lymphocyte proliferation kinetics and genotoxic findings in a pilot study on individuals chronically exposed to arsenic in Mexico. Mutat Res. 1991;250:477–82. doi: 10.1016/0027-5107(91)90204-2. [DOI] [PubMed] [Google Scholar]
- 26.Sikorski EE, Burns LA, McCoy KL, Stern M, Munson AE. Suppression of splenic accesory cell function in mice exposed to gallium arsenide. Toxicol Appl Pharmacol. 1991;110:143–56. doi: 10.1016/0041-008x(91)90297-r. [DOI] [PubMed] [Google Scholar]
- 27.Sikorski EE, McCay JA, White KL, Bradley SG, Munson AE. Immunotoxicity of the semiconductor gallium arsenide in female B6C3F1 mice. Fundam Appl Toxicol. 1989;13:843–58. doi: 10.1016/0272-0590(89)90338-2. [DOI] [PubMed] [Google Scholar]
- 28.Savabieasfahani M, Lochmiller RL, Rafferty DP, Sinclair JA. Sensitivity of wild cotton rats (Sigmodon hispidus) to the immunotoxic effects of low-level arsenic exposure. Arch Environ Contam Toxicol. 1998;34:289–96. doi: 10.1007/s002449900320. [DOI] [PubMed] [Google Scholar]
- 29.Steffensen IL, Mesa OJ, Andruchow E, Namork E, Hylland K, Andersen RA. Cytotoxicity and accumulation of Hg, Ag, Cd, Cu, Pb and Zn in human peripheral T and B lymphocytes and monocytes in vitro. Gen Pharmacol. 1994;25:1621–33. doi: 10.1016/0306-3623(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 30.Yan H, Carter CE, Xu C, et al. Cadmium-induced apoptosis in the urogenital organs of the male rat and its suppression by chelation. J Toxicol Environ Health. 1997;52:149–68. doi: 10.1080/00984109708984058. [DOI] [PubMed] [Google Scholar]
- 31.Chess L, Schlossman SF. Methods for the separation of unique human lymphocyte subpopulations. In: Rose NR, Friedman H, editors. Manual of clinical immunology. Washington, DC: American Society for Microbiology; 1976. pp. 79–85. [Google Scholar]
- 32.Ormerod MG. Analysis of DNA-general methods. In: Ormerod MG, editor. Flow cytometry. A practical approach. Oxford: IRL Press; 1994. pp. 119–35. [Google Scholar]
- 33.Cox DH. Arsine evolution–electrothermal atomic absorption method for the determination of nanogram levels of total arsenic in urine and water. J Anal Toxicol. 1980;4:207–11. doi: 10.1093/jat/4.4.207. [DOI] [PubMed] [Google Scholar]
- 34.Gougeon ML, Lecoeur H, Dulioust A, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 35.Horikawa K, Nakakuma H, Kawaguchi T, et al. Apoptosis resistance of blood cells from patients with paroxysmal nocturnal hemoglobinuria, aplastic anemia, and myelodisplastic syndrome. Blood. 1997;90:2716–21. [PubMed] [Google Scholar]
- 36.Bustamante J, Dock L, Vahter M, Fowler B, Orrenius S. The semiconductor elements arsenic and indium induce apoptosis in rat thymocytes. Toxicology. 1997;118:129–36. doi: 10.1016/s0300-483x(96)03607-4. [DOI] [PubMed] [Google Scholar]
- 37.Yañez L, Carrizales L, Zanatta MT, Mejia JJ, Batres L, Diaz-Barriga F. Arsenic–cadmium interaction in rats: toxic effects in the heart and tissue metal shifts. Toxicology. 1991;67:227–34. doi: 10.1016/0300-483x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- 38.Xu C, Johnson JE, Singh PK, Jones MM, Yan H, Carter CE. In vivo studies of cadmium-induced apoptosis in testicular tissue of the rat and its modulation by a chelating agent. Toxicology. 1996;107:1–8. doi: 10.1016/0300-483x(95)03195-l. [DOI] [PubMed] [Google Scholar]
- 39.Habeebu S, Liu J, Klaasen CD. Cadmium-induced apoptosis in mouse liver. Toxicol Appl Pharmacol. 1998;149:203–9. doi: 10.1006/taap.1997.8334. [DOI] [PubMed] [Google Scholar]
- 40.Lohmann RD, Beyersmann D. Cadmium and zinc mediated changes of the Ca2+-dependent endonuclease in apoptosis. Biochem Biophys Res Commun. 1993;190:1097–103. doi: 10.1006/bbrc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 41.Lohmann RD, Beyersmann D. Effects of zinc and cadmium on apoptotic DNA fragmentation in isolated bovine liver nuclei. Environ Health Perspect. 1994;102(Suppl. 3):269–71. doi: 10.1289/ehp.94102s3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberto A, Marks N, Evans HL, Guidotti AJ. Lead (Pb2+) promotes apoptosis in newborn rat cerebellar neurons: pathological implications. J Pharmacol Exp Ther. 1996;277:435–42. doi: 10.1163/2211730x96x00234. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Briones S, Portales-Perez DP, Baranda L, de la Fuente H, Rosenstein Y, Gonzalez-Amaro R. Stimulation through CD50 preferentially induces apoptosis of TCR1+ human peripheral blood lymphocytes. Cell Adhes Commun. 1998;6:465–72. doi: 10.3109/15419069809010795. [DOI] [PubMed] [Google Scholar]
- 44.Driesback RH. Handbook of poisoning: prevention, diagnosis and treatment. 11. Los Altos, CA: Lange Medical Publications; 1980. pp. 241–5. [Google Scholar]
- 45.Schwartz RA. Arsenic and the skin. Int J Dermatol. 1997;36:241–50. doi: 10.1046/j.1365-4362.1997.00101.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–53. [PubMed] [Google Scholar]


