Abstract
To determine the biological functions of membrane expressed CD45 isoforms on polymorphonuclear neutrophils (PMN), the monoclonal IgG F(ab′)2 antibody against CD45, CD45RA or CD45RO was used as surrogate ligand for binding with these molecules on PMN. We found 99·5 ± 3·2%, 42·3 ± 5·8% and 96·7 ± 2·6% PMN expressed CD45, CD45RA and CD45RO molecules on the cell surface, respectively. The interaction of CD45, CD45RA or CD45RO with its specific antibody on PMN enhanced phagocytosis markedly (34–83% increase), mainly via increased expression of complement receptor type 3 (CR3, CD11b) on the cells. The production of IL-8 by PMN was also increased significantly after binding with antibodies (anti-CD45 > anti-CD45RO > anti-CD45RA). Anti-CD45RA and anti-CD45RO, but not anti-CD45, enhanced TNF-α mRNA expression and decreased protein tyrosine phosphorylation of PMN. However, only anti-CD45RO suppressed Src family protein tyrosine kinase p56lck expression in the cells. These results suggest that the cross-linking of CD45 isoforms by their specific antibodies stimulated different PMN activities by differential suppression on protein tyrosine phosphorylation and Src family tyrosine kinase p56lck.
Keywords: leucocyte-common antigen, PMN phagocytosis, proinflammatory cytokines, protein tyrosine kinase p56lck
INTRODUCTION
The CD45 molecule, referred to as ‘leucocyte-common antigen’, is a family of high molecular weight transmembrane protein tyrosine phosphatase (PTPase) expressed on all nucleated haematopoietic cells [1,2]. CD45 is expressed as one of the eight potential isoforms that vary in molecular weight from 180kDa (CD45RO) to 220kDa (CD45RA) due to alternative mRNA splicing of up to three exons, 4–6, encoding a variable amino-terminal domain rich in O-linked sugars [3]. In human peripheral CD4+T lymphocytes, CD45RA+ and CD45RO+ subsets have been confirmed as representing the naive and memory T cells, respectively [4]. Many authors have reported the possible roles of CD45 and its isoforms in T and B cell differentiation [5–10], natural killer and cytotoxic T lymphocyte functions [10–12], cytokine production of mononuclear cells [13–15] and TCR-associated signalling in T cells [16,17]. Recently, CD45 has been demonstrated to be crucial in the activation of p56lck member of the Src family tyrosine kinase during T cell activation [18–23]. Mature PMN have long been regarded as the terminally differentiated cells incapable of protein synthesis. However, a number of authors found that PMN expressed many important cytokine and chemokine mRNA, including IL-1, IL-6, IL-8, IL-10, IL-12, TNF-α, G-CSF, GM-CSF, MIP-1α, MIP-1β and MCP-2 constitutionally or by stimulation [24,25]. Ontogenically, CD45RO isoform was little expressed on immature myeloid cells but became increasingly dense towards the terminal stage of myeloid maturation. By contrast, the high molecular weight isoform CD45RA disappears virtually from the mature myeloid cells [26,27]. Unexpectedly, only a few studies have discussed the biological roles of CD45 isoforms in human mature granulocytes in the literature. Liles et al.[28] reported that the respiratory burst induced by neutrophil activators was enhanced by CD45 cross-linking with the antibody and cross-linker. Harvath et al.[29] demonstrated that CD45 epitopes, after interacting with leukotriene B4 (LTB4) and complement component C5a receptor-associated molecules, regulated the chemotactic responses of PMN. Recently, Gao et al.[30] demonstrated that CD45 could modulate human neutrophil functions such as decreased ADCC activity but increased IL-6 production through FcγRIIa. In the present study, we incubated normal human PMN with IgG F(ab′)2 antibody against CD45, CD45RA or CD45RO as surrogate ligand and found the interactions exerted a profound modulating effect on PMN functions. The molecular basis of the neutrophil-stimulating effects by anti-CD45 isoform antibodies was discussed.
MATERIALS AND METHODS
Antibodies and reagents
Monoclonal mouse antibodies against human CD45 (clone J-33, IgG1) CD45RA (clone ALB11, IgG1) and CD45RO (clone UCHL1, IgG2a) were purchased from ImmunoTech (Marseille Cedex 9, France). The isotype-matched mouse non-specific IgG were obtained from Sigma Immunochemical Corp. (St Louis, MO, USA). These immunoglobulin G molecules were pepsin digested (enzyme:IgG ratio = 1:50 by weight) at pH 3·5 for 20h at 37°C. The digested fragments were then absorbed with protein A-conjugated agarose beads (Sigma) at room temperature for 1 h. The non-absorbed IgG F(ab′)2 supernatants were proved free of intact IgG molecules and Fcγ fragments analysed by 10% SDS-PAGE. The purified IgG F(ab′)2 portions of anti-CD45 isofroms and mouse IgGs were used in some experiments in comparison with whole IgG antibody molecules. FITC-labelled monoclonal antibodies against human complement receptor type 1(CR1, C3bR/CD35), type 3 (CR3, CD11b) and FcγRIII (CD16) were purchased from Ancell Corporation (Bayport, MN, USA). Polymyxin B (PMB), bacterial lipopolysacchride (LPS, Eschericia coli serotype 0111:B4), cytochalasin B and Limulus amoebocyte coagulation test kits were obtained from Sigma Chemical Company. Mouse monoclonal antiphosphotyrosine (clone PT 20, IgG2b) and antip56lck (clone 28, IgG2a) were purchased from Transduction Laboratories (Lexington, KY, USA).
Isolation of neutrophils, lymphocytes and monocytes from normal human peripheral blood
Heparinized venous blood obtained from normal individuals was mixed with one-quarter volume of 2% dextran solution (molecular weight 500000Da) and incubated at room temperature for 30 min. Leucocyte-enriched supernatant was collected and diluted with the same volume of Hanks’ balanced salt solution (HBSS). After Ficoll-Hypaque (specific gravity 1·077–1·078) density gradient centrifugation at 150g for 20 min, the mononuclear cells (MNC) were aspirated from the interphase and the PMN were obtained from the bottom. The contaminating red blood cells in PMN suspensions were lysed by incubating with chilled 0·83% NH4Cl solution at 4°C for 10 min. The adherent cells (monocytes/macrophages) in MNC suspension were harvested by scraping with a rubber policeman after incubation in Petri dishes at 37°C in 5% CO2–95% air for 60 min. The same procedure was repeated twice for obtaining the highly pure adherent and non-adherent cells (lymphocytes) from MNC. The concentration of PMN, lymphocytes and monocytes was adjusted to 2 × 106/ml in 10% fetal bovine serum in RPMI-1640 (10% FBS-RPMI). The viability of three cell populations was greater than 95% confirmed by trypan blue dye exclusion. The purity of PMN and lymphocytes was ≥95% confirmed by Wright's stain. The purity of monocytes was ≥93% confirmed by non-specific esterase staining kit (Sigma). The culture medium, cell suspensions and cell-cultured supernatants were confirmed free of bacterial endotoxin contamination as detected by L. amebocyte coagulation test kit.
Detection of CD45, CD45RA and CD45RO expression on the surface of different cell populations by flow cytometry
Freshly isolated PMN, lymphocytes and monocytes (1 × 106/ml) were incubated with 5 μl of monoclonal antibody (50 μg/ml) against human CD45, CD45RA, CD45RO or isotype-matched mouse non-specific IgG as primary antibody in an ice-bath for 30 min. After three washes with PBS, pH 7·2, the cell suspensions were stained with FITC-labelled goat anti-mouse IgGs (Jackson ImmunoResearch Laboratory Inc., West Grove, PA, USA) in an ice-bath for another 30 min. After several time-washes, both percentage (%) and mean fluorescence intensity (MFI, denoted by mean channel number) of the positive cells were measured by FACSort flow cytometry (Becton-Dickinson Immunocytometry Systems, Mountain View, CA, USA).
Agglutinating activity of monoclonal antibody against CD45 isoforms on PMN and MNC
Fifty microlitres of PMN or MNC suspension (5 × 106/ml), 10 μl of anti-CD45, anti-CD45RA or anti-CD45RO monoclonal antibody (50 μg/ml) and 40 μl of PBS, pH 7·2 were mixed in a round-bottomed microwell and incubated at room temperature for 4 h. The cell agglutination was observed by eye.
Measurement of PMN phagocytosis by flow cytometry
We followed the method reported by Shalaby et al.[31]. Briefly, PMN (1 × 106/ml) were preincubated with 10 μl of IgG F(ab′)2 antibody against CD45 isoforms (50 μg/ml) for 45 min. Commercially available fluoresbrit carboxylate microsphere (0·75 μm in diameter, Polysciences Inc., Washington, PA, USA) that were opsonized previously with fresh normal human serum at 37°C for 60 min were then added to the antibody-pretreated PMN (PMN:beads = 1:100) and incubated for another 45 min at 37°C in 5% CO2–95% air. After incubation, the cells were centrifuged at 300 g for 10min three times to remove the free beads followed by fixation with 2·5% paraformaldehyde. Both percentage and mean fluorescence intensity (MFI) of PMN with phagocytosis were detected by FACSort flow cytometry (Becton-Dickinson) after subtracting the non-specific binding of opsonized beads with PMN. The non-specific binding of beads was detected by pretreatment of PMN with cytochalasin B (10 ng/ml) for 30min before reacting with opsonized beads. We found the non-specific binding of opsonized beads with PMN was usually less than 5%.
Measurement of phagocytosis-related membrane receptors by flow cytometry
Direct immunofluorescence antibody method was used to measure the phagocytosis-related membrane receptors expression on PMN including complement receptor type 1 (CR1), type 3 (CR3) and IgG Fc receptor type III (FcγRIII). Briefly, PMN (1 × 106/ml) were preincubated with IgG F(ab′)2 antibody against CD45, CD45RA or CD45RO at room temperature for 60min. These cells were then stained with FITC-labelled monoclonal mouse anti-human CR1, anti-CR3 or anti-FcγRIII in an ice-bath for 30 min. Both percentage and MFI of positive PMN were analysed by FACSort flow cytometry (Becton-Dickinson).
Preparation of PMN cultured supernatants
PMN suspension, 0·25 ml (2 × 106/ml), 0·01 ml of individual IgG F(ab′)2 anti-CD45 isoform antibody or mouse non-specific IgG F(ab′)2 (50 μg/ml), and 0·24 ml 10% FBS-RPMI or 0·19 ml 10% FBS-RPMI + 0·05 ml polymyxin B (50 ng/ml) were mixed in a conical tube and incubated at 37°C in 5% CO2–95% air for 24 h. The cell-free cultured supernatants were harvested and checked for bacterial endotoxin contamination by L. amebocyte coagulation test before use.
Quantification of IL-8 in the PMN cultured supernatants by ELISA
Commercially available IL-8 ELISA kit (Quantikine, R&D System, Minneapolis, MN, USA) was used for measuring the concentration of IL-8 in PMN cultured supernatants. The detailed procedures are described in the manufacturer's instruction booklet. The minimal detectable concentration of IL-8 was 18·1 pg/ml.
Reverse transcription-polymerase chain reaction (RT-PCR) detection of different cytokine mRNA expression in PMN
Total cellular RNA extraction and cDNA synthesis
PMN (1 × 107/ml) were incubated with antibody against CD45, CD45RA, CD45RO or mouse IgG (50 μg/ml) at 37°C in 5% CO2–95% air for 2 h. The total cellular RNA was then extracted according to the method of Chomczynski and Sacchi [32]. cDNA was synthesized by priming 1 μg/ml of total RNA at 42°C for 1h in a final volume of 20 μl containing 1 μg of oligo-dT primer (Pharmacia Fine Chemicals, Piscataway, NJ, USA), 200 nmol of each dNTP (Pharmacia) and MMLV reverse transcriptase (Bethesda Research Laboratories, Gaithersburg, MD, USA) at 200 U/ng RNA.
Amplification of cDNA by PCR
An aliquot of cDNA was amplified by PCR using oligonucleotide paired primers specific for human IL-1β, IL-4, IL-8, TNF-α or IFN-γ. Primers for human glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were used to amplify the cDNA of ubiquitous molecule in the cells as internal control. The nucleotide sequences of these pair primers is demonstrated below:
Human IL-1β: 5′ATG GCA GAA GTA CCT AAG CTC GC 3′ (sense);
5′A CAC AAA TTG CAT GGT GAA GTC AGT T 3′ (antisense).
Human IL-4: 5′ CGG CAA CTT TGA CCA CGG ACA CAA GTG GGA TA 3′ (sense);
5′ ACG TAC TCT GGT TGG CTT CCT TCA CAG GAC AG 3′ (antisense).
Human IL-8: 5′ ATG ACT TCC AAG CTG GCC GTG GCT 3′ (sense);
5′ T CTC AGC CCT CTT CAA AAA CTT CTC 3′ (antisense).
Human TNF-α: 5′ GAG TGA CAA GCC TGT AGC CCA TGT TGT AGC A3′ (sense);
5′ GCA ATG ATC CCA AAG TAG ACC TGC CCA GAC 3′ (antisense).
Human IFN-γ: 5′ ACC ACA GTC CAT GCC ATC AC 3′ (sense);
5′TCC ACC ACC CTG TTG CTG TA3′ (antisense).
Human G3PDH: 5′ACC ACA GTC CAT GCC ATC AC 3′ (sense);
5′TCC ACC ACC CTG TTG CTG TA3′ (antisense).
A HYBAID OmniGene DNA Thermal Cycler (Teddington, Middlesex, UK) was run for 26 cycles for denaturation at 94°C for 1 min and annealing/extension at 65°C for 2 min in the case of G3PDH. Thirty-five cycles of denaturation at 95°C for 1 min and annealing/extension at 60°C for 2 min were carried out for the cytokines. The cDNA fragments amplified by these sets of primers were 802 bp of IL-1β, 344 bp of IL-4, 289 bp of IL-8, 444 bp of TNF-α and 427 bp of IFN-γ. The PCR products were electrophoresed in 1·8% agarose gel with ϕx174 digested by HaeIII enzyme as calibration markers.
Detection of protein tyrosine phosphorylation and protein tyrosine kinase p56lck by Western blot
Cell lysates were prepared from anti-CD45 isoform antibody or mouse non-specific IgG-treated PMN (5 × 106/ml) by the method reported elsewhere in the literature. The protein concentration of cell lysates was adjusted to 2 mg/ml and the cell lysates were probed by mouse monoclonal anti-phosphotyrosine or anti-p56lck antibody in Western blot analysis. The antigen–antibody complexes were detected by an enhanced chemiluminescence (ECL) protein detection system (Amersham, Aylesbury, UK).
Statistical analysis
Results represent mean ± s.d. throughout the study. Statistical significance was assessed by Student's paired t-test for multiple comparisons.
RESULTS
Phenotypic expression of CD45 isoforms on normal human neutrophils, lymphocytes and monocytes
The phenotypic expression of CD45 isoforms on normal human neutrophils, lymphocytes and monocytes was detected by flow cytometry. We found almost all the leucocytes expressed CD45; 42·3 ± 5·8% and 96·7 ± 2·6% PMN expressed CD45RA and CD45RO molecules on the cell surface, respectively. A representative case is shown in Fig. 1. By contrast, 69·2 ± 6·6% and 37·8 ± 5·5% lymphocytes expressed CD45RA and CD45RO; 34·1 ± 4·4% and 76·1 ± 7·6% monocytes/macrophages expressed CD45RA and CD45RO (data not shown).
Fig. 1.

A representative case showing CD45 isoform expression on normal human PMN. Cells were stained with (1) secondary antibody, (2) anti-CD45, (3) anti-CD45RA and (4) anti-CD45RO. Both percentage and mean fluorescence intensity (denoted by mean channel number) of CD45 isoforms on human PMN were measured by flow cytometry.
Agglutinating activity of anti-CD45 isoform antibodies on human PMN and MNC
We noted that only anti-CD45RO showed a weak agglutinating effect towards PMN. It is possible that the immunochemical properties of anti-CD45RO antibody per se together with the high density of CD45RO on PMN were agglutinated by this unique antibody.
Effect of IgG F(ab′)2 anti-CD45 isoform antibodies on neutrophil phagocytosis
Preincubation of individual IgG F(ab′)2 anti-CD45 isoform antibody with PMN for 45 min enhanced PMN phagocytosis remarkably in both percentage (34–83% increase) and mean fluorescence intensity (Fig. 2). The enhanced neutrophil phagocytosis by the antibody was due mainly to the increased expression of CR3, but not CR1 or FcγRIII, on PMN surface (Fig. 3). However, when PMN were preincubated with anti-CD45 IgG F(ab′)2 and then reacted with anti-CR3 (CD11b) antibody, the neutrophil phagocytosis was remarkably suppressed (Fig. 2, panel 5). It suggests that although CR1, CR3 and FcγRIII are all involved in PMN phagocytosis, CR3 seems much more important than the other two receptors to mediate neutrophil phagocytosis.
Fig. 2.
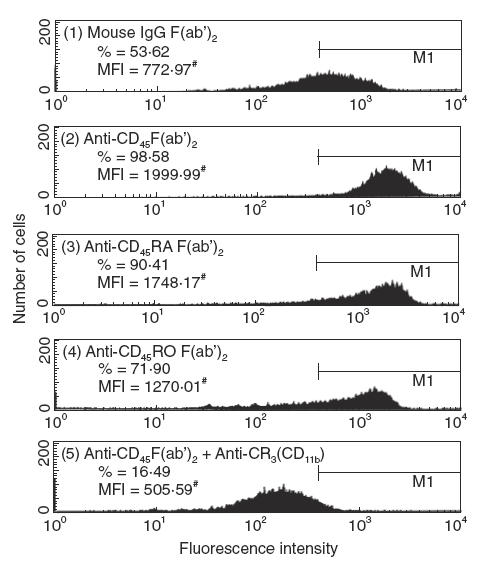
Effect of antibody against of CD45, CD45RA or CD45RO on neutrophil phagocytosis. Human PMN (1 × 106/ml) were preincubated with 50 μg/ml IgG F(ab′)2 fragments of different anti-CD45 isoform antibodies for 45 min before the measurement of phagocytosis. (1) mouse non-specific IgG F(ab′)2, (2) anti-CD45, (3) anti-CD45RA, (4) anti-CD45RO and (5) anti-CD45+ anti-CR3. Both percentage (%) and mean fluorescence intensity (MFI, denoted by mean channel number) of positive cells were detected by FACSort flow cytometry. The three antibodies enhanced PMN phagocytosis markedly in both percentage and MFI compared to mouse IgG control. Remarkable suppression of PMN phagocytosis by combined treatment of anti-CD45 and anti-CR3 antibodies was shown in (5). The same experiment was repeated five times with a similar tendency.
Fig. 3.
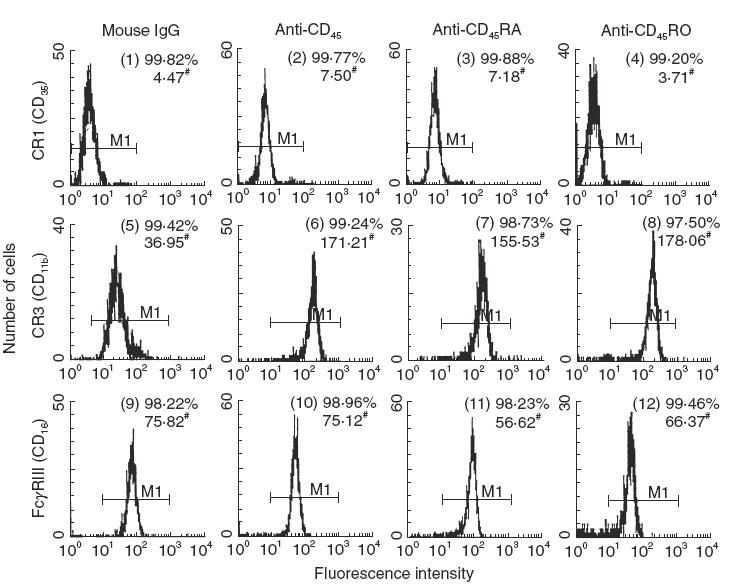
Effect of IgG F(ab′)2 antibody against CD45, CD45RA or CD45RO on the membrane expression of phagocytosis-related receptors; complement receptor type 1 (CR1), type 3 (CR3) and IgG Fc receptor type III (FcγRIII) on human PMN. PMN (1 × 106/ml) were preincubated with IgG F(ab′)2 antibody or non-specific mouse IgG F(ab′)2 for 60 min and then stained with FITC-labelled goat antimouse IgG. Both percentage (%) and mean fluorescence intensity (MFI, denoted by mean channel number) of positive cells were detected by flow cytometry. All three antibodies enhanced CR3 expression compared to mouse IgG F(ab′)2 control. The same experiment was repeated four times with a similar tendency.
Effect of IgG F(ab′)2 anti-CD45 isoform antibodies on IL-8 production of PMN
Many important cytokines/chemokines can be produced by PMN either spontaneously or by stimulation in vitro including IL-1β, IL-3, IL-6, IL-8 IL-12, TNF-α, TGF-α/β1, GRO-α, MIP-1α/1β, IP-10 and CINC-1/2β/3 [24,25]. Among these, IL-8 is the most prominent cytokine produced by PMN. We found the three anti-CD45 isoform IgG F(ab′)2 antibodies stimulated PMN to produce an increased amount of IL-8 rather than mouse IgG F(ab′)2. The addition of polymyxin B abrogated the enhancing effect of IL-8 production by LPS, but not by anti-CD45 isoform antibodies (Fig. 4).
Fig. 4.

Effect of anti-CD45 isoform antibodies IgG F(ab′)2 (50 μg/ml) on IL-8 production of normal human PMN (1 × 106/ml) after incubation with PMN for 24 h. Bacterial lipopolysaccharide (LPS, E. coli serotype 0111:B4, 100 ng/ml) was used as positive control. Mouse IgG F(ab′)2 was used as negative control. Polymyxin B (PMB, 50 ng/ml) was added to the cell suspensions and abrogated significantly the enhancing effects of LPS, but not IgG F(ab′)2 anti-CD45 isoform antibody-mediated enhancement. *P < 0·01 and **P < 0·002 compared to mouse IgG (50 μg/ml) control, calculated by Student's paired t-test for multiple comparisons.
Effect of anti-CD45 isoform antibodies on different cytokine mRNA expression in PMN
In an attempt to determine how many cytokines in human PMN can be induced by anti-CD45 isoform antibodies, the mRNA of IL-1β, IL-4, IL-8, TNF-α and IFN-γ in PMN were detected by RT-PCR after incubation with different antibodies for 2 h. As shown in Fig. 5, normal human PMN expressed mRNA of IL-1β and IL-8 spontaneously, but not TNF-α, IL-4 or IFN-γ. After 2h incubation with anti-CD45RA or anti-CD45RO, TNF-α mRNA was induced.
Fig. 5.

Effect of antibody against CD45, CD45RA or CD45RO on IL-1β, IL-4, IL-8, TNF-α and IFN-γ mRNA expression of human PMN detected by RT-PCR after preincubation of PMN with respective antibody (50 μg/ml) for 2 h. Lanes 1–6: incubation with mouse non-specific IgG (50 μg/ml); lanes 7–12: incubation with anti-CD45; lanes 13–18: incubation with anti-CD45RA; and lanes 19–24: incubation with anti-CD45RO. Lane 1: G3PDH (452 bp, as internal control), lane 2: IL-1β (802 bp); lane 3: TNF-α (695 bp); lane 4: IL-8 (289 bp); lane 5: IL-4 (344 bp); lane 6: IFN-γ (452 bp); lane 7: G3PDH; lane 8: IL-1β; lane 9: TNF-α; lane 10: IL-8; lane 11: IL-4; lane 12: IFN-γ; lane 13: G3PDH; lane 14: IL-1β; lane 15: TNF-α; lane 16: IL-8; lane 17: IL-4; lane 18: IFN-γ; lane 19: G3PDH; lane 20: IL-1β; lane 21: TNF-α; lane 22: IL-8; lane 23: IL-4; and lane 24: IFN-γ. The expression of TNF-α mRNA in PMN was enhanced by anti-CD45RA and anti-CD45RO antibodies. The same experiment was repeated three times with a similar tendency.
Effect of anti-CD45 isoform antibodies on protein tyrosine phosphorylation and p56lck expression in PMN
Phosphorylation of tyrosine residues in a protein molecule is the result of activation on its respective protein tyrosine kinase. Functionally, CD45 and its isoforms are tyrosine phosphatase that can dephosphorylate the cytoplasmic phosphoproteins. As expected, many cytoplasmic proteins in PMN have already been phosphorylated in the tyrosine residues as the cells are metabolically active even in a non-stimulated state. After incubation with antibody against anti-CD45RA or CD45RO for 10 min, a remarkable decrease in 180 and 100 kDa phosphoproteins was found (Fig. 6). Interestingly, only anti-CD45RO suppressed the p56lck (Fig. 7).
Fig. 6.

Detection of protein tyrosine phosphorylation in different PMN lysates after incubation with antibody (50 μg/ml) against CD45, CD45RA or CD45RO for 10 min probed by anti-phosphotyrosine antibody in Western blot analysis. Lane 1: incubation with mouse non-specific IgG (50 μg/ml); lane 2: incubation with LPS (100 ng/ml); lane 3: incubation with anti-CD45; lane 4: incubation with anti-CD45RA; and lane 5: incubation with anti-CD45RO. Two bands with 180 and 100 kDa were diminished markedly after incubation with anti-CD45RA and anti-CD45RO, but not with anti-CD45. The same experiment was repeated three times with a similar tendency.
Fig. 7.

Detection of Src family protein tyrosine kinase p56lck in different PMN lysates after incubation with antibody (50 μg/ml) against CD45, CD45RA or CD45RO for 10 min probed by antip56lck antibody in Western blot analysis. Lane 1: incubation with mouse non-specific IgG (50 μg/ml); lane 2: incubation with LPS (100 ng/ml); lane 3: incubation with anti-CD45; lane 4: incubation with anti-CD45RA; lane 5: incubation with anti-CD45RO; and lane 6: Jurkat cell lysate as a positive control for p56lck molecule supplied by the kit. Only anti-CD45RO suppressed p56lck expression in PMN. The same experiment was repeated three times with a similar tendency.
DISCUSSION
The main purpose of the present study is to define the biological functions of membrane-expressed CD45 isoforms on PMN. Because the natural ligands for CD45 isoforms have not yet been found, anti-CD45 monoclonal antibodies have been used as surrogate ligand for this purpose [33]. Hoffmeyer et al.[34] and Gao et al.[30] have shown that CD45 may play a role in FcγR-mediated signalling and immune functions in human neutrophils after co-cross-linking with CD45. The use of whole anti-CD45 isoform antibodies may react with Fcγ-receptors, especially low-affinity FcγRIIa and FcγRIIIb, if aggregated antibodies exist. To avoid the unwanted effects, we used IgG F(ab′)2 fragments of anti-CD45 isoform antibodies in some experiments instead of the intact IgG antibody to determine the effects of these antibodies on neutrophil functions (Figs 2, 3, 4). We found a similar effectiveness in these two preparations. It is possible that the IgG antibodies we used did not cause co-cross-linking of CD45 and FcγR molecules because of negligible (anti-CD45 and anti-CD45RA) or very weak agglutinating activity (anti-CD45RO) on PMN. Three original findings were observed in this study: (1) the three anti-CD45 isoform antibodies stimulated PMN phagocytosis mainly via increased expression of CR3 on the cell surface. (2) Anti-CD45RA and anti-CD45RO enhanced both IL-8 and TNF-α gene expression whereas anti-CD45 only increased IL-8 production of PMN. (3) Both anti-CD45RA and anti-CD45RO decreased protein tyrosine phosphorylation, but only anti-CD45RO suppressed Src family protein tyrosine kinase p56lck expression in PMN.
It is conceivable that two biological functions in lymphocytes are clearly dependent on CD45 and its isoforms. The primary target of CD45 is the Src family protein kinase p56lck that is associated with CD4 and CD8 [35–37]. CD45 and isoforms can act either as positive or negative regulators of the kinase activity of p56lck. Dephosphorylation of the C-terminal inhibitory site (site 1) tyrosine in the p56lck molecule by interaction with CD45 molecules changed the kinase confirmation into an active structure. In conjunction with TCR activation, the dephosphorylated Src family kinase is then phosphorylated on a second stimulatory site (site 2) to become an active form to promote the catalytic function of the kinase in the lymphocytes [38,39]. In an in vitro study, Mustelin et al.[21] reported that the incubation of purified CD45 with p56lck increased the catalytic activity of the kinase more than twofold. This finding indicates that expression of CD45 in T lymphocytes is required to regulate positive tyrosine phosphorylation of the carboxyl-terminal tyrosine residue of p56lck in order to maintain the kinase in an active state. Whether a similar-acting mechanism of CD45 isoforms operates in neutrophils as well as in lymphocytes has not yet been reported in the literature.
Our results suggest that the binding of CD45 isoform molecules with specific antibody on PMN dephosphorylates the inhibitory site 1 in the C-terminal Src family kinase domain. Autophosphorylation of the stimulatory site 2 in the molecule occurs subsequently in PMN, as PMN are usually committed to activation. Because the activated protein tyrosine kinase p56lck with catalytic activity does not underlie dephosphorylation by CD45 isoform, this active form kinase phosphorylates certain transcription factors and transduces a positive signal to active PMN. As a consequence, PMN was activated to increase production/expression of IL-8 and TNF-α and phagocytosis. Because CD45 and its two isoforms are functional protein tyrosine phosphatase, decreased cytoplasmic protein tyrosine phosphorylation in PMN after activation by antibody was inevitable, as in lymphocytes. However, we note that the binding of CD45 with its specific antibody did not decrease tyrosine phosphorylation, as demonstrated in Fig. 6. The real cause of this inconsistency is not clear. It remains possible that the difference in cell physiology between PMN and lymphocytes is responsible.
In normal bone marrow myeloid cells, CD45RA gradually disappeared in parallel to cell maturation and only 3–15% PMN expressed CD45RA [26]. However, our results show that approximately 40% normal human PMN expressed CD45RA. The increased CD45RA expression on PMN may be due to non-specific activation during cell separation that elicits translocation of cytoplasmic CD45RA molecule to the surface membrane. The PMN-stimulating activty of anti-CD45 isoform antibodies was not due to bacterial endotoxin contamination because all the reagents, culture media, cell suspension or cell cultured supernatants used in the experiments were proved negative using the L. amebocyte coagulation test. In addition, the increased PMN IL-8 production by anti-CD45 isoform antibody was not affected by polymyxin B, different from the LPS-induced enhancement.
Another interesting finding revealed that only anti-CD45RO antibody suppressed p56lck expression in PMN. We noted that the monoclonal anti-p56lck antibody was induced by immunization with a 21·3-kDa fragment of the human p56lck N-terminal domain, corresponding to amino acid residues 1–191. This epitope is far from the C-terminal catalytic kinase domain of the Lck. Theoretically, there should be no changes of p56lck expression in anti-CD45 isoform antibody-treated PMN. However, considering the weak agglutinating activity of anti-CD45RO on PMN, it remains possible that the agglutination of PMN by anti-CD45RO antibody may increase the interaction of CD45RO and p56lck and finally accelerates the decay of p56lck molecules.
Our results indicate that the three anti-CD45 isoform antibodies possessed a wide spectrum of stimulating effects on neutrophils. These results are similar to those of Liles et al.[28] and Gao et al.[30]. Liles demonstrated that cross-linking of CD45 resulted in a 30-fold increase in respiratory burst of normal neutrophils induced by FMLP, GM-CSF or TNF-α. They postulated that CD45 mediated coupling of specific cell surface receptors to downstream tyrosine kinase-dependent signal-transducing pathways in activated neutrophils. Hoffmeyer et al.[34] further identified that the specific surface receptors that can be coupled by anti-CD45 were low-affinity FcγRIIa and FcγRIIIb. In addition, Gao et al.[30] showed clearly that cross-linking of CD45 enhenced IL-6 production of PMN. The co-cross-linking of CD45 and FcγRII further increased IL-6 production of PMN. However, the FcγRII cross-linking-mediated protein tyrosine phosphorylation and F-actin polymerization were down-regulated inversely in PMN after co-cross-linking with CD45. Our results showed that anti-CD45 isoform antibodies stimulated IL-8 and TNF-α gene expression of PMN. Undoubtedly, CD45 ligation may play an essential role in proinflammatory cytokine gene expression and production that led to inflammatory reactions. Whether CD45-mediated human neutrophil stimulation is through FcγR is not explored in this study. The reason why anti-CD45RO mediated broader effects on neutrophils than anti-CD45 and anti-CD45RA is also not clear at present. We speculate that the immunochemical property of anti-CD45RO per se rendered the difference because (1) only the antibody can agglutinate PMN weakly and (2) the IgG subclass of anti CD45RO (clone UCHL1 IgG2a) was different from anti-CD45 (clone J-33, IgG1) and anti-CD45RA (clone ALB11, IgG1) that may differ in co-cross-linking activity with FcγR on PMN. In conclusion, we are the first to identify the biological functions of CD45 and its two isoforms, CD45RA and CD45RO, on PMN. Our results suggest that binding of these three CD45 isoforms with their specific antibodies activate different PMN functions by differential suppression on protein tyrosine phosphorylation and p56lck protein tyrosine kinase.
Acknowledgments
This study was supported by the grants from National Science Council (NSC88-2314-B-002–398) and Yen-Tjing-Ling Research Foundation (CI-89-4-3) Taiwan.
REFERENCES
- 1.Thomas ML, Lefrancois L. Differential expression of the leukocyte-common antigen family. Immunol Today. 1988;9:320–6. doi: 10.1016/0167-5699(88)91326-6. [DOI] [PubMed] [Google Scholar]
- 2.Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–69. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 3.Justement LB. The role of CD45 in signal transduction. Adv Immunol. 1997;66:1–65. doi: 10.1016/s0065-2776(08)60595-7. [DOI] [PubMed] [Google Scholar]
- 4.Young JL, Ramage JM, Hill Gaston JS, Beverley PCL. In vitro responses of human CD45RObrightRA− and CD45RO−RAbright T cell subsets and their relationship to memory and naive T cells. Eur J Immunol. 1997;27:2383–9. doi: 10.1002/eji.1830270937. [DOI] [PubMed] [Google Scholar]
- 5.Lodbetter JA, Rose LM, Spooner CE, Beatty PG, Martin PJ, Clark EA. Antibodies to leukocyte common antigen p220 influence human T cell proliferation by modifying IL-2 receptor expression. J Immunol. 1985;135:1819–25. [PubMed] [Google Scholar]
- 6.Bernabeu C, Carrera AC, De LandaZuri MO, Sanchez-Madrid F. Interaction between the CD45 antigen and phytohemagglutinin inhibitory effect on the lectin-induced T cell proliferation by anti-CD45 monoclonal antibody. Eur J Immunol. 1987;17:1461–6. doi: 10.1002/eji.1830171012. [DOI] [PubMed] [Google Scholar]
- 7.Pingel JT, Thomas ML. Evidence that leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989;58:1055–65. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittler RS, Greenfield RS, Schacter BZ, Richard NF, Hoffmann MK. Antibodies to the leukocyte common antigen (T200) inhibit an early phase in the activation of resting B cell. J Immunol. 1987;138:3159–66. [PubMed] [Google Scholar]
- 9.Smeland EB, Hote H, Blomhoff HK, et al. Inhibition of polyphosphoinositide breakdown and c-myc induction accompanying inhibition of human B-cell activation by two monoclonal antibodies against the common antigen (CD45) Scand J Immunol. 1990;31:583–91. doi: 10.1111/j.1365-3083.1990.tb02809.x. [DOI] [PubMed] [Google Scholar]
- 10.Newman W, Fast LD, Rose LM. Blockade of NK cell lysis is a property of monoclonal antibodies that bind to distinct regions of T-200. J Immunol. 1993;131:1742–7. [PubMed] [Google Scholar]
- 11.Targen SR, Newman W. Definition of a ‘trigger’ stage in the NK cytolytic reaction sequence by a monoclonal antibody to the glycoprotein T-200. J Immunol. 1983;131:1149–53. [PubMed] [Google Scholar]
- 12.Giezeman-Smits KM, Gorter A, van Vlierberghe RLP, et al. The regulatory role of CD45 on rat NK cells in target cell lysis. J Immunol. 1999;163:71–6. [PubMed] [Google Scholar]
- 13.Bottomly K, Lugman M, Greenbaum L, et al. A monoclonal antibody murine CD45R distinguished CD45+ T cell population that produce different cytokines. Eur J Immunol. 1989;19:617–23. doi: 10.1002/eji.1830190407. [DOI] [PubMed] [Google Scholar]
- 14.Xu X-L, Chong S-F. Cross-linking of CD45 on NK cells stimulates p56lck-mediated tyrosine phosphorylation and IFN-γ production. J Immunol. 1995;155:5241–8. [PubMed] [Google Scholar]
- 15.Shen F, Xu X-L, Graf LH, Chong S-F. CD45-cross-linking stimulates IFN-γ production in NK cells. J Immunol. 1995;154:644–52. [PubMed] [Google Scholar]
- 16.Spertini F, Wang AVT, Chatila T, Geha RS. Engagement of the common leukocyte antigen CD45 induces homotypic adhesion of activated human T cells. J Immunol. 1994;153:1593–602. [PubMed] [Google Scholar]
- 17.Hall SR, Heffernan BM, Thompson NT, Rowan WC. CD4+CD45RO+T cells differ in their TCR-associated signaling responses. Eur J Immunol. 1999;29:2098–106. doi: 10.1002/(SICI)1521-4141(199907)29:07<2098::AID-IMMU2098>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Ledbetter JA, Tonks NK, Fisher EH, Clark EA. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T and B cells. Proc Natl Acad Sci USA. 1988;85:8628–32. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deans JP, Shaw J, Pearse MJ, Pilarski LM. CD45R as a primary signal transducer stimulates IL-2 and IL-2R mRNA synthesis by CD3−4−8− thymocytes. J Immunol. 1989;143:2425–30. [PubMed] [Google Scholar]
- 20.Koretzky GA. Role of the CD45 tyrosine phosphatase in signal transduction in the immune system. FASEB J. 1993;7:420–6. doi: 10.1096/fasebj.7.5.8462784. [DOI] [PubMed] [Google Scholar]
- 21.Mustelin T, Coggeshall KM, Altman A. Rapid activation of the T-cell tyrosine protein kinase p56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci USA. 1989;86:6302–6. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schraven B, Kirchgessner H, Gaber B, Samstag Y, Meuer S. A functional complex is formed in human T lymphocyte between the protein tyrosine phosphatase CD45, the protein tyrosine kinase p56lck and pp32, a possible common substrate. Eur J Immunol. 1991;21:2469–77. doi: 10.1002/eji.1830211025. [DOI] [PubMed] [Google Scholar]
- 23.Arendt CW, Hsi G, Ostergaard HL. Immobilized antibodies to CD45 induce rapid morphologic changes and increased tyrosine phosphorylation of p56lck-associated proteins in T cells. J Immunol. 1995;155:5095–103. [PubMed] [Google Scholar]
- 24.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophils in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 25.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell CW, Patterson WP, Toalson BM, Yesus YW. Surface and cytoplasmic expression of CD45 antigen isoforms in normal and malignant myeloid cell differentiation. Am J Clin Pathol. 1991;95:180–7. doi: 10.1093/ajcp/95.2.180. [DOI] [PubMed] [Google Scholar]
- 27.Lacal P, Pulido R, Sanchez-Madrid F, Mollinedo F. Intracellular location of T200 and Mo1 glycoproteins in human neutrophils. J Biol Chem. 1991;263:9946–51. [PubMed] [Google Scholar]
- 28.Liles WC, Ledbetter JA, Waltersdorph AW, Klebanoff SJ. Cross-linking of CD45 enhances activation of the respiratory burst in response to specific stimuli in human phagocytes. J Immunol. 1995;155:2175–84. [PubMed] [Google Scholar]
- 29.Harvath LH, Balke JA, Christiansen NP, Russel AA, Skubitz KM. Selected antibodies to leukocyte common antigen (CD45) inhibit human neutrophil chemotaxis. J Immunol. 1991;146:949–57. [PubMed] [Google Scholar]
- 30.Gao H, Henderson A, Flynn DC, Landreth KS, Ericson SG. Effects of the protein tyrosine phosphatase CD45 on FcγRIIa signaling and neutrophil function. Exp Hematol. 2000;28:1062–70. doi: 10.1016/s0301-472x(00)00513-0. [DOI] [PubMed] [Google Scholar]
- 31.Shalaby MR, Aggarwal BB, Rainderknecht E, Svedersky LP, Finkel BS, Palladino MA., Jr Activation of human polymorphonuclear neutrophil function by interferon-γ and tumor necrosis factor. J Immunol. 1985;135:2069–73. [PubMed] [Google Scholar]
- 32.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guadinidium–thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.Clement LT, Yamashita N, Martin AM. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CDR antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988;141:1464–72. [PubMed] [Google Scholar]
- 34.Hoffmeyer F, Witte K, Gebhardt U, Schmidt RE. The low affinity FcγRIIa and FcγRIIIb on polymorphonuclear neutrophils are differentially regulated by CD45 phosphatase. J Immunol. 1995;155:4016–23. [PubMed] [Google Scholar]
- 35.Thomas ML, Brown EJ. Positive and negative regulation of Src-family membrane kinase by CD45. Immunol Today. 1999;20:406–11. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 36.Ostergaard HL, Trowbridge IS. Coclustering CD45 with CD4 or CD8 alters the phosphorylation and kinase activity of p56lck. J Exp Med. 1990;172:347–50. doi: 10.1084/jem.172.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagi S, Sugawara H, Kurosaki M, Sabe H, Yamamura H, Kurosaki T. CD45 modulated phosphorylation of both autophosphorylation and negative regulatory tyrosines of Lyn in B cells. J Biol Chem. 1996;271:30487–92. doi: 10.1074/jbc.271.48.30487. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 39.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Lck. Nature. 1997;385:602–9. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]


