Abstract
Leptin, the Ob gene product, is an adipocyte hormone that centrally regulates weight control. In addition, other effects of leptin in peripheral tissues have been described. Thus, leptin has been found to regulate reproduction, haematopoiesis and immune function. We have found recently that leptin has a stimulatory effect on human peripheral blood mononuclear cells (PBMC). Monocytes are activated by leptin alone whereas T lymphocytes need a suboptimal stimulus of PHA or ConA before further activation by leptin. These effects are mediated by the long isoform of the leptin receptor, which has been shown to trigger signalling in PBMC. In fact, we have found that human leptin stimulates Janus kinase (JAK)-signal transducer and activator of transcription (STAT), phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways in PBMC. In order to assess possible regulation of the long isoform of the leptin receptor (Ob-R) in mononuclear cells upon activation, we have studied the expression of Ob-R by RT-PCR and Western blotting in PBMC activated in vitro by PHA or ConA and in vivo in HIV-infected patients. We have found that in vitro activation and in vivo HIV infection correlates with an increase in leptin receptor expression in PBMC. Moreover, the leptin receptor is tyrosine phosphorylated in PBMC from HIV-infected patients, suggesting that the leptin receptor is activated. These results are consistent with the suggested role of leptin in modulating the immune response.
Keywords: cell activation HIV, leptin receptor, lymphocytes, PBMC
INTRODUCTION
Leptin, the 16-kDa non-glycosylated protein product of the ob gene [1], is a hormone synthesized mainly in adipose cells [2], although it can also be expressed in placenta and stomach [3,4]. It regulates weight control in a centralized manner by interacting with its cognate receptor in the hypothalamus, so increasing the basal metabolic rate and reducing food intake [5–8]. Leptin is released into the circulation, and plasma levels correlate with total body fat mass [9]. However, there is increasing evidence that leptin has other effects apart from those related to energy homeostasis, including regulation of neuroendocrine, reproductive, haematopoietic and immune functions [10].
The primary amino acid sequence of leptin indicated that it could belong to a long-chain helical cytokine family, such as IL-2 [11]. In fact, the leptin receptor (Ob-R) shows sequence homology to members of the class I cytokine receptor (gp130) superfamily [12] which includes the receptor for IL-6, leucocyte inhibitory factor (LIF) and granulocyte colony stimulating factor (G-CSF). Moreover, Ob-R has been shown to have signalling capabilities in line with IL-6-type cytokine receptors [13]. Ob-R expression is not limited to the hypothalamus but is distributed widely [12,14] and is found expressed on haematopoietic cells [15]. In this context, a role for leptin in haematopoiesis and the immune system at the stem cell level has been proposed [16].
Obese leptin-deficient ob/ob mice and db/db mice, in which the leptin receptor is truncated, display immune dysfunction and lymphoid organ atrophy, affecting thymic size and cellularity similar to that observed in starved animals and malnourished humans [17–19]. Thus, such animals have reduced levels of peripheral T and B cells [17] suggesting that leptin may have a role in lymphopoiesis. Furthermore, leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity when administered to ob/ob mice [18]. Moreover, human leptin deficiency caused by a missense mutation also produces immune system dysfunction [20]. In conjunction with this, leptin can induce proliferation, differentiation, and functional activation of haematopoietic cells and these functions may explain the role of adipose tissue present in the marrow cavity [16].
Leptin enhances cytokine production (GM-CSF and G-CSF) in murine peritoneal macrophages [21], and phenotypic abnormalities have been found in macrophages from leptin-deficient, obese mice [22]. Furthermore, leptin up-regulates both phagocytosis and the production of proinflammatory cytokines by murine macrophages [23]. In this context, an immunoregulatory role for leptin on macrophage function as a proinflammatory signal has gained physiological importance because the inflammatory cytokines (TNF-α and IL-1) can raise mouse leptin levels in vivo, resulting in anorexia and weight loss [24]. Leptin has not, however, been proved to be the main signal for the anorectic effects of inflammation but a role for leptin in regulating immunity, inflammation and haematopoiesis has been accepted [25].
We have found recently that leptin is able to promote activation and proliferation of human monocytes and to enhance activation and proliferation of preactivated T lymphocytes [26,27]. Moreover, leptin can induce the synthesis of proinflammatory cytokines by human monocytes and the production of Th1-type cytokines by human T lymphocytes cultured in vitro[26,27]. Thus, leptin increases the production of IL-6 and TNF-α by monocytes, whereas it enhances IL-2 and INF-γ production by PHA-stimulated T lymphocytes [26,27].
These effects of leptin on human peripheral blood mononuclear cells (PBMC) are mediated by the leptin receptor which is present in peripheral blood monocytes and T lymphocytes [26–29]. Both the long and short isoforms of Ob-R are present in PBMC, although only the long isoform is supposed to mediate full signalling in target cells. Thus, we have also found that human leptin can trigger signal transduction activating Janus kinase (JAK)-signal transducer and activator of transcription (STAT), phosphatidylinositol 3-kinase (PI3K), and mitogen activated protein kinase (MAPK) pathways in human PBMC [28,29].
To assess further the role of leptin in the immune response, we have studied expression of the long isoform of the leptin receptor in response to in vitro activation of PBMC by PHA and Con A and in vivo in HIV-infected patients.
MATERIALS AND METHODS
Materials
Human recombinant leptin was from R&D Systems (Minneapolis, MN, USA). Antibodies against the long isoform of leptin receptor (C-terminal), β-actin and antiphosphotyrosine were from Santa Cruz (Santa Cruz, CA, USA).
Patients
HIV-infected patients were from the Internal Medicine Department (AIDS Unit) and were selected by their similar clinical characteristics, low viral load and intermediate number of CD4+ T cells (Table 1). Informed consent was obtained from the patients and the studies had the approval from the ethical committee of the Virgen Macarena University Hospital.
Table 1.
Clinical features of the patients (n = 11)
| Age in years (median, range) | 34 (12–38) |
| Male gender | 7 |
| HIV transmission | 7 |
| Parenteral drugs | 3 |
| Sexual | |
| Vertical transmission | 1 |
| Time from HIV-infection diagnosis in years(mean, range) | 9 (2–12) |
| CDC classification | |
| A2 | 9 |
| B3 | 2 |
| CD4 cells/mm3 (median, range) | 321 (212–651) |
| Undetectable viral load | 7 |
| Log HIV viral load in copies/ml in patients withdetectable (mean, range) | 4·92 (2·15–5·79) |
| HAART | 9 |
| Co-infections | |
| Any | 7 |
| Hepatitis C virus | 7 |
| Hepatitis B virus | 1 |
Data are expressed in number of patients except where indicated. HAART: highly active antiretroviral therapy.
Cell preparation and culture
Peripheral blood mononuclear cells (PBMC) obtained from normal donors (six healthy subjects, three men and three women, aged 26–32) and HIV-infected patients were isolated from heparinized venous blood by density-gradient sedimentation over Ficoll-Hypaque (Seromed Biochrom KG, Berlin, Germany), as described previously [30,31]. Cells were then washed twice in phosphate buffered saline (PBS) and resuspended in RPMI 1640 supplemented with 25 mm HEPES, 2 mml-glutamine, 100 μU/ml penicillin, 100 μg/ml streptomycin and amphotericin B (2·5 μg/ml) (complete medium without serum) (all from Biological Industries, Beit Haemek, Israel) as described previously [32]. PBMC were cultured at 37°C in an atmosphere containing 5% CO2. Cells (5 × 105) were treated with different stimuli. Following treatment, the cells were washed with cold PBS and solubilized for 30 min at 4°C in lysis buffer containing 20 mm Tris, pH 8, 1% nonidet P-40, 137 mm NaCl, 1 mm MgCl2, 1 mm CaCl2, 1 mm dithiothreitol (DTT), 10% glycerol, 1 mm phenylmethylsulphonyl fluoride and 0·4 mm sodium orthovanadate [33]. After centrifugation, the soluble cell lysates were used for the study. Protein concentration was determined by a kit from Bio-Rad (Richmond, CA, USA), using bovine serum albumin as a standard.
Immunoprecipitation and Western blotting analysis
Soluble cellular lysates (0·5 mg of protein) were precleared with 50 μl of protein A-Sepharose (Pharmacia, Uppsala, Sweden) for 2h at 4°C by end-over-end rotation. The precleared cellular lysates were incubated with appropriate antibodies for 3h at 4°C [33,34]. Next, 50 μl of protein A-Sepharose was added to immune complexes and incubation was continued for 2h at 4°C. The immunoprecipitates were washed three times with lysis buffer and 40 μl of SDS-stop buffer containing 100 mmol/l DTT added. The soluble cellular lysates were also denatured in SDS-stop buffer containing 100 mmol/l DTT. Both lysates and immunoprecipitate samples were boiled for 5 min and the resultant products resolved by SDS-PAGE and transferred electrophoretically onto nitrocellulose membranes [33,34]. The membranes were blocked with Tris-buffered saline-0·05% Tween 20 (TBST) containing 5% non-fat dry milk for 1h at 23°C. The blots were then incubated with primary antibody for 1 h, washed in TBST and incubated further with secondary antibodies linked to horseradish peroxidase. Bound horseradish peroxidase was visualized by a highly sensitive chemiluminescence system (SuperSignal from Pierce, Rockfield, IL, USA) [34]. The bands obtained in the blots were scanned and analysed by the PCBAS 2·0 program.
OB-R mRNA detection by RT-PCR
Total RNA from PBMC (1 × 106 cells) was extracted using the QuickPrep Total RNA extraction kit (AmershamPharmacia Biotech, Barcelona, Spain). First-strand cDNA synthesis was performed using an oligo-dT primer (kit from Roche Molecular Biochemicals, Barcelona, Spain) and this was then used for detection of OB-R messenger RNA (mRNA) by RT-PCR as described previously [35]. The sequences of primers and hybridization probes for OB-R have been used previously for the detection of OB-R expression [35]. β-Actin mRNA expresion was used as an internal control.
Statistical analysis
Values are expressed as means ± s.e.m. Student's t-test was used for comparisons, with differences being considered significant at P < 0·05.
RESULTS
We have recently shown expression of the long isoform of the leptin receptor in PBMC by RT-PCR [28], the isoform through which leptin can trigger full signal transduction [28,29]. In order to study differences in the levels of leptin receptor expression, we employed RT-PCR to investigate the effect of PBMC activation induced by in vitro incubation with lectins (PHA or Con A). As shown in Fig. 1, stimulation of PBMC with 8 μg/ml PHA or 10 μg/ml Con A for 24h increased expression of the leptin receptor, as assessed by RT-PCR normalized by the amount of retrotranscribed RNA, and adjusted for β-actin levels. Densitometric analysis of the bands showed that activation of PBMC by either PHA or Con A increased expression of the leptin receptor to about twice that of control cells.
Fig. 1.
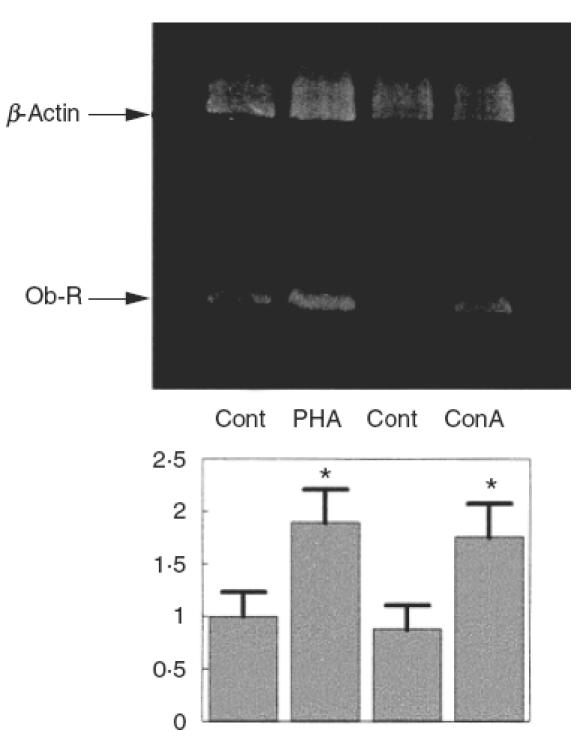
Expression of the long isoform of the leptin receptor in PBMC in response to PHA or Con A. PBMC were cultured in complete medium for 24h in the presence or absence of 8 μg/ml PHA or 10 μg/ml Con A. Total RNA extracted from PBMC was reverse transcribed and OB-R expression determined by RT-PCR amplification. An ethidium bromide-stained gel of the PCR products is shown. The lower bands correspond to the 338 bp PCR product of OB-R. The upper bands correspond to the 764 bp PCR product of β-actin that were used as internal controls to check the total RNA amount in each sample. A representative experiment of five is shown. OB-R bands were quantified and normalized with the β-actin bands. The lower panel shows the means ± s.e.m. of the quantitative data. *P < 0·01 versus control.
In order to assess whether the increase in leptin receptor mRNA expression was correlated with an increase in the amount of leptin protein produced, we next analysed PBMC by SDS-PAGE and immunoblotting using an antibody that recognizes the C-terminal part of the long isoform of the leptin receptor. As shown in Fig. 2, PBMC activation by either PHA or Con A for 24h resulted in an increase in the amount of leptin receptor, by about twice as much as control samples, suggesting that the up-regulation of mRNA expression in activated PBMC results in a corresponding increase in the amount of receptor protein produced.
Fig. 2.
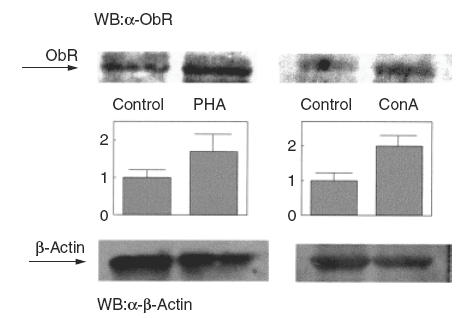
Ob-Rb protein is increased in activated PBMC. PBMC were cultured as described in the legend to Fig. 1, and stimulated either with PHA (8 μg) or Con A (10 μg). Cells were lysed and analysed by SDS-PAGE and immunoblotting with anti-Ob-R. Representative immunoblots of four independent experiments are shown. OB-R bands were quantified and normalized against the β-actin bands. The middle panel shows the means ± s.e.m. of the quantitative data. *P < 0·01 versus control.
Leptin has been shown to have an immunoregulatory role in human mononuclear cells [26–29] and expression of the leptin receptor is up-regulated following PBMC activation (Figs 1 and 2). In order to test whether the leptin receptor may be involved in some pathophysiological conditions, we determined the expression of leptin receptor mRNA in PBMC from HIV-infected patients. As shown in Fig. 3, PBMC from HIV+ patients had increased expression levels of leptin receptor mRNA. Normalized data showed that HIV+ patients expressed about twice as much leptin receptor mRNA as control subjects. When the amount of leptin receptor protein was assessed by specific immunoblotting, PBMC from HIV+ patients showed more than twice (2·4) as much receptor protein as PBMC from control subjects (Fig. 4).
Fig. 3.
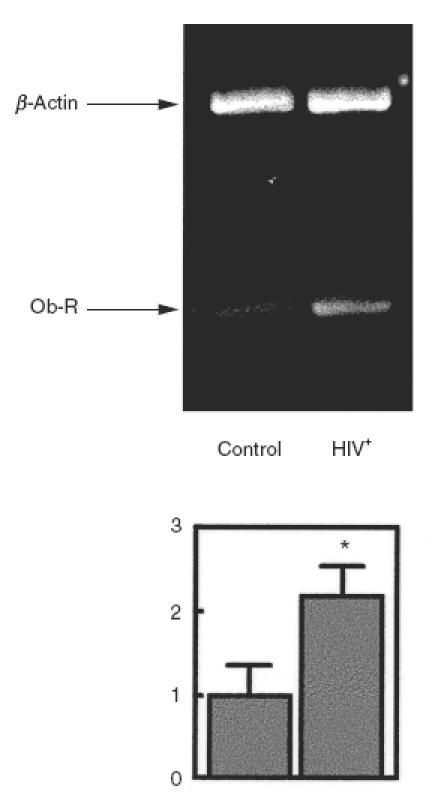
Increased expression of the long isoform of the leptin receptor in PBMC from HIV infected patients. Total RNA extracted from PBMC of HIV infected patients was reverse transcribed and OB-R expression determined by RT-PCR amplification. An ethidium bromide-stained gel of the PCR products is shown. The lower bands correspond to the 338 bp PCR product of OB-R. The upper bands correspond to the 764 bp PCR product of β-actin that were used as internal controls to compare the total RNA amount in each sample. OB-R bands were quantified and normalized against the β-actin bands. The lower panel shows the means ± s.e.m. of the quantitative data. *P < 0·01 versus control.
Fig. 4.
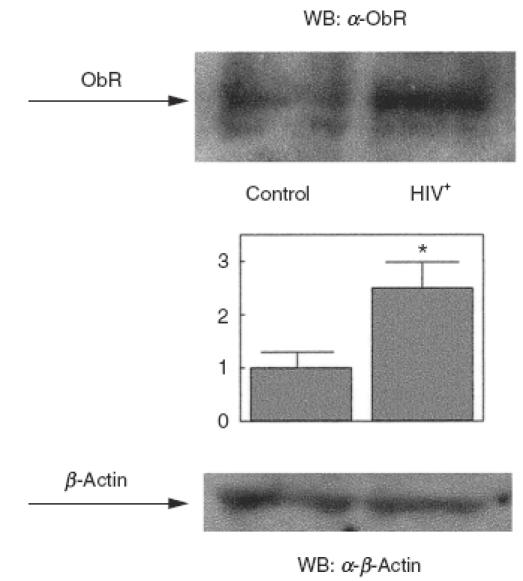
Ob-Rb in protein is increased in PBMC from HIV-infected patients. PBMC were obtained from HIV-infected patients. Cells were lysed and analysed by SDS-PAGE and immunoblotting with anti-Ob-R. A representative experiment is shown. OB-R bands were quantified and normalized against the β-actin bands. The middle panel shows the means ± s.e.m. of the quantitative data. *P < 0·01 versus control.
Because activated PBMC (in vitro or in vivo) have increased expression of the leptin receptor, we sought to check whether the leptin receptor was activated under these conditions, i.e. whether or not the leptin receptor was tyrosine phosphorylated. We found that PBMC from HIV+ patients do indeed contain tyrosine phosphorylated leptin receptors, whereas PBMC from control subjects lack such phosphorylation (Fig. 5). As shown in Fig. 5, in vitro activation of PBMC from control donors by 8 μm PHA or 10 μm Con A did not induce tyrosine phosphorylation of the leptin receptor.
Fig. 5.
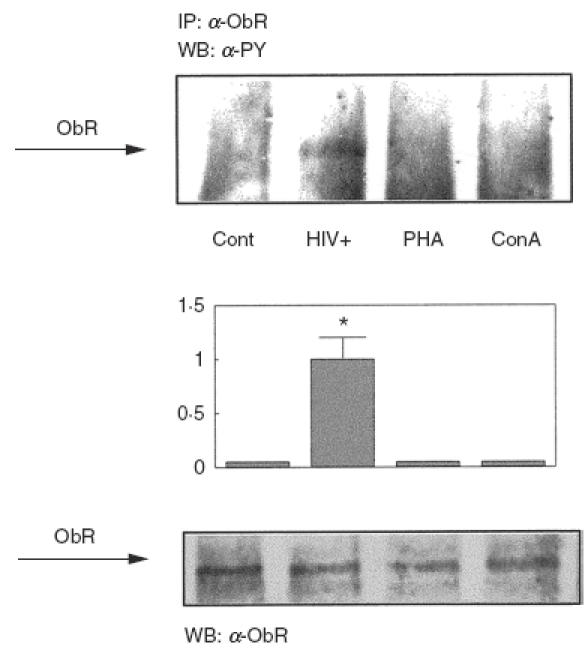
Tyrosine phosphorylation of Ob-Rb in activated PBMC and HIV-infected patients. PBMC from control donors were cultured and activated in vitro with PHA or Con A as described in the legend to Fig. 1, or they were obtained from HIV-infected patients and analysed without stimulation. Cells were lysed and the soluble cell lysates were immunoprecipitated with a specific antibody against the C-terminal part of the long isoform of the leptin receptor. Samples were then analysed by SDS-PAGE and immunoblotted with antiphosphotyrosine antibodies. Representative immunoblots are shown. The amount of Ob-Rb immunoprecipitated was controlled by Western blotting with anti-Ob-R. The middle panel shows the means ± s.e.m. of the quantitative data. *P < 0·001 versus control.
DISCUSSION
Both leptin and its receptor share structural and functional similarities with the IL-6 family of cytokines [11,12]. The leptin receptor also has signalling capabilities comparable with IL-6-type cytokine receptors [13]. In fact, leptin has been shown to induce proliferative activity in monocytes, lymphocytes, leukaemia cells and haematopoietic progenitors [21,25–27]. Moreover, leptin increases cytokine production, the activation of monocytes and T lymphocytes, wound healing, angiogenesis and haematopoiesis [15,16,23,25–27]. Consistent with these effects, leptin production is increased during infection and inflammation [24,25,36]. Therefore, leptin has been proposed to have a role as a proinflammatory cytokine [23,25–27]. In fact, leptin has been associated with a stress-related (acute-phase) reaction [37], and leptin has been suggested to be the link connecting the thrifty and the cytokine genotype/phenotype [28,29].
Human circulating monocytes and lymphocytes express the leptin receptor [26,27], and this receptor is able to trigger signalling beginning with activation of JAK activity and tyrosine phosphorylation of the receptor itself [28,29]. In this context, we wanted to assess regulation of leptin receptor expression in PBMC in response to in vitro activation. We have found that activation of PBMC by PHA or Con A up-regulates expression of the leptin receptor. Considering the stimulatory activity of leptin in PBMC, this effect of activation on leptin receptor expression may be useful as a positive feed-back mechanism. In line with this, lymphocytes have been shown to respond to leptin only when co-stimulated with either PHA or Con A [27], whereas monocytes can be activated directly by leptin. The need of lymphocytes for costimulation may be partly explained by the effect of activation increasing leptin receptor expression. These data suggest that the leptin receptor may be regulated in a similar way to other cytokine receptors such as IL-2 receptor [38].
One of the first signalling steps in leptin receptor activation is its own tyrosine phosphorylation. Thus, we used the phosphorylation level of the leptin receptor as a test for receptor activation. Even though the leptin receptor expression level was up-regulated in PBMC by lectin stimulation, the receptors did not show any increase in tyrosine phosphorylation.
However, we also checked leptin receptor expression under some in vivo conditions that result in PBMC activation, such as HIV infection; infection with HIV has been associated with elevated IL-6 levels and production [39,40], and activation of lymphomonocytes is one of the hallmarks of HIV infection [41,42]. In this context, we found that PBMC from HIV-infected subjects have increased expression of the leptin receptor, similar to the levels seen in in vitro activated PBMC from healthy controls. Moreover, the leptin receptor is tyrosine phosphorylated in PBMC from HIV+ subjects suggesting that the leptin receptor is not only up-regulated but also activated. Because PBMC from control donors had increased expression but not activated leptin receptors when stimulated with lectins, one possible explanation for the discrepancy is that HIV infection in PBMC may itself induce activation of the leptin receptor. In vitro infection of mononuclear cells with HIV would confirm this hypothesis, although this remains to be investigated.
Plasma HIV RNA levels and immune activation have been related in HIV infection [43,44]. We have studied PBMC from patients with a low virus load, suggesting that the effect of HIV infection on leptin receptor expression does not need a high virus load. However, there is still a fairly broad range of HIV loads within the patients studied, which do not correlate with the narrow range of leptin receptor expression levels observed in PBMC from these patients. Whether a higher virus load may lead to a higher level of leptin receptor expression requires further investigation. Problems are associated with such studies, however, because a high virus load may have a deleterious effect on PBMC promoting altered stimulation and apoptosis and resulting in lower numbers of T lymphocytes. It was for this reason that we chose patients with a low viral load and an intermediate number of CD4+ T cells.
Most of the HIV-infected patients in this study had HCV co-infection. Nevertheless, both co- and singly infected patients had similar expression levels of the leptin receptor, suggesting that HIV infection itself is sufficient to account for this effect. We do not know whether HCV infection in HIV seronegatives may also lead to the same result. Although one may speculate that a possible increase in leptin receptor expression is a result of any viral infection that activates PBMC, this hypothesis needs further study.
In conclusion, we have demonstrated that leptin receptor expression is induced in PBMC both upon in vitro activation by lectins and under in vivo activation conditions, such as HIV infection. These results support further the hypothesis of leptin as a stimulatory cytokine, and point to a possible role for leptin in the pathophysiology of human HIV infection.
Acknowledgments
This work was supported by the University Hospital Virgen Macarena, Sevilla, Servicio Andaluz de Salud, Andalucía, Spain and Plan Andaluz de Investigación, Junta de Andalucía, Spain.
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homolog. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Maffei M, Fei H, Lee GH, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA. 1995;92:6957–60. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–33. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 4.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–3. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 5.Flier JS. The adipocyte: storage depot or node on the energy information superhighway? Cell. 1995;80:15–8. doi: 10.1016/0092-8674(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 6.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 7.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 8.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein. evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 9.Frederich RC, Löllmann B, Hamann A, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–63. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 11.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995;373:13–8. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 13.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 15.Cioffi J, Shafer AW, Zupancic TJ, et al. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–8. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 16.Bennet BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Mathews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–80. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 17.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 18.Howard JK, Lord GM, Matarese G, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–9. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faggioni R, Jones-Carson J, Reed DA, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–72. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 21.Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93:14564–8. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee F-YJ, Li Y, Yang EK, et al. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Pysiol. 1999;276:C386–C394. doi: 10.1152/ajpcell.1999.276.2.C386. [DOI] [PubMed] [Google Scholar]
- 23.Loffreda S, Rai R, Yang SQ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 24.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 26.Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 27.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–6. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- 29.Martín-Romero C, Sánchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- 30.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- 31.Lucas M, Sánchez-Margalet V, Sanz A, Solano F. Protein kinase C activation promotes cell survival in mature lymphocytes prone to apoptosis. Biochem Pharmacol. 1994;47:1994. doi: 10.1016/0006-2952(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 32.García-Mauriño S, González-Haba MG, et al. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: a possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J Immunol. 1997;159:574–81. [PubMed] [Google Scholar]
- 33.Sung CK, Sanchez-Margalet V, Goldfine ID. Role of p85 subunits of phosphatidylinositol-3-kinase as an adaptor molecule linking the insulin receptor, p62 and GTPase-activating protein. J Biol Chem. 1994;269:12503–7. [PubMed] [Google Scholar]
- 34.Sánchez-Margalet V, Najib S. Sam68 is a substrate of the insulin receptor and associates with the SH2 domains of p85 PI3K. FEBS Lett. 1999;455:307–10. doi: 10.1016/s0014-5793(99)00887-x. [DOI] [PubMed] [Google Scholar]
- 35.Jin L, Burguera BG, Couce ME, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role of leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–11. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- 36.Grunfeld C, Zhao C, Fuller J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–7. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickup JC, Chusney GD, Mattock MB. The innate immune response and type 2 diabetes: evidence that leptin is associated with a stress-related reaction. Clin Endocrinol. 2000;52:107–12. doi: 10.1046/j.1365-2265.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 38.Lai KN, Leung JC, Lai FM. Soluble interleukin 2 receptor release, interleukin 2 production, and interleukin 2 receptor expression in activated T-lymphocytes in vitro. Pathology. 1991;23:224–8. doi: 10.3109/00313029109063570. [DOI] [PubMed] [Google Scholar]
- 39.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–4. [PubMed] [Google Scholar]
- 40.Berman MA, Zaldivar F, Jr, Imfeld KL, Kenney JS, Sandborg CL. HIV-1 infection of macrophages promotes long-term survival and sustained release of interleukins 1 alpha and 6. AIDS Res Hum Retroviruses. 1994;10:529–39. doi: 10.1089/aid.1994.10.529. [DOI] [PubMed] [Google Scholar]
- 41.Abbate I, Dianzani F, Capobianchi MR. Activation of signal transduction and apoptosis in healthy lymphomonocytes exposed to bystander HIV-1-infected cells. Clin Exper Immnol. 2000;122:374–80. doi: 10.1046/j.1365-2249.2000.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navikas V, Link J, Persson C, et al. Increased mRNA expression of IL-6, IL-10, TNF-alpha, and perforin in blood mononuclear cells in human HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:484–9. [PubMed] [Google Scholar]
- 43.Salazar-Gonzalez JF, Martinez-Maza O, Aziz N, et al. Relationship of plasma HIV-RNA levels of TNF-alpha and immune activation products in HIV infection. Clin Immunol Immunopathol. 1997;84:36–45. doi: 10.1006/clin.1997.4364. [DOI] [PubMed] [Google Scholar]
- 44.Sulkowski MS, Chaisson RE, Karp CL, Moore RD, Margolick JB, Quinn TC. The effect of acute infectious illnesses on plasma human immunodeficiency virus (HIV) type 1 load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis. 1998;178:1642–8. doi: 10.1086/314491. [DOI] [PubMed] [Google Scholar]


