Abstract
Glomerulonephritis with organized microtubular monoclonal immunoglobulin deposits (GOMMID) and glomerulonephritis related to type I cryoglobulin are well-known but rare complications of B cell derived chronic lymphocytic leukaemia. In these disorders, monoclonal Ig have never been studied at the molecular level. We conducted a pathological and molecular analysis in a patient with chronic lymphocytic leukaemia, glomerulonephritis and a single circulating monoclonal Ig. Unusual IgG1κ kidney deposits were observed. The heavy and light chain variable region sequences of that cryoprecipitating monoclonal Ig were characterized. Light microscopy revealed glomerulonephritis typical of cryoglobulinaemia, with neutrophil and macrophage infiltration, endocapillary hyperplasia and few protein thrombi. Electron microscopic study clearly evidenced numerous subepithelial mixed granular and organized deposits with a unique microtubular organization, reminiscent of the GOMMID. The Ig molecule sequence revealed alterations of charge and hydrophobicity potentially promoting a crystal-like aggregation and the aggregation of microtubules.This description suggests that common mechanisms are involved in various forms of precipitation and/or deposition of complete Ig molecules, with a variable extent of organization and with a possible overlap between pathological patterns of either glomerulonephritis with microtubular deposits or type I cryoglobulinic glomerulonephritis.
Keywords: cryoglobulin, primary structure, renal deposits
INTRODUCTION
Glomerulonephritis with organized microtubular monoclonal immunoglobulin deposits (GOMMID) have been reported in a number of patients with chronic lymphocytic leukaemia (CLL) [1–6], most of them without cryoglobulinaemia and with low levels of circulating monoclonal Ig, detectable only with sensitive techniques, or undetectable. In some patients, cytoplasmic inclusions with identical substructures were also present within circulating leukaemic cells [2]. A clear picture has now emerged from a number of studies and demonstrated that microtubular deposits usually involve monoclonal Ig, although the condition first referred to as immunotactoid glomerulopathy (IT) was mistakenly defined as involving only polyclonal Ig [7]. Microtubules seen in GOMMID differ from fibrils seen in pseudoamyloid fibrillary glomerulonephritis (FG) not only by their mean diameter (10–60nm versus 10–20nm, respectively), but mostly their pro-tein content (monoclonal Ig versus polyclonal IgG4) and their respective parallel versus random arrangement [8–10]. However, monotypic IgG have occasionally been reported in cases supposedly classified as FG [11,12].
Type I cryoglobulinaemia results usually in membranoproliferative glomerulonephritis, eventually associated with organized subendothelial, mesangial deposits and protein thrombi with microtubular organization in most cases. Subepithelial deposits are scarce or absent. A singular form of monoclonal Ig organized deposits, defining cryocrystalglobulinaemia, is characterized by highly organized crystalline substructures affecting various organs, especially the kidneys and the synovia [13]. This complication of various B cell-derived immunoproliferative disorders [13–17] features immunoglobulin crystallization within both monoclonal B cells and deposits.
Strikingly, primary structure data concerning the monoclonal Ig responsible for deposits are lacking both for GOMMID and for type I cryoglobulinic glomerulonephritis. In the current report, we have characterized a monoclonal IgG1κ cryoglobulin in a patient with CLL-associated glomerulonephritis. Primary sequences of the heavy and light chain variable domains have been determined at the cDNA level, while pathological examination of the kidneys revealed lesions related to type I cryoglobulinaemia but associated with unusual microtubular subepithelial deposits typical of GOMMID.
PATIENT AND METHODS
Case report
The patient, JAN, a 52-year-old Caucasian man, was admitted because of massive proteinuria. He had a 19-year history of B cell-derived CLL, treated with chlorambucil during less than 3 years. Leukaemic cells expressed CD5, CD19, CD20, IgG and κ chains (99% of cells); they also partially expressed CD23 (82%) and FMC7 (72%). Five years after the beginning of CLL, a microscopic haematuria appeared. Proteinuria was detected since 1996 and slowly increased.
At admission, physical examination showed extensive oedema and mild spleen enlargement but no adenopathy. The lungs, heart, skin, joints and neurological examination was normal. Urinalysis showed 10·5 g/24-h proteinuria and 200000 red blood cells/mm3. The haemoglobin level was 12·5g/dL, the white cell count was 22100/mm3 with 68% lymphocytes. The patient had normal serum levels of creatinine (1·1mg/dL); total serum protein level was 4·3g/dL, albumin 2·74g/dL and gammaglobulin 0·15g/dL. Serum level of IgG was 0·14g/dL, corresponding to a monoclonal IgGκ band, while polyclonal IgM, IgA and IgG were undetectable. Search for a cryoglobulin on warm separated serum was positive and corresponded to the monoclonal IgGκ.
Evolution under 6 months treatment with chlorambucil (4mg/day) was favourable and 18 months later the patient had no peripheral oedema, white cell count 5300/mm3 with 37% lymphocytes, total serum protein level 5·6g/l, albumin 3·8g/dL and proteinuria level down to 2·6g/24 h, while the monoclonal cryoglobulin persisted as a weak monoclonal band.
Pathological studies
Paraffin-embedded kidney biopsy samples were cut into 4 μm-thick slices. Organs were studied for the presence of deposited Ig by immunofluorescence with fluorescein-conjugated antibodies against human IgA, IgM (ICN, Costa Mesa, CA, USA), IgG, κ, λ, C1q, C3, fibrinogen, CD20 and CD68 (Dako, Carpinteria, CA, USA). For ultrastructural studies, small samples of kidney were fixed in 2·5% glutaraldehyde. Sections were then stained with uranyl acetate and examined using a Jeol 100 CX electron microscope.
For electron microscopy, peripheral blood lymphocytes were fixed with 4% paraformaldehyde plus 2·5% glutaraldehyde. Thin sections of resin-embedded cells were cut with the Ultracut S (Reichert, Austria), double-contrasted with uranyl acetate and lead citrate and viewed in an electron microscope.
Cryoprecipitate was fixed with 2·5% glutaraldehyde and embedded in araldite.
Immunochemical analysis
Serum and urine specimens from the patient were analysed by agarose gel immunofixation using a panel of anti-γ, -α or -μ heavy chain and anti-κ or -λ light chain antisera.
Molecular biology study
Total RNA was extracted from the patient's peripheral blood lymphocytes and used as template for synthesizing single-stranded cDNA by extending an oligo(dT) primer (Amersham Pharmacia Biotech, Orsay, France) with reverse transcriptase (Life Technologies SARL, Cergy Pontoise, France). To determine the subgroup of Ig light chain of the patient, a series of PCR amplifications was performed with the cDNA as a template, a 3′ primer complementary to the upstream part of the Cκ exon and four different 5′ primers representing consensus sequences of leader regions for each Vκ subgroup. The 3′ primer was 5′-CGG GAA GAT GAA GAC AGA TGG TGC ACC-3′ and the four 5′ primers were: VκI (5′-ATG GAC ATG AGG GTC CCC GCT-3′), VκII (5′-ATG AGG CTC CCT GCT CAG CTC-3′), VκIII (5′-ATG GAA GCC CCA GCG CAG CTT-3′) and VκIV (5′-ATG GTG TGG CAG ACC CAG GTC-3′). To determine the heavy chain sequence, we used a-3′ primer complementary to human γ genes (CH1 exon) and six different 5′ primers corresponding to consensus sequences of variable VH subgroup leader region. The 3′ primer was 5′-GCT CTT GGA GGA GGG TGC CAG-3′, and the six VH primers were VH1 (5′-CCA TGG ACT GGA CCT GGA-3′), VH2 (5′-ATG GAC ATA CTT TGT TCC AC-3′), VH3 (5′-ATG GAG TTT GGG CTG AGC T-3′), VH4 (5′-ATG AAA CAC CTG TGG TTC TTC CTC CT-3′), VH5 (5′-ATG GGG TCA ACC GCC ATC C-3′) and VH6 (5′-ATG TCT GTC GTC TCC TTC CTC AT-3′).
PCR amplification for Vκ was performed with Taq DNA polymerase (Amersham Pharmacia Biotech) as described [18]. PCR amplification protocol for VH was: denaturation at 94°C for 2min, followed by 35 cycles consisting of denaturation at 94°C for 30s, annealing at 55°C for 30 s, elongation at 72°C for 30 s, and a final elongation step at 72°C for 12 min. The PCR products were cloned into pCRII-TOPO plasmid (Invitrogen, Groningen, the Netherlands). DNA sequencing was performed by the dideoxy method using Big-dye terminators (Applied Biosystems) and a capillary electrophoresis system (Applied Biosystems).
Molecular modelling
We compared the computer-generated tertiary structures of the variable regions of JANH and JANκ with the tertiary structures of their germline counterparts. Three-dimensional structures were obtained using Swiss-Model v3·5 automated comparative protein modelling server (GlaxoSmithKline, Geneva, Switzerland). The generated tertiary structures were then superimposed using the Swiss-PDB Viewer molecular modelling software (Glaxo Wellcome Experimental Research, Geneva, Switzerland).
RESULTS
Pathological studies
Renal biopsy was performed: light microscopy revealed 10 glomeruli, one of which was sclerotic. The predominant abnormality was intraglomerular hypercellularity with mesangial matrix expansion (Fig. 1a) and voluminous pseudothrombi; periodic acid Schiff-positive and Congo red-negative deposits laid against the inner side of the glomerular capillary wall and silver methenamine impregnation showed segmental and focal double-contour appearance of the glomerular capillary wall. Interstitium was nodularly infiltrated by CD20+ leukaemic lymphocytes. Tubular epithelial cells appeared normal, interstitial fibrosis was very mild and vessels displayed no abnormality. Immunohistology revealed deposition of IgGκ, C1q and C3 along the capillary wall in all 10 glomeruli studied; immunofluorescence staining was negative for IgA, IgM and λ light chain. The anti-CD68 monoclonal antibody stained numerous macrophages in glomerular capillary lumens.
Fig. 1.
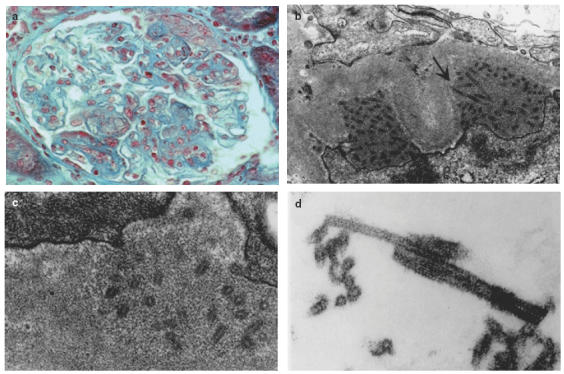
Mixed deposits involving the IgG1κ cryoglobulin. (a) Renal biopsy, light microscopy (original magnification ×200); glomerulus from patient JAN with endocapillary proliferative changes and subendothelial deposits. (b) Renal biopsy, electron microscopy (original magnification ×15000). Osmiophilic subepithelial deposits made up of mixed microgranular material and 55 nm diameter microtubules in cross and longitudinal sections. (c) Renal biopsy, electron microscopy (original magnification ×20000). Microtubules 50–55 nm in diameter with a 15–20 nm central lumen and a 15–17·5 nm thick wall. Smaller substructures in lamina densa are collagenous in nature. (d) In vitro formed cryoprecipitate, electron microscopy (original magnification ×50000). The same 55 nm microtubules are found without any other substructure or amorphous/granular material.
Electron microscopy was performed on glomeruli unfortunately devoid of endoluminal pseudothrombi, thrombi or voluminous subendothelial deposits. Numerous but not diffuse osmiophilic subepithelial deposits were found. The more voluminous deposits were formed by a mixed ground of microgranular material and thick microtubules 55 nm in external diameter, with a lumen of 25nm and a 15-nm-thick wall (Fig. 1b,c). Microtubules 15nm in diameter were seen in the lamina densa within zones of duplication or mesangium and were collagenous in nature. Microtubules 55nm in diameter were also seen individually in the subepithelial aspect of few capillary walls in the lamina rara externa, between the lamina densa and partially fused podocytes processes.
Neither crystalline inclusions nor microtubules were seen in endothelial cell cytoplasm, in circulating mononuclear macrophage and in peripheral blood lymphocyte cytoplasms.
Electron microscopy of cryoprecipitate showed only microtubules 50–55 nm in external diameter, 15–20 nm in internal diameter with wall 15–17·5nm in thickness (Fig. 1d). A periodic cross-striation 12·5nm in periodicity was seen in longitudinal section of microtubules.
Immunochemistry
Serum electrophoresis showed that the monoclonal immunoglobulin was the only Ig detectable in the serum as polyclonal Ig were severely depressed. This monoclonal peak also appeared as the sole component of the cryoglobulin precipitate. Immunotyping with monospecific antisera indicated that this type I cryoglobulin was an IgGκ.
Molecular biology studies
Using circulating leukaemic cells from the patient, RNA was isolated and cDNA sequences corresponding to the expressed H and κ L chain genes were obtained. Products of three independent RT-PCR amplifications of V domains of both chains were cloned and sequenced. VH and VL sequences obtained in the three independent experiments were perfectly identical, confirming the monoclonal character of the proliferation and of the cloned IgG1κ cDNAs. Because the monoclonal IgGκ was the only detectable immunoglobulin in the patient serum and tissue deposits, its sequence could be deduced unambiguously from Ig cDNA sequences obtained from leukaemic cells.
The complete amino acid sequence of both heavy (JANκ) and light chain (JANH) is presented (Fig. 2). The JANH sequence belonged to the γ1 class and its variable region was made up of a VH4 subgroup segment while the L chain was related to the VκIII subgroup. Searches for homologies were made on the latest available release of V BASE or IMmunoGeneTics (IMGT) databases. Rearranged genes were assigned to their closest germline counterparts (VH4·41 joined to DXP4[ 19] and JH3 for the H chain and VκA27/3–20 [20] for the L chain) by both nucleotide and protein sequence alignments.
Fig. 2.
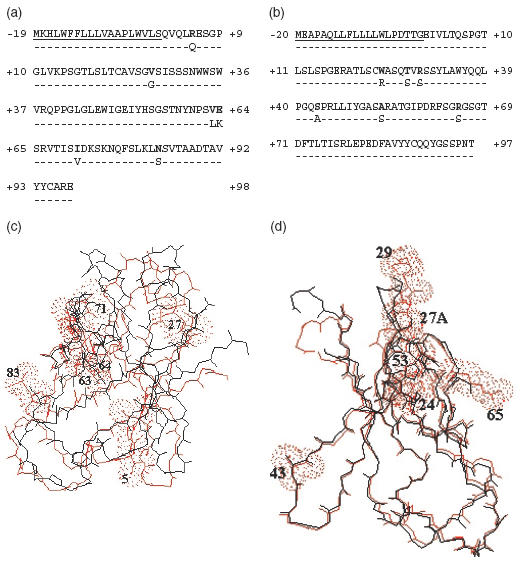
Light and heavy chain variable domain structures. Alignment of the primary sequences of the VH domain with the germinally encoded VH4·41/DXP4/JH3 sequence (a) and of the Vκ domain with the germinally encoded VκΑ27/Jκ2 sequence (b). Dashes indicate identities. Residues potentially involved in cryoprecipitation or microtubule formation are in bold. Amino acids are numbered according to Kabat [32]. (c) and (d) Molecular modelization of the of H (c) and L (d) Ig variable domains. Computer-generated tertiary structures were superimposed with the variable domains from closely related H and L chain sequences using a previously determined molecular structure of a complete Fab fragment (Tr1·9 Fab [33]).
The amino acid sequence of the JANH chain showed several point mutations which could modify the conformation of its variable domain (Fig. 2a). The invariant residue Gln at position 5 in the FR1 region is replaced by an Arg residue, which would not be expected to alter the polarity. Val27 (FR1) in the JANH chain has not been reported previously. Two substitutions affected neighbouring invariant residues in the CDR2: Val63 replaced Leu, while Glu64 replaced Lys and introduced a change in the polarity. In the FR3, Ile71 replaced the invariant Val, which would not be expected to alter the hydrophobicity at this position. In the same region, a point mutation at position 82a changes the invariant Ser into a positively charged Asn residue. Comparison of the JANH sequence with previously established three-dimensional molecular models indicate that Arg5, Val27, Val63, Glu64 and Asn83 are solvent-exposed (Fig. 2c).
Several unique amino acid substitutions occurred in the JANκ chain sequence compared to other κIII proteins (Fig. 2b). Trp at position 24 (CDR1) in JANκ is present in only one other κIII protein, AL 700 [21], while the majority contain Arg. This substitution could alter the charge and polarity at this position. Ser27a is changed to a Thr. The Arg29 replacing Ser (CDR1) has been reported previously in protein B5G10 κ CL [22]. This substitution alters the charge at this position. In the FR2, Ser43 replaced the hydrophobic residue Ala. In the CDR3 region, Ala53 has not been reported in other proteins and is normally Ser. Arg65 has never been reported and is normally Ser, as in HG2B10 κ CL [22] and HIV-s5 CL [23], while the majority contain Ser. This replacement results in inverting the charge at this position. The last substitution found in JANκ sequence consisted in the presence of an Asn96 residue, which has been described previously in only one κIII protein, MoAb112 CL [24]. Comparison of the JANκ sequence with previously established three-dimensional molecular models indicate that Trp24, Thr27A, Arg29, Ser43, Ala43 and Arg65 are solvent-exposed (Fig. 2d).
DISCUSSION
Cryoglobulin kidney lesions correspond typically to membranoproliferative glomerulonephritis with subendothelial deposits and a peculiar infiltration by monocytes and polymorphs. In the most severe cases in the acute phase, there is occlusion of the capillary lumen by the same immunoglobulin constituents of the cryoprecipitate. Such aspects most often involve type II cryoglobulins, but have also been found associated with type I cryoglobulins, where amorphous eosinophilic, PAS-positive deposits occluding glomerular capillary lumens have also been reported occasionally [25–27]. Subepithelial deposits have rarely been reported, but altogether there are few reports of detailed immunohistochemical and electron microscopy studies in type I cryoglobulinaemia [28]. Type I cryoglobulins have also been studied rarely in human regarding immunological and structural properties [29] and no sequence data are available about such proteins.
GOMMID is featured by the formation of subepithelial deposits with microtubular structures (20–60 nm in diameter) made up of monoclonal Ig [1–6]. GOMMID occur in the course of monoclonal immunoproliferative B cell disorders, most often CLL, or in a few cases without overt lymphoid or plasma cell proliferation or without detectable serum monoclonal component. Again in GOMMID, we lack any sequence information about pathogenic Ig yielding nephritogenic deposits in patients.
We report here an observation of mixed granular and microtubular organized deposits at the ultrastructural level in patient JAN affected with type I cryoglobulin and B cell-derived CLL. This observation shows that the same monoclonal immunoglobulin can eventually lead to various aspects of tissue deposition and renal pathogenicity, with the co-existence of amorphous (microgranular) and organized microtubular deposits. The combination of immunochemical studies on the serum monoclonal component and cryoprecipitate, of immunohistochemical studies on kidney deposits and of mRNA sequence determination from the leukaemic cells allowed us to show that the same IgG1κ produced by leukaemic cells was responsible for cryoprecipitation and mixed granular and organized deposition into the kidneys.
In mouse, although the precise mechanisms of cryoprecipitation are not understood, convincing data support an electrostatic model. The exclusive presence of IgG3 within monoclonal type I murine cryoglobulins and also the enrichment of the IgG3 subclass within mixed type II cryoglobulins have been ascribed to a strong tendency of this isotype to self-aggregate due to a more positively charged CH2 domain ]30, 31[. Monoclonal IgG3 cryoglobulins with a wide range of antigen specificities have been reported, and γ3 constant regions would promote electrostatic interactions and self-aggregation of Ig independently of their antigen-binding site. However, not all monoclonal IgG3 behave as cryoglobulins and not all cryoglobulins induce kidney lesions; it is thus clear that V region sequences also play a role in self-aggregation, precipitation and nephritogenicity, noticeably by providing additional positively charged residues. More specifically, residues Glu6 and Lys23 of the heavy chain variable domain have been postulated to play a role in precipitation in a study comparing six IgG3 cryoglobulins to several noncryoprecipitating monoclonal IgG3 [31]. A recent study has provided additional insights into the role of V regions by showing that a crystal-cryoglobulin forming highly organized glomerular deposits could be converted into a ‘classical’ type I cryoglobulin yielding granular amorphous thrombi, through a limited mutation in the VL domain [26].
In IgG1κJAN, the cumulative effects of all substitutions described above create together additional positive charges in some areas of the V domains and may enhance hydrophobicity in other areas, thus being implicated in the deposition properties and cryoprecipitability. In particular, some of the mutated amino acid residues are exposed at the surface of the Ig molecule and could generate electrostatic interactions: Arg5, Glu64 and Asn82a in the VH region and Arg29 and Arg65 in the Vκ domain. Other substitutions may promote hydrophobic interactions such as the solvent-exposed residues Val27, Val63 in the VH region, and Trp24 and Ala43 in the Vκ domain. Interestingly, the VH domain also featured Glu6, as for all studied murine cryoglobulins. The above-mentioned residues exposed at the surface of the Ig molecule may define contact areas essential for monomer interactions and for precipitation, and may together promote the microtubular arrangement observed by electronic microscopy.
The herein reported observation suggests strongly that common mechanisms favouring interactions between Ig monomers due to electrostatic charges and/or hydrophobicity may promote nucleation of Ig and underlie the process of deposition and cryoprecipitation in various patterns. Depending upon the structure and the arrangement of monomers within aggregates, tissue binding and deposition would then occur at various locations and with a variable extent and type of organization.
Clearly, additional molecular studies will be useful for a better understanding of the pathophysiology of immunoglobulin cryoprecipitation.
Acknowledgments
This work was supported by grants from Ligue Contre le Cancer (Comité Régional de la Haute-Vienne), Conseil Régional du Limousin, Association pour la Recherche sur le Cancer (grant 9121), AREN Poitou-Charentes. HRG was supported by a fellowship from Association pour la Recherche sur le Cancer. We thank Josette Nicaud and Isabelle Poulidor for technical help.
REFERENCES
- 1.Gilboa N, Durante D, Guggenheim S, et al. Immune deposit nephritis and single-component cryoglobulinaemia associated with chronic lymphocytic leukemia. Nephron. 1979;24:223–31. doi: 10.1159/000181721. [DOI] [PubMed] [Google Scholar]
- 2.Touchard G, Preud’homme JL, Aucouturier P, et al. Nephrotic syndrome associated with chronic lymphocytic leukemia: an immunological and pathological study. Clin Nephrol. 1989;31:107–16. [PubMed] [Google Scholar]
- 3.Rollino C, Coppo R, Mazzucco G, et al. Monoclonal gammopathy and glomerulonephritis with organized microtubular deposits. Am J Kidney Dis. 1990;15:276–80. doi: 10.1016/s0272-6386(12)80775-x. [DOI] [PubMed] [Google Scholar]
- 4.Touchard G, Bauwens M, Goujon JM, Aucouturier P, Patte D, Preud’homme JL. Glomerulonephritis with organized microtubular monoclonal immunoglobulin deposits. In: Grunfeld JP, Bach JF, Kreis H, Maxwell MH, editors. Advances in nephrology. St Louis: Mosby Yearbook; 1994. p. 149. [PubMed] [Google Scholar]
- 5.Moulin B, Ronco PM, Mougenot B, et al. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127–35. doi: 10.1038/ki.1992.270. [DOI] [PubMed] [Google Scholar]
- 6.Ronco P. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355–77. doi: 10.1046/j.1523-1755.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 7.Korbet SM, Schwartz MM, Rosenberg BF, et al. Immunotactoid glomerulopathy. Medicine (Baltimore) 1985;64:228–43. doi: 10.1097/00005792-198507000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Alpers CE. Immunotactoid (microtubular) glomerulopathy. Am J Kidney Dis. 1992;19:185–91. doi: 10.1016/s0272-6386(12)70132-4. [DOI] [PubMed] [Google Scholar]
- 9.Fogo A, Qureshi N, Horn RG. Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy. Am J Kidney Dis. 1993;22:367–77. doi: 10.1016/s0272-6386(12)70138-5. [DOI] [PubMed] [Google Scholar]
- 10.Ferluga D, Hvala A, Vizjak A, et al. Immunotactoid glomerulopathy with unusually thick extracellular microtubules and nodular glomerulosclerosis in a diabetic patient. Pathol Res Pract. 1995;191:585–96. doi: 10.1016/s0344-0338(11)80879-4. [DOI] [PubMed] [Google Scholar]
- 11.Grove P, Neale PH, Peck M, et al. Monoclonal immunoglobulin G1-kappa fibrillary glomerulonephritis. Mod Pathol. 1998;11:103–9. [PubMed] [Google Scholar]
- 12.Strom EH, Hurwitz N, Mayr AC, et al. Immunotactoid-like glomerulopathy with massive fibrillary deposits in liver and bone marrow in monoclonal gammopathy. Am J Nephrol. 1996;16:523–8. doi: 10.1159/000169053. [DOI] [PubMed] [Google Scholar]
- 13.Papo T, Musset L, Bardin T, et al. Cryocrystalglobulinaemia as a cause of systemic vasculopathy and widespread erosive arthropathy. Arthritis Rheum. 1996;39:335–40. doi: 10.1002/art.1780390225. [DOI] [PubMed] [Google Scholar]
- 14.Von Bonsdorff B, Groth H, Packalen T. On the presence of a high molecular crystallizable protein in the blood serum in myeloma. Folia Hematol. 1938;59:184. [Google Scholar]
- 15.Grossman J, Abraham GN, Leddy JP, et al. Crystalglobulinaemia. Ann Intern Med. 1972;77:395–400. doi: 10.7326/0003-4819-77-3-395. [DOI] [PubMed] [Google Scholar]
- 16.Ball NJ, Wickert W, Marx LH, et al. Crystalglobulinaemia syndrome: a manifestation of multiple myeloma. Cancer. 1993;71:1231–4. doi: 10.1002/1097-0142(19930215)71:4<1231::aid-cncr2820710410>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Albert L, Inman R, Gordon DA, et al. Cryocrystalglobulinaemia mimicking rheumatoid arthritis and vasculitis. J Rheumatol. 1996;23:1272–7. [PubMed] [Google Scholar]
- 18.Decourt C, Cogne M, Rocca A. Structural peculiarities of a truncated V kappa III immunoglobulin light chain in myeloma with light chain deposition disease. Clinical and Experimental Immunology. 1996;106:357–61. doi: 10.1046/j.1365-2249.1996.d01-841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng N, Marcus D. Polymorphism of human immunoglobulin VH4 germ-line genes. Eur J Immunol. 1992;22:1075–82. doi: 10.1002/eji.1830220430. [DOI] [PubMed] [Google Scholar]
- 20.Straubinger B, Huber E, Lorenz W, et al. The human Vκ locus: characterization of a duplicated region encoding 28 different immunoglobulin genes. J Mol Biol. 1988;199:23–34. doi: 10.1016/0022-2836(88)90376-2. [DOI] [PubMed] [Google Scholar]
- 21.Toft KG, Olstad OK, Sletten K. AL protein and light chain related amyloidosis. In: Natvig JB, Forre O, Husby G, et al., editors. Amyloid and amyloidosis. Dordrecht: Kluwer Academic Publishers; 1990. pp. 169–72. [Google Scholar]
- 22.Weng NP, Yu-Lee LY, Sanz I, et al. Structure and specificities of anti-ganglioside autoantibodies associated with motor neuropathies. J Immunol. 1992;149:2518–29. [PubMed] [Google Scholar]
- 23.Barbas CF, Collet TA, Amberg W, et al. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993;230:812–23. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani L, Wilder RL, Casali P. Human rheumatoid B-1a (CD5+ B) cells make somatically hypermutated high affinity IgM rheumatoid factors. J Immunol. 1993;151:473–88. [PMC free article] [PubMed] [Google Scholar]
- 25.Ishimura E, Nishizawa Y, Shoji S, et al. Heat-insoluble cryoglobulin in a patient with essential type I cryoglobulinaemia and massive cryoglobulin-occlusive glomerulonephritis. Am J Kidney Dis. 1995;26:654–7. doi: 10.1016/0272-6386(95)90604-5. [DOI] [PubMed] [Google Scholar]
- 26.Rengers JU, Touchard G, Decourt C, et al. Heavy and light chain primary structures control IgG3 nephritogenicity in an experimental model for cryocrystalglobulinaemia. Blood. 2000;95:3467–72. [PubMed] [Google Scholar]
- 27.Provot F, Bridoux F, Vanhille P, et al. Spectrum of glomerular disease in type I cryoglobulinaemia. J Am Soc Nephrol. 2000;11:95. [Google Scholar]
- 28.Monga G, Mazzucco G, Casanova S, et al. Ultrastructural glomerular findings in cryoglobulinemeic glomerulonephritis. Appl Pathol. 1987;5:108–15. [PubMed] [Google Scholar]
- 29.Abraham GN, Podell DN, Wistar RJ, et al. Immunological and structural properties of human monoclonal IgG cryoglobulins. Clinical & Experimental Immunology. 1979;36:63–70. [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelmoula M, Spertini F, Shibata T, et al. IgG3 is the major source of cryoglobulins in mice. J Immunol. 1989;143:526–32. [PubMed] [Google Scholar]
- 31.Panka DJ, Salant DJ, Jacobson BA, et al. The effect of VH residues 6 and 23 on IgG3 cryoprecipitation and glomerular deposition. Eur J Immunol. 1995;25:279–84. doi: 10.1002/eji.1830250146. [DOI] [PubMed] [Google Scholar]
- 32.Johnson G, Kabat EA, Wu TT. Kabat database of proteins of immunological interest. In: Herzenberg LA, Weir WM, Blackwell C, editors. Weirs handbook of experimental immunology. 5. Cambridge, MA: Blackwell Science Inc.; 1996. p. 6.1. [Google Scholar]
- 33.Chacko S, Padlan EA, Portolano S, et al. Structural studies of human autoantibodies. Crystal structure of a thyroid peroxidase autoantibody Fab. J Biol Chem. 1996;271:12191–8. doi: 10.1074/jbc.271.21.12191. [DOI] [PubMed] [Google Scholar]


