Abstract
Leucocytes infiltrate into renal tissue and are involved in the pathogenesis of crescentic glomerulonephritis. The initial event in the process of leucocyte infiltration is characterized by selectin-mediated leucocyte rolling on endothelial surface. Role of selectins in pathogenesis of glomerulonephritis has still been controversial. Sulphated glycolipids and sulphated polysaccharides interfere with the binding of P- and L-selectin with carbohydrate ligands on endothelial cells or on leucocytes. Here we evaluated the role of selectins and the preventive effects of sulphated colominic acid (SCA), a synthetic sulphated polysaccharide, on experimental crescentic glomerulonephritis in Wistar-Kyoto (WKY) rats. Crescentic glomerulonephritis was induced by injection of nephrotoxic serum (NTS) in WKY rats. Rats subsequently received intraperitoneal injection of saline, neutralizing or non-neutralizing monoclonal antibody (mAb) to rat P-selectin and L-selectin, SCA (5 or 10mg/kg/day) or nonsulphated colominic acid (CA) (10mg/kg/day) for 2 weeks. Localization of P-, E-selectin, ligands for L-selectin and intraglomerular leucocytes was examined by immunohistochemistry. Gene expression of platelet-derived growth factor (PDGF) B chain in glomeruli was quantified using real-time RT-PCR. P-selectin was highly expressed on glomerular endothelial cells after injection of NTS, whereas E-selectin and L-selectin ligands were not detected. Anti-P-selectin mAb, but not anti-L-selectin mAb, significantly reduced glomerular infiltration of macrophages, crescent formation, and proteinuria. SCA also reduced proteinuria, macrophage infiltration, and crescent formation in a dose-dependent manner. Furthermore, SCA suppressed gene expression of PDGF B chain in glomeruli. Our results indicate that P-selectin partially mediate glomerular infiltration of macrophage in experimental crescentic glomerulonephritis. Moreover, SCA may inhibit intraglomerular infiltration of macrophages by interfering with P-selectin-dependent adhesion pathway, and progression of experimental crescentic glomerulonephritis.
Keywords: selectin, crescentic, glomerulonephritis, sulphated colominic acid, platelet-derived, growth factor
INTRODUCTION
Leucocyte infiltration into tissues occurs through multiple and sequential steps and various cell adhesion molecules including selectins, immunoglobulin superfamily molecules and integrins are important in such process. Selectins and their carbohydrate-containing ligands mediate leucocyte rolling on the vascular endothelium, i.e. the first step of leucocyte adhesion to endothelial cells [1]. Selectin family consists of three adhesion molecules, P-, L- and E-selectin. P-selectin is expressed on the surface of activated endothelial cells and in platelets [2,3]. Similarly, E-selectin is expressed on the activated endothelium in the early stages of inflammation [4]. In contrast to P- and E- selectin, L-selectin is constitutively expressed on the surface of leucocytes and binds to the natural ligands on endothelial cells [5].
Both P- and L-selectin bind to sulphated sugar chains on the ligands. Several studies reported that sulphatide, a sulphated glycolipid, specifically interacts with L-selectin by binding to its sulphated sugar chains [6,7]. Furthermore, sulphated polysaccharides have been shown to interfere with the adhesive interactions of selectins with their natural ligands on endothelial cells or on leucocytes [8,9]. For instance, sulphatide can interfere with the binding of P-selectin to neutrophils [10]. We also reported the preventive effect of sulphatide, which binds both P- and L-selectin, on mononuclear cell infiltration into renal interstitium after unilateral ureteral obstruction [11]. Sulphated colominic acid (SCA), a synthetic sulphated polysaccharide, interferes with the binding of P-selectin to neutrophils in vitro[9]. SCA also exhibits a potent antiviral activity against human immunodeficiency virus type 1 [12,13]. Recently, SCA has been demonstrated to block P-selectin-dependent infiltration of leucocytes in acute lung injury induced by cobra venom factor [9].
Leucocytes contribute to the glomerular injury seen in a variety of glomerulonephritis through the release of inflammatory cytokines including platelet derived growth factor (PDGF) [14], nitric oxide [15], proteases and other inflammatory mediators [16]. Accumulating evidence suggests that leucocyte adhesion molecules including β2 integrins and immunoglobulin superfamily molecules such as intercellular adhesion molecule-1 (ICAM-1) mediate leucocyte recruitment in various types of glomerulonephritis [17–20]. Anti-ICAM-1 antibody prevents leucocyte recruitment into renal tissue and ameliorates tissue injury in crescentic glomerulonephritis [17,20]. However, the role of selectins in glomerular leucocyte recruitment is still controversial [21,22].
To elucidate the specific role of selectins in the pathogenesis of crescentic glomerulonephritis, we employed nephrotoxic serum (NTS) nephritis in Wistar-Kyoto (WKY) rats as animal model. This model is characterized by accumulation of various inflammatory cells in the glomeruli, including macrophages, CD4 and CD8 lymphocytes, which are known to play key roles in the pathogenesis of crescentic glomerulonephritis [17]. For inhibition of the selectin-dependent pathways, specific blocking antibodies and SCA were used. Our results demonstrated the importance of P-selectin-dependent leucocyte adhesive pathway and preventive potential of SCA on crescentic glomerulonephritis in WKY rats.
MATERIALS AND METHODS
Preparation of nephrotoxic serum
Normal WKY rat kidneys were perfused with physiologic saline through a catheter placed in the aorta. Renal cortical tissue was removed, homogenized and diluted with physiologic saline at about 20% suspension. Two milliliters of renal cortical homogenate were emulsified with an equal volume of Freund's complete adjuvant (Difco Laboratories, Detroit, MI). This emulsion was injected subcutaneously into rabbits once a week for one month. Seven days after last injection, the rabbits were bled from the inferior vena cava under anaesthesia. The sera were decomplementized for 30min at 56°C and absorbed with freshly harvested rat erythrocytes. Preliminary immunohistochemical experiments showed that intraperitoneal injection of 1·0ml of the prepared NTS into WKY rats, which weighed about 140g, resulted in linear binding of rabbit IgG along the glomerular basement membrane (GBM).
Preparation of sulphated colominic acid
SCA and nonsulphated colominic acid (CA) were prepared in Marukin Chuyu Co. Ltd. (Kyoto, Japan) (Fig. 1). The preparation of SCA was reported previously [12,13]. The sodium salt with an average molecular mass of 24000 (n = 50) was used in the present study. The compound was dissolved in saline before use.
Fig. 1.
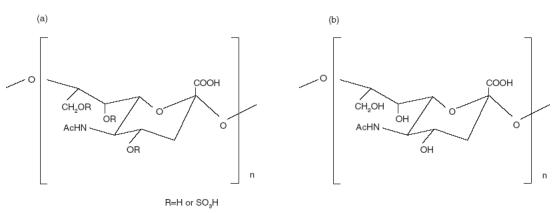
Chemical structures of (a) sulphated colominic acid and (b) colominic acid.
Experimental protocol
Female WKY rats (140g) were obtained from Charles River Japan (Atsugi, Kanagawa, Japan). All rats were fed standard chow and water ad libitum. WKY rats were randomly divided into nine groups of 8 rats each (Table 1). One group of rats (saline group) was administered saline every day after intraperitoneal injection of 1·0 ml of NTS, which induced glomerulonephritis. To confirm the role of selectins, 2mg/kg/day of mouse neutralizing anti-rat-P-selectin monoclonal antibody (mAb) (ARP2-4) [23,24] or the F(ab′)2 fragment of neutralizing anti-rat-L-selectin mAb (HRL3) [25] was administered intraperitoneally to 8 rats each every day after NTS injection. Mouse IgG was administered intraperitoneally at 2mg/kg/day every day as a control for ARP2-4, and a non-neutralizing mAb to rat-L-selectin (HRL2) as a control for HRL3 [25]. SCA (5mg/kg/day and 10mg/kg/day) or CA (10mg/kg/day) was administered every day by intraperitoneal injection for 14 days. As normal controls, one group of rats were injected with 1·0ml of normal rabbit serum instead of NTS (normal control group). Twenty-four-hour urine collections were obtained from rats that were individually housed in metabolic cages. Rats were fasted during the collection period, but were allowed free access to water. On days 1, 4, 8, 11 and 14, urine samples were collected and urinary protein levels were measured by the pyrogallol red method.
Table 1.
Serum creatinine level and creatinine clearance of each treatment group in WKY rats with crescentic glomerulonephritis on day 14
| Group | Serum creatinine level (mg/dl) | Creatinine clearance (μl/min/100g rat) |
|---|---|---|
| Saline | 0·64 ± 0·19 | 368·1 ± 89·7 |
| CA (10mg/kg) | 0·58 ± 0·18 | 356·2 ± 137·5 |
| SCA (5mg/kg) | 0·49 ± 0·06 | 391·8 ± 53·2 |
| SCA (10mg/kg) | 0·46 ± 0·09 | 409·4 ± 86·4 |
| mouse IgG (2mg/kg) | 0·56 ± 0·11 | 424·0 ± 134·2 |
| ARP 2–4 (2mg/kg) | 0·63 ± 0·16 | 376·2 ± 133·5 |
| HRL2 (2mg/kg) | 0·64 ± 0·09 | 368·1 ± 89·7 |
| HRL3 (2mg/kg) | 0·58 ± 0·12 | 388·5 ± 100·2 |
| Normal control | 0·43 ± 0·05 | 484·7 ± 91·8 |
Data are mean ± SEM of 8 rats in each group. Numbers in parentheses represent the daily dose of the drug. There are no statistically significant differences.
CA, colominic acid; SCA, sulphated colominic acid; mouse IgG, used as a control for ARP 2–4; ARP 2–4, neutralizing anti-rat P-selectin mAb; HRL2, non-neutralizing anti-rat L-selectin mAb used as a control for HRL3; HRL3, neutralizing anti-rat L-selectin mAb.
At day 14, the rats were sacrificed and both kidneys were removed. Portions of these tissues were processed for light microscopy, immunofluorescence staining and immunoperoxidase staining. Serum creatinine level and creatinine clearance were measured on day 14.
All experimental protocols described in the present study were approved by the Ethics Review Committee for Animal Experimentation of Okayama University Medical School.
Histopathological examination
Immunofluorescence staining
P-selectin, E-selectin, ligands for L-selectin, and ICAM-1 were detected by the indirect immunofluorescence method as described previously [18, 26–28]. Briefly, sections were fixed with cold acetone for 3 min and stained with mouse anti-P-selectin antibody (ARP2-3) [23,24], anti-E-selectin antibody (Y-18; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), the soluble fusion protein of rat L-selectin with human IgG (LEC-IgG) [27], and anti-rat ICAM-1 antibody (1A29) [29] for 24h at 4°C. Then, the sections were stained with fluorescein isothiocyanate (FITC)-labelled goat anti-mouse IgG antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA), FITC-labelled swine anti-goat IgG antibody (Cedarlane Laboratories, Ontario, Canada), FITC-labelled goat anti-human IgG antibody (Cappel, Aurora, OH) or FITC-labelled goat anti-mouse IgG antibody (Jackson Immunoresearch Laboratories) for 30min at room temperature, respectively. The sections were washed in PBS, mounted with PermaFluor (Shandon, Pittsburgh, PA, USA) and examined under a fluorescence microscope (LSM-510, Carl Zeiss, Jena, Germany).
In order to identify the localization of P-selectin positive cells in the glomeruli, serial sections were prepared and incubated with anti-rat endothelial cell mAb (OX-43; BMA Biomedicals, Rheinstrasse, Switzerland) as described previously [30]. Then, the section was stained with FITC-labelled goat anti-mouse IgG antibody (Jackson Immunoresearch Laboratories), and observed using a fluorescence microscope (LSM 510; Carl Zeiss, Jena, Germany). As negative control, the second antibody alone, FITC-labelled goat anti-mouse IgG antibody, was used.
Immunoperoxidase staining
Leucocyte infiltration in the glomerulus was examined by immunoperoxidase staining using a Vectastain ABC kit and Avidin Biotin Blocking Kit (Vector Laboratories, Inc., Burlingame, CA, USA) as described previously [26]. In brief, the frozen sections (4-μm thick) were fixed with cold acetone for 3 min and nonspecific protein binding was blocked by incubation with normal goat serum and avidin for 20 min The sections were first incubated with mouse mAbs against rat leucocyte common antigen (OX-1), mouse anti-rat monocyte/macrophage (ED1), rat CD4 and rat CD8 (Serotec, Oxford, UK) in a solution containing biotin, for 60min at room temperature. Then the sections were incubated with biotin-labelled goat anti-mouse IgG antibody (Jackson Immunoresearch Laboratories) for 30min at room temperature. Endogenous peroxidase activity was blocked by incubating the sections in methanol containing 0·3% H2O2 for 30min After that, the sections were incubated with ABC reagent, containing avidin and biotinylated horseradish peroxidase, for 30min at room temperature. Peroxidase activity was visualized by 3,3-diaminobenzidine and hydrogen peroxide. The sections were then counterstained with Mayer's haematoxylin. The nuclei of OX-1, ED1, CD4, CD8 positive cells in 50 glomeruli per kidney were counted under high magnification (×400).
Light microscopy
The renal tissue was fixed in 10% formalin and embedded in paraffin. Paraffin sections (4-μm thick) were then stained with periodic acid-Schiff's reagent (PAS). Fifty glomeruli in each rat were examined and crescent formation with two or more layers of cells along Bowman's capsule was counted.
Quantitative real-time reverse transcription-polymerase chain reaction
Glomeruli were isolated from the kidney cortex using a sieving mesh. Total RNA was extracted from the glomeruli by using an RNeasy Midi kit using the instructions provided by the manufacturer (Qiagen, Valencia, CA, USA). Two micrograms of total RNA from each sample was used for reverse transcription using a reverse transcription-PCR kit (Perkin Elmer, Foster City, CA, USA). Quantitative real-time RT-PCR was used to quantify the amounts of PDGF B chain and β-actin mRNAs. RT-PCR experiments were repeated twice under identical conditions to verify the results. cDNA was diluted 1:10 with autoclaved deionized water and 5 μl of the diluted cDNA was added to the Lightcycler-Mastermix (0·5 μm of specific primer, 3 mm MgCl2 and 2 μl Master SYBR Green, Roche Diagnostics, Mannheim, Germany). This reaction mixture was filled up with water to a final volume of 20μl. PCR reactions were carried out in a real-time PCR cycler (Lightcycler; Roche Diagnostics). The program was optimized and performed finally as denaturation at 95°C for 10 min followed by 40 cycles of amplification (95°C for 15 s; 59°C for 4 s; 72°C for 9 s in PDGF B chain and 22 s in β-actin). The temperature ramp rate was 20°C/s. At the end of each extension step, the fluorescence of each sample was measured to allow the quantification of the PCR product. After completion of the PCR, the melting curve of the product was measured by temperature gradient from 60 to 95°C at 0·2°C/s with continuous fluorescence monitoring to produce a melting profile of the primers. The amounts of PCR products were normalized with a housekeeping gene (β-actin) to calculate the relative expression ratios for PDGF B chain mRNA. The following oligonucleotide primers specific for rat PDGF B chain (Gene Bank accession no. Z14117) and β-actin (accession no. V01217) were used: PDGF, 5′-AGGTGTTCCAGATCTCGC-3′ (sense) and 5′-GTCACTGTGGCCTTCTTG-3′ (antisense); β-actin, 5′-TTGTAACCAACTGGGACGATATGG-3′ (sense) and 5′-ATCGGAACCGCTCATTGCC-3′ (antisense).
Statistical analysis
All values are expressed as the mean ± SEM. Differences between groups were examined for statistical significance using anova followed by Scheffe's test. A P-value less than 0·05 denoted the presence of a statistically significant difference.
RESULTS
Metabolic data
Urinary protein excretion on days 1, 4, 8, 11 and 14 were measured in saline group, ARP 2–4 group, mouse IgG group and normal control group (Fig. 2a). Before day 4, there was no difference between the groups with regard to urinary protein excretion. However, after day 8, the urinary protein excretion of saline group increased significantly compared with that in the normal control group. In the saline group, urinary protein excretion on day 14 was over 200mg/day. Administration of ARP 2–4 (anti-P-selectin) significantly decreased the level of urinary protein on day 14 to 137·1 ± 11·1mg/day compared with that in saline (215·8 ± 19·0mg/day) and mouse IgG groups (212·4 ± 23·7mg/day). On the other hand, there was no difference in urinary protein excretion between HRL3 (anti-L-selectin; neutralizing) and HRL2 (anti-L-selectin; non-neutralizing) groups (HRL3, 180·2 ± 16·0mg/day; HRL2, 214·7 ± 30·1mg/day) (Fig. 2b). In SCA and CA groups, there was no difference between the groups in urinary protein excretion before day 4. However, after day 8, the urinary protein excretion of SCA groups decreased significantly compared with that in CA group in a dose-dependent manner (SCA, 5mg/kg/day, 140·2 ± 11·5mg/day; SCA, 10mg/kg/day, 90·0 ± 16·2mg/day; CA, 209·9 ± 20·6mg/day) (Fig. 2c). In contrast, no differences in serum creatinine level and creatinine clearance were noted in SCA (5mg/kg/day) group, SCA (10mg/kg/day) group, CA group and saline group (Table 1). There was no difference in blood leucocyte count in CA, SCA (5mg/kg/day), SCA (10mg/kg/day) and saline group on day 14 (3480 ± 1907/μl, 3975 ± 1204/μl, 3840 ± 1205/μl, 3600 ± 1474/μl, respectively).
Fig. 2.
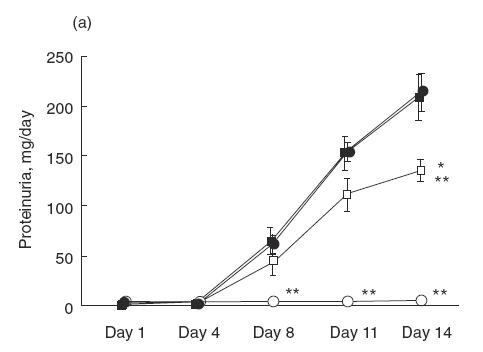
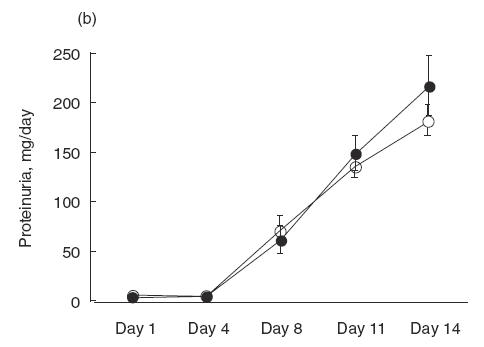
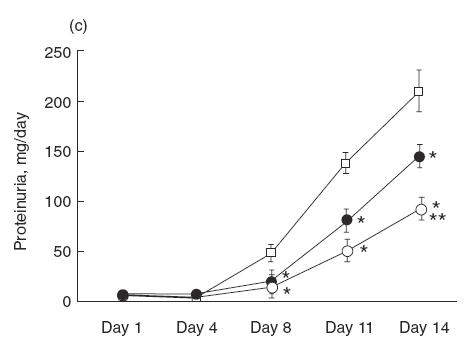
Urinary protein excretion over 24h measured on days 1, 4, 8, 11 and 14 after the injection of NTS. (a) Saline (•), anti-P-selectin monoclonal antibody (mAb) (ARP 2–4)(□), mouse IgG (▪) and normal control (○) group. Urinary protein excretion in saline group significantly increases after day 8, compared with normal control group. Administration of ARP 2–4 decreases urinary protein excretion on day 14 compared with mouse IgG group. Data are mean ± SEM of 8 rats in each group. *P < 0·01 versus mouse IgG group, ** P < 0·01 versus saline group. (b) Anti-L-selectin mAb (HRL3; ○) and non-neutralizing anti-L-selectin mAb (HRL2; •) group. There is no difference in urinary protein excretion between the groups. (c) Sulphated colominic acid (SCA: 5mg/kg/day; • and 10mg/kg/day; ○) and nonsulphated colominic acid CA (10mg/kg/day; □) group. Urinary protein excretion significantly decreases in SCA group after day 8, compared with CA group, in a dose-dependent manner. Data are mean ± SEM of 8 rats in each group. *P < 0·01 versus CA group, ** P < 0·01 versus SCA (5mg/kg/day) group.
Expression of P-selectin in the glomerulus
Indirect immunofluorescence study showed little or no expression of P-selectin in the glomerulus before injection of NTS (Fig. 3a). In contrast, P-selectin expression was detected in the glomerulus from day 1 in the saline group (Fig. 3b). Expression of P-selectin was gradually intensified at day 4 (Fig. 3c), day 8 (Fig. 3d), day 11 (Fig. 3e) and day 14 (Fig. 3f). E-selectin and the ligands for L-selectin were not detected in the glomeruli of both normal control and saline groups. When serial sections were stained with anti-P-selectin antibody and OX-43 (anti-rat endothelial cell mAb), P-selectin expression was detected mainly in glomerular endothelial cells (Fig. 4a–d). No staining was observed in the glomerulus both in normal and saline groups when the secondary antibody alone was used as negative control.
Fig. 3.
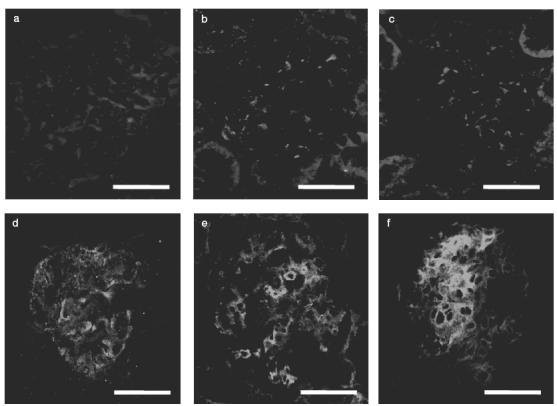
Expression profile of P-selectin in representative glomeruli from a rat of the saline group. Indirect immunofluorescence study showed little or no expression of P-selectin in the glomerulus before injection of NTS (a). In contrast, P-selectin expression was detected in the glomerulus from day 1 in the saline group (b). Expression of P-selectin was gradually intensified at day 4 (c), day 8 (d), day 11 (e) and day 14 (f). Scale bar = 50 μm.
Fig. 4.
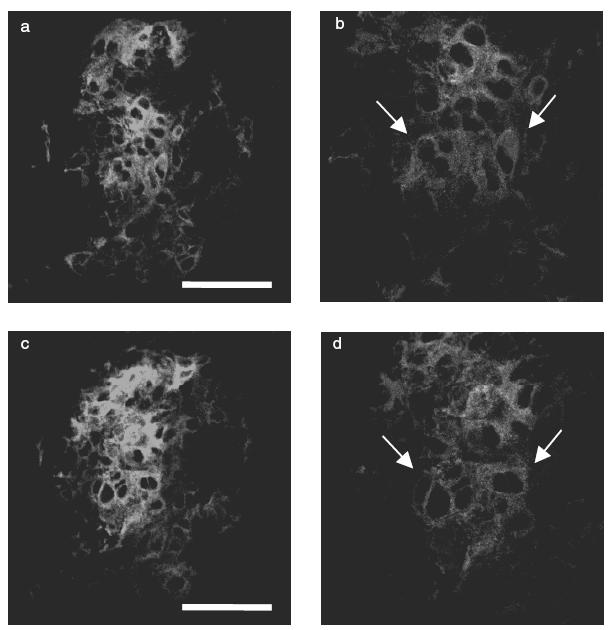
Immunofluorescence micrographs showing expression of OX-43 (a, b) and P-selectin (c, d) in representative glomeruli from a rat of the saline group on day 14. (a) Indirect immunofluorescence staining for endothelial cell. (b) Higher magnification of (a), arrow indicates endothelial cell. (c) Indirect immunofluorescence staining for P-selectin in a serial section of (a). (d) Higher magnification of (c), arrow indicates P-selectin positive cell. Scale bar = 50 μm.
Expression of ICAM-1 in the glomerulus
ICAM-1 expression was increased in the glomeruli of saline group (Fig. 5a), ARP 2–4 group (Fig. 5b), SCA (10mg/kg/day) group (Fig. 5c) as compared with normal control rats (Fig. 5d). There are no differences in ICAM-1 expression in ARP 2–4 group and SCA group as compared with saline group.
Fig. 5.
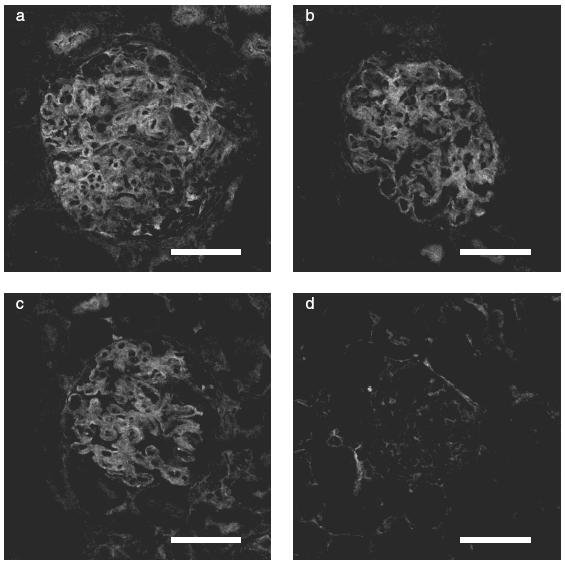
Expression of ICAM-1 in representative glomeruli of WKY rats with crescentic glomerulonephritis on day 14. a-d: Immunofluorescence-stained sections (a) saline group, (b) ARP 2–4 group, (c) SCA (10mg/kg/day) group, (d) normal control group. ICAM-1 expression in the glomerulus was detected at day 14, and there was no difference in the saline group (a), ARP 2–4 group (b) and SCA group (c). There was little or no expression of ICAM-1 in the glomerulus in normal control group (d). Scale bar = 50μm.
Histopathological changes
The numbers of total leucocytes (OX-1 positive cells), macrophages (ED1 positive cells), CD4- and CD8-positive cells were estimated in the glomeruli of each group on day 14 (Table 2). In the saline group, total numbers of leucocytes, macrophages, CD4 and CD8 positive cells were higher than in normal control group. In ARP 2–4 group, total leucocytes and macrophages in the glomeruli were lower than in mouse IgG group and saline group. There were no difference in total leucocytes and macrophages between HRL3 group and HRL2 group. Total leucocytes and macrophages in the glomeruli were significantly lower in SCA groups than in CA group. Macrophage counts in the glomerulus on day 14 were significantly lower in SCA groups than in CA group and the effect of SCA on these cells was dose-dependent. Immunoperoxidase staining showed that ED1 positive cells infiltrated in the glomerulus on day 14 in CA, SCA (5mg/kg/day) and SCA (10mg/kg/day) groups (Fig. 6a–c). The numbers of CD4- and CD8-positive cells were not different in ARP 2–4, mouse IgG, HRL3, HRL2, SCA (5mg/kg/day), SCA (10mg/kg/day), CA and saline group.
Table 2.
Densities (number/glomerulus) of total leucocytes (OX-1), macrophages (ED1), CD4 and CD8 positive cells, and crescent (%) in glomeruli of each treatment group in WKY rats with crescentic glomerulonephritis on day 14
| Group | OX-1 | ED1 | CD4 | CD8 | Crescent |
|---|---|---|---|---|---|
| Saline | 20·5 ± 0·8 | 17·7 ± 0·8 | 2·0 ± 0·1 | 1·1 ± 0·1 | 43·4 ± 1·8 |
| CA (10mg/kg) | 20·1 ± 0·4 | 18·0 ± 0·3 | 2·0 ± 0·1 | 1·0 ± 0·1 | 42·5 ± 1·4 |
| SCA (5mg/kg) | 17·9 ± 0·4*† | 14·4 ± 0·6*† | 1·9 ± 0·1 | 0·9 ± 0·1 | 35·1 ± 0·7*† |
| SCA (10mg+kg) | 15·9 ± 0·4*†‡ | 12·6 ± 0·5*†‡ | 1·8 ± 0·1 | 0·9 ± 0·1 | 26·5 ± 1·2*†§ |
| mouse IgG (2mg/kg) | 20·2 ± 0·4 | 18·1 ± 0·3 | 2·2 ± 0·1 | 1·2 ± 0·1 | 43·7 ± 1·2 |
| ARP 2–4 (2mg/kg) | 16·3 ± 0·3*¶ | 14·2 ± 0·4*¶ | 2·0 ± 0·1 | 1·0 ± 0·1 | 28·8 ± 1·4*¶ |
| HRL2 (2mg/kg) | 20·6 ± 0·5 | 17·6 ± 0·5 | 2·3 ± 0·1 | 1·2 ± 0·1 | 42·3 ± 1·3 |
| HRL3 (2mg/kg) | 19·5 ± 0·6 | 17·2 ± 0·6 | 2·3 ± 0·1 | 1·2 ± 0·1 | 39·4 ± 1·8 |
| Normal control | 1·2 ± 0·1** | 0·7 ± 0·1** | 0·4 ± 0·1** | 0·2 ± 0·1** | 0** |
Data are mean ± SEM of 8 rats in each group. Numbers in parentheses represent the daily dose of the drug.
P < 0·01 versus saline group
P < 0·01 versus CA group
P < 0·05 versus SCA (5mg/kg) group
P < 0·01 versus SCA (5mg/kg) group
P < 0·01 versus mouse IgG group
P < 0·01 versus saline, CA,SCA (5mg/kg), SCA (10mg/kg), mouse IgG, ARP 2–4, HRL2, and HRL3 group.
CA, colominic acid; SCA, sulphated colominic acid; mouse IgG, used as a control for ARP 2–4; ARP 2–4, neutralizing anti-rat P-selectin mAb; HRL2, non-neutralizing anti-rat L-selectin mAb used as a control for HRL3; HRL3, neutralizing anti-rat L-selectin mAb.
Fig. 6.
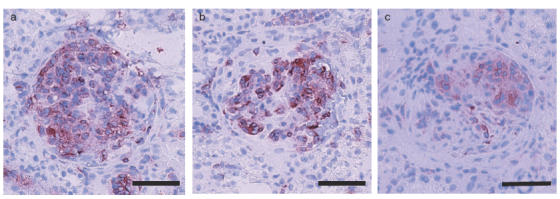
Identification and quantification of ED1-positive cells in representative glomeruli of WKY rats with crescentic glomerulonephritis on day 14. a–c: Immunoperoxidase-stained sections (a) CA group, (b) SCA (5mg/kg/day) group, (c) SCA (10mg/kg/day) group. Scale bar = 50 μm.
Crescent formation was less in ARP 2–4 group compared with mouse IgG group and saline group (ARP 2–4, 28·8 ± 1·4%; mouse IgG, 43·8 ± 1·2%; saline, 43·4 ± 1·8%), however, there were no difference in crescent formation between HRL3 group and HRL2 group (HRL3, 39·4 ± 1·8%; HRL2, 42·3 ± 1·3%) (Table 2). It was also less in SCA groups compared with CA group and saline group in a dose-dependent manner (SCA, 5mg/kg/day, 35·1 ± 0·7%; SCA, 10mg/kg/day, 26·5 ± 1·2%; CA, 42·5 ± 1·4%; saline, 43·4 ± 1·8%) (Table 2). Light microscopy showed no crescent formation in the glomeruli of normal control rats (Fig. 7a). In the saline (Fig. 7b) and CA (Fig. 7c) groups, severe mesangial hypercellularity, mesangial matrix expansion, necrotizing lesions and cellular crescent formation were observed in the kidneys harvested on day 14. On the other hand, histopathological changes in the kidneys were improved in SCA group (10mg/kg/day) (Fig. 7d).
Fig. 7.
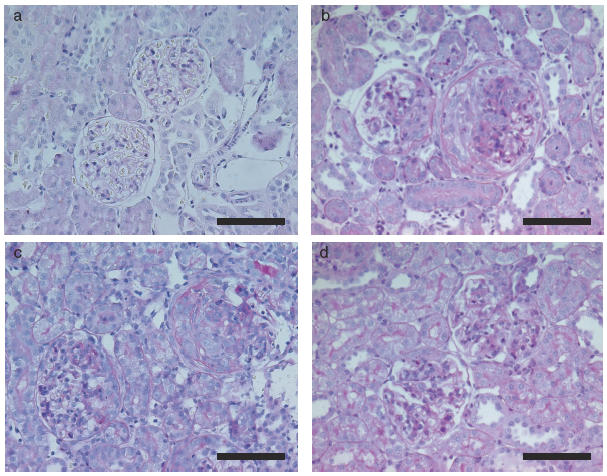
Histopathological findings of the kidney in crescentic glomerulonephritis in WKY rats on day 14. (a) Normal control group, (b) Saline group, (c) CA group, (d) SCA (10mg/kg/day) group (PAS staining, scale bar = 100μm). Note severe mesangial hypercellularity, mesangial matrix expansion, necrotizing lesions and cellular crescent formation are observed in the kidneys of saline and CA groups. In comparison, note the improvement in histopathological changes in the SCA group.
Expression of PDGF B chain mRNA in glomerulus
Real-time RT-PCR was used to quantify the relative abundance of PDGF B chain and β-actin mRNA in the glomeruli. PDGF B chain mRNA expression was significantly lower in SCA groups than in CA group, and the effect of SCA was dose-dependent (Fig. 8).
Fig. 8.
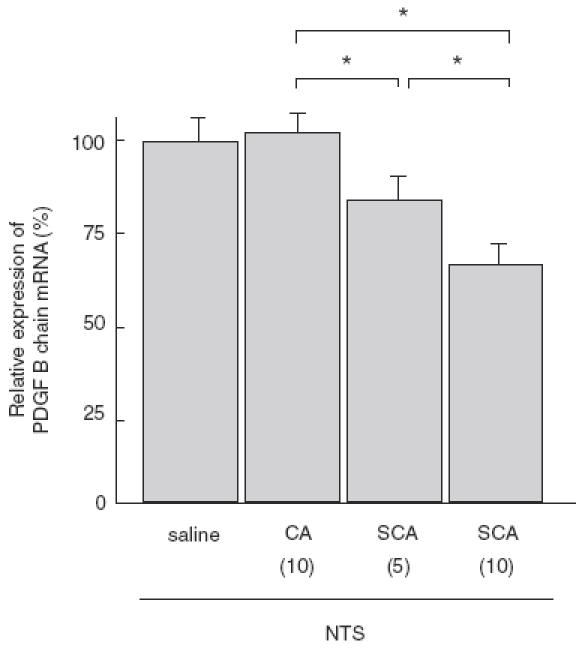
Quantitative real-time RT-PCR analysis of relative expression of PDGF B chain mRNA in glomeruli of WKY rats with crescentic glomerulonephritis on day 14. Numbers in parentheses represent the daily dose of each drug in mg/kg. Relative expression of PDGF B chain mRNA is significantly reduced in SCA groups, relative to CA group, in a dose-dependent manner. Data are mean ± SEM of 8 rats in each group. *P < 0·01.
DISCUSSION
The adhesive interaction between leucocytes and endothelium is critically involved in the progression of inflammation. While leucocytes are only minimally adherent to the endothelium under normal circumstances, stimulation with cytokines enhances their adhesiveness to endothelial cells. The enhanced adhesive interaction is dependent on cell adhesion molecules expressed on endothelial cells such as P-selectin. Accumulation of CD4 positive T lymphocytes, monocytes, and neutrophils is reduced significantly in the inflammatory response at sites of contact hypersensitivity in P-selectin-deficient mice [2]. Neutrophil accumulation into the inflammatory site in the early stage of the arthus reaction is inhibited by anti-P-selectin mAb [23]. These results suggest that P-selectin plays an important role in leucocyte recruitment into the inflammatory sites.
The role of P-selectin in the pathogenesis of glomerulonephritis has still been controversial [21,22]. We and other investigators showed increased expression of P-selectin in the glomeruli of patients with renal diseases including crescentic glomerulonephritis and diabetic nephropathy [31,32]. Huang et al.[33] demonstrated that the expression of both P-selectin and monocyte chemoattractant protein-1 was up-regulated in the glomeruli of rats with anti-GBM antibody-induced glomerulonephritis. Furthermore, treatment with an anti-P-selectin antibody prevented proteinuria and intraglomerular neutrophil accumulation in mouse anti-GBM glomerulonephritis [34] and in rat concanavalin A-induced immune complex glomerulonephritis [35]. On the other hand, Papayianni et al.[36] reported that glomerular leucocyte recruitment was not attenuated by anti-P-selectin antibody in rat model of immune complex glomerulonephritis.
L-selectin, which is expressed on leucocytes, binds to natural ligands on endothelial cells. We previously reported that soluble L-selectin-IgG fusion protein binds to the distal tubules but not to glomeruli in the rat kidney suggesting that the ligands for L-selectin exist in distal tubular epithelial cells but not in glomeruli under normal conditions [11,27]. We further demonstrated that anti-L-selectin antibody prevented infiltration of macrophages into the renal interstitium after unilateral ureteral obstruction indicating that leucocyte infiltration into interstitium is L-selectin-dependent in rats with unilateral ureteral obstruction [11].
WKY rats are known to be more susceptible to anti-GBM antibody than other strains, and injection of this antibody enhances macrophage infiltration into the glomeruli, progressive proteinuria and crescentic glomerulonephritis [17]. In this model, ICAM-1 mediates glomerular infiltration of leucocytes mainly composed of macrophages, which play a central role in progression of crescentic glomerulonephritis [17,37]. However, the involvement of selectins in glomerular leucocyte infiltration has been uncertain in this model. In the present study, we showed over expression of P-selectin on glomerular endothelial cells in this model, however, anti-E-selectin antibody and soluble L-selectin-IgG fusion protein did not react in the glomeruli suggesting that E-selectin and ligands for L-selectin are not expressed in the glomeruli in this model. Recently, Ito et al.[38] reported the same results of ours that P-selectin was highly expressed in glomeruli, but E-selectin and L-selectin ligands were not detected in model of acute thrombotic glomerulonephritis. On the other hand, anti-P-selectin mAb reduced leucocyte infiltration into the glomeruli, while anti-L-selectin mAb did not change glomerular leucocyte infiltration and crescent formation. These results suggest that P-selectin but not L-selectin mediates leucocyte infiltration into glomeruli in this model. In contrast to our results, Papayianni et al.[36] reported that glomerular leucocyte recruitment was not attenuated by anti-P-selectin antibody in rats with immune-complex glomerulonephritis. The different result between our study and the previous report [36] might be due to either differences in the blocking potential of anti-P-selectin antibodies or differences in the type of infiltrated leucocytes; neutrophil infiltration into glomeruli was predominant in their model, while intraglomerular inflammatory cells were mainly macrophages in our model.
SCA is known to interfere with the binding of P-selectin, but not E-selectin, to neutrophils in vitro[9] The present results showed that urinary protein excretion in CA group was >200mg/day, while administration of SCA at 5 or 10mg/kg/day decreased urinary protein excretion and macrophage infiltration in the glomeruli. However, the number of CD4 and CD8-positive cells did not change after treatment with SCA. Anti-P-selectin antibody and SCA did not alter the expression of ICAM-1 in the glomeruli. Furthermore, leukopenia was not seen in rats treated with SCA suggesting that SCA did not activate leucocytes. These results suggest that SCA prevents the progression of rat crescentic glomerulonephritis by inhibiting P-selectin-dependent macrophage infiltration into glomeruli. De Vriese et al.[39] recently reported that fucoidan F7, which interferes with L- and P-selectin-dependent adhesion in vitro, does not seem to prevent the development of anti-GBM glomerulonephritis in rats. However, they did not examine the effects of anti-selectin antibodies in their experiment. Although the exact cause of the discrepancy between their results and ours is not clear at present, it might be due to differences in experimental model.
As for the importance of lymphocytes in this model, Fujinaka et al. described that CD8-positive cells play a crucial role in glomerular accumulation of macrophages through stimulation of ICAM-1 and induction of inflammatory cytokines in WKY rats administered with anti-GBM antibody [37]. They showed that accumulation of CD8-positive cells increases from day 3 to day 6 after induction of nephritis. Although glomerular accumulation of CD8-positive cells and ICAM-1 expression were not changed on day 14 in our experiment, it might be possible that anti-P-selectin antibody and SCA altered the accumulation of CD8-positive cells resulting in decrease in glomerular macrophage accumulation.
PDGF is thought to contribute to the progression of renal injury in mesangial proliferative glomerulonephritis [14,40,41], crescentic glomerulonephritis [42,43] and diabetic nephropathy [44]. The glomerular expression of PDGF B chain mRNA decreased following administration of SCA. Macrophages are one of the major sources of cytokines including PDGF. Considered together, it seems that SCA can reduce glomerular expression of PDGF B chain by inhibiting macrophage infiltration, although the direct effect of SCA on PDGF production from platelets or mesangial cells remains to be determined.
In conclusion, we have demonstrated in the present study that SCA ameliorates rat crescentic glomerulonephritis. Our results suggest that P-selectin partially mediate glomerular infiltration of macrophages and SCA may inhibit P-selectin-dependent macrophage infiltration in the glomeruli in experimental rat crescentic glomerulonephritis.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (C) (11671036 to K Shikata) from the Ministry of Education, Science, Culture, Sports and Technology of Japan.
REFERENCES
- 1.Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91:379–87. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramaniam M, Saffaripour S, Watson SR, Mayadas TN, Hynes RO, Wagner DD. Reduced recruitment of inflammatory cells in a contact hypersensitivity response in P-selectin-deficient mice. J Exp Med. 1995;181:2277–82. doi: 10.1084/jem.181.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–46. [PubMed] [Google Scholar]
- 5.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 6.Imai Y, True DD, Singer MS, Rosen SD. Direct demonstration of the lectin activity of gp90MEL, a lymphocyte homing receptor. J Cell Biol. 1990;111:1225–32. doi: 10.1083/jcb.111.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y, Toda Y, Tamatani T, et al. Sulfated glycolipids are ligands for a lymphocyte homing receptor, L-selectin (LECAM-1), Binding epitope in sulfated sugar chain. Biochem Biophys Res Commun. 1993;190:426–34. doi: 10.1006/bbrc.1993.1065. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan MS, Miyasaka M, Suzuki Y, et al. Anti-inflammatory effects of sulfatides in selectin-dependent acute lung injury. Int Immunol. 1995;7:1107–13. doi: 10.1093/intimm/7.7.1107. [DOI] [PubMed] [Google Scholar]
- 9.Mulligan MS, Warner RL, Lowe JB, et al. In vitro and in vivo selectin-blocking activities of sulfated lipids and sulfated sialyl compounds. Int Immunol. 1998;10:569–75. doi: 10.1093/intimm/10.5.569. [DOI] [PubMed] [Google Scholar]
- 10.Todderud G, Alford J, Millsap KA, Aruffo A, Tramposch KM. PMN binding to P-selectin is inhibited by sulfatide. J Leukoc Biol. 1992;52:85–8. doi: 10.1002/jlb.52.1.85. [DOI] [PubMed] [Google Scholar]
- 11.Shikata K, Suzuki Y, Wada J, et al. L-selectin and its ligands mediate infiltration of mononuclear cells into kidney interstitium after ureteric obstruction. J Pathol. 1999;188:93–9. doi: 10.1002/(SICI)1096-9896(199905)188:1<93::AID-PATH305>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Yang DW, Ohta Y, Yamaguchi S, Tsukada Y, Haraguchi Y, Hoshino H, Amagai H, Kobayashi I. Sulfated colominic acid: an antiviral agent that inhibits the human immunodeficiency virus type 1 in vitro. Antiviral Res. 1996;31:95–104. doi: 10.1016/0166-3542(96)00957-6. [DOI] [PubMed] [Google Scholar]
- 13.Ushijima H, Perovic S, Leuck J, Rytik PG, Muller WE, Schroder HC. Suppression of PrP(Sc)- and HIV-1 gp120 induced neuronal cell death by sulfated colominic acid. J Neurovirol. 1999;5:289–99. doi: 10.3109/13550289909015815. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RJ, Floege J, Couser WG, Alpers CE. Role of platelet-derived growth factor in glomerular disease. J Am Soc Nephrol. 1993;4:119–28. doi: 10.1681/ASN.V42119. [DOI] [PubMed] [Google Scholar]
- 15.Cattell V, Cook T, Moncada S. Glomeruli synthesize nitrite in experimental nephrotoxic nephritis. Kidney Int. 1990;38:1056–60. doi: 10.1038/ki.1990.312. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Gejyo F, Kuroda T, Kazama JJ, Imai N, Kimura H, Arakawa M. Effects of a novel elastase inhibitor, ONO-5046, on nephrotoxic serum nephritis in rats. Kidney Int. 1998;53:1201–8. doi: 10.1046/j.1523-1755.1998.00872.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki K, Yaoita E, Yamamoto T, Tamatani T, Miyasaka M, Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993;150:1074–83. [PubMed] [Google Scholar]
- 18.Wada J, Shikata K, Makino H, et al. The critical role of intercellular adhesion molecule-1 in Masugi nephritis in rats. Nephron. 1996;73:264–72. doi: 10.1159/000189050. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan MS, Johnson KJ, Todd RF, et al. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J Clin Invest. 1993;91:577–87. doi: 10.1172/JCI116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikawa K, Guo YJ, Miyasaka M, Tamatani T, Collins AB, Sy MS, McCluskey RT, Andres G. Antibodies to intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 prevent crescent formation in rat autoimmune glomerulonephritis. J Exp Med. 1993;177:667–77. doi: 10.1084/jem.177.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefkowith JB. Leukocyte migration in immune complex glomerulonephritis. role of adhesion receptors. Kidney Int. 1997;51:1469–75. doi: 10.1038/ki.1997.201. [DOI] [PubMed] [Google Scholar]
- 22.Mayadas TN, Mendrick DL, Brady HR, et al. Acute passive anti-glomerular basement membrane nephritis in P-selectin-deficient mice. Kidney Int. 1996;49:1342–9. doi: 10.1038/ki.1996.190. [DOI] [PubMed] [Google Scholar]
- 23.Ohnishi M, Koike H, Kawamura N, Tojo SJ, Hayashi M, Morooka S. Role of P-selectin in the early stage of the Arthus reaction. Immunopharmacology. 1996;34:161–70. doi: 10.1016/0162-3109(96)00127-0. [DOI] [PubMed] [Google Scholar]
- 24.Tojo SJ, Yokota S, Koike H, et al. Reduction of rat myocardial ischemia and reperfusion injury by sialyl Lewis x oligosaccharide and anti-rat P-selectin antibodies. Glycobiology. 1996;6:463–9. doi: 10.1093/glycob/6.4.463. [DOI] [PubMed] [Google Scholar]
- 25.Tamatani T, Kitamura F, Kuida K, et al. Characterization of rat LECAM-1 (L-selectin) by the use of monoclonal antibodies and evidence for the presence of soluble LECAM-1 in rat sera. Eur J Immunol. 1993;23:2181–8. doi: 10.1002/eji.1830230920. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, Shikata K, Makino H, Sugimoto H, Ota Z. Glomerular expression of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in patients with various forms of glomerulonephritis. Laboratory Invest. 1996;75:403–12. [PubMed] [Google Scholar]
- 27.Tamatani T, Kuida K, Watanabe T, Koike S, Miyasaka M. Molecular mechanisms underlying lymphocyte recirculation. III. Characterization of the LECAM-1 (L-selectin)-dependent adhesion pathway in rats. J Immunol. 1993;150:1735–45. [PubMed] [Google Scholar]
- 28.Makino H, Gibbons JT, Reddy MK, Kanwar YS. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane – I. J Clin Invest. 1986;77:142–56. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamatani T, Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2:165–71. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto H, Shikata K, Hirata K, et al. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes. 1997;46:2075–81. doi: 10.2337/diab.46.12.2075. [DOI] [PubMed] [Google Scholar]
- 31.Segawa C, Wada T, Takaeda M, et al. In situ expression and soluble form of P-selectin in human glomerulonephritis. Kidney Int. 1997;52:1054–63. doi: 10.1038/ki.1997.428. [DOI] [PubMed] [Google Scholar]
- 32.Hirata K, Shikata K, Matsuda M, Akiyama K, Sugimoto H, Kushiro M, Makino H. Increased expression of selectins in kidneys of patients with diabetic nephropathy. Diabetologia. 1998;41:185–92. doi: 10.1007/s001250050888. [DOI] [PubMed] [Google Scholar]
- 33.Huang XR, Kitching AR, Tipping PG, Holdsworth SR. Interleukin-10 inhibits macrophage-induced glomerular injury. J Am Soc Nephrol. 2000;11:262–9. doi: 10.1681/ASN.V112262. [DOI] [PubMed] [Google Scholar]
- 34.Tipping PG, Huang XR, Berndt MC, Holdsworth SR. A role for P selectin in complement-independent neutrophil-mediated glomerular injury. Kidney Int. 1994;46:79–88. doi: 10.1038/ki.1994.246. [DOI] [PubMed] [Google Scholar]
- 35.Zachem CR, Alpers CE, Way W, Shankland SJ, Couser WG, Johnson RJ. A role for P-selectin in neutrophil and platelet infiltration in immune complex glomerulonephritis. J Am Soc Nephrol. 1997;8:1838–44. doi: 10.1681/ASN.V8121838. [DOI] [PubMed] [Google Scholar]
- 36.Papayianni A, Serhan CN, Phillips ML, Rennke HG, Brady HR. Transcellular biosynthesis of lipoxin A4 during adhesion of platelets and neutrophils in experimental immune complex glomerulonephritis. Kidney Int. 1995;47:1295–302. doi: 10.1038/ki.1995.184. [DOI] [PubMed] [Google Scholar]
- 37.Fujinaka H, Yamamoto T, Feng L, et al. Crucial role of CD8-positive lymphocytes in glomerular expression of ICAM-1 and cytokines in crescentic glomerulonephritis of WKY rats. J Immunol. 1997;158:4978–83. [PubMed] [Google Scholar]
- 38.Ito I, Yuzawa Y, Mizuno M, Nishikawa K, Tashita A, Jomori T, Hotta N, Matsuo S. Effects of a new synthetic selectin blocker in an acute rat thrombotic glomerulonephritis. Am J Kidney Dis. 2001;38:265–73. doi: 10.1053/ajkd.2001.26085. [DOI] [PubMed] [Google Scholar]
- 39.De Vriese AS, Endlich K, Elger M, et al. The role of selectins in glomerular leukocyte recruitment in rat anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol. 1999;10:2510–7. doi: 10.1681/ASN.V10122510. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura A, Gordon K, Alpers CE, et al. Demonstration of PDGF B-chain mRNA in glomeruli in mesangial proliferative nephritis by in situ hybridization. Kidney Int. 1991;40:470–6. doi: 10.1038/ki.1991.234. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda M, Shikata K, Makino H, Sugimoto H, Ota K, Akiyama K, Hirata K, Ota Z. Gene expression of PDGF and PDGF receptor in various forms of glomerulonephritis. Am J Nephrol. 1997;17:25–31. doi: 10.1159/000169067. [DOI] [PubMed] [Google Scholar]
- 42.Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int. 1998;54:416–25. doi: 10.1046/j.1523-1755.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- 43.Tang WW, Feng L, Loskutoff DJ, Wilson CB. Glomerular extracellular matrix accumulation in experimental anti-GBM Ab glomerulonephritis. Nephron. 2000;84:40–8. doi: 10.1159/000045537. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa H, Sasahara M, Haneda M, Koya D, Hazama F, Kikkawa R. Immunohistochemical characterization of glomerular PDGF B-chain and PDGF beta-receptor expression in diabetic rats. Diabetes Res Clin Pract. 2000;48:87–98. doi: 10.1016/s0168-8227(99)00144-8. [DOI] [PubMed] [Google Scholar]


