Abstract
Co-stimulatory blockade may be a promising strategy for tolerance induction in transplantation. In allogeneic bone marrow transplantation (BMT) for leukaemia treatment, however, preservation of the graft-versus-leukaemia (GVL) effect is another critical requirement for clinical application. In this study, we have compared the effect on GVL of using CD28 and CD40 co-stimulatory blockades as graft-versus-host disease (GVHD) prophylaxis in a murine allogeneic BMT model with simultaneous transfer of BCL1 leukaemia. Despite the relative improvement of GVHD as assessed by survival and body weight in both treatment regimes, treatment with anti-CD154 moAb clearly diminished the GVL effect, whereas treatment with anti-CD80 and CD86 MoAbs maintained this effect. Although T cell-mediated effector function at 14 days post-BMT assessed by IFNγ expression and cytotoxicity against host alloantigen was comparable between both co-stimulatory blockades, IL-12 mRNA expression was preferentially reduced by CD40 blockade. Our results suggest the differential involvement of the CD28 and CD40 co-stimulatory pathways in the development of GVHD and GVL effects. CD28 blockade may be a favourable strategy for tolerance induction in leukaemia patients undergoing BMT.
Keywords: co-stimulation, GVL, GVHD, IL-12, tolerance
INTRODUCTION
An optimal T cell activation requires antigen stimulation and co-stimulation. CD28 and CD40 co-stimulation may play crucial roles in the interaction between T cells and antigen-presenting cells (APC). Blockade of either CD28 or CD40 pathway with MoAbs or recombinant fusion proteins (e.g. CTLA-4Ig) inhibits lethal acute graft-versus-host disease (GVHD) efficiently [1–5]. The simultaneous blockade of the CD28 and CD40 pathways successfully induces long-term acceptance of allografts [6–8], suggesting that a combined co-stimulatory signal blockade has a great promise for clinical application. However, in allogeneic bone marrow transplantation (BMT) for leukaemia treatment, the therapeutic requirement is not a complete immunosuppression but rather a moderate suppression with a preserved graft-versus-leukaemia (GVL) effect.
On the one hand, CD28 and CD40 are involved in antitumour immune responses. Transduction of CD154, CD80 or CD86 into leukaemia cells induces host antileukaemia responses mediated by T and NK cells [9–13]. Therefore, blockade of these molecules may reduce antileukaemia immune responses after BMT. On the other hand, Blazar et al.[14] demonstrated that the GVL effect against leukaemia cells in delayed lymphocyte infusion (DLI) post-BMT was eliminated by treatment with anti-CD80 MoAbs, suggesting involvement of the CD28-CD80 pathway in the GVL effect. The depletion of T cells from the bone marrow (BM) graft prevents GVHD, but increases the risk of leukaemia relapse, suggesting the involvement of donor T cells in both the GVH reaction and the GVL effect. Several recent reports demonstrated the differential effects between GVHD prophylaxis and the GVL effect by IL-2 [15], a metalloproteinase inhibitor [16], and IL-11 [17]. These observations suggested that the molecules and effector cells involved in the GVH reaction and the GVL effect are not completely identical.
In this study, we have compared the effect on GVL of GVHD prophylaxis using either treatment with anti-CD154 MoAb or a combination of anti-CD80 and CD86 MoAbs in a murine model of fully major histocompatibility complex (MHC)-mismatched allogeneic BMT with a simultaneous injection of leukaemia cells. We further compared the effector function of T cells, NK cells and APC in the recipient mice after performing these two types of co-stimulatory blockades.
MATERIALS AND METHODS
Mice
Female C57BL/6 (B6, H-2b), BALB/c (H-2d), and BALB/c nu/nu mice were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). Donors for BM were 4–5-week-old and those for splenocytes were 12 weeks old and recipients were 8–10-weeks old at the time of BMT. All mice procedures were reviewed and approved by the Animal Care and Use Committee in the National Children's Medical Research Center.
Monoclonal antibodies
Hybridomas producing anti-mouse CD80 (RM80, rat IgG2a) and CD86 (PO3, rat IgG2b) MoAbs were generated as described previously [18]. A hybridoma producing anti-mouse CD154 MoAb (MR1, hamster IgG) was obtained from the American Type Culture Collection (Rockville, MD, USA). RM80 and PO3 were purified from ascites as described [19] and MR1 was purified from hybridoma supernatant using protein A MAPS II Kit (Japan Bio-Rad, Tokyo, Japan). The pyrogen level was <0·01 ng/μg protein as determined by a Limulus amebocyte lysate assay. MoAbs against the following antigens were used for immunofluorescence analysis: CD3 (145–2C11, hamster IgG), CD4 (RM4-5, rat IgG2a), CD8 (53–6·7, rat IgG2a), CD45R/B220 (RA3–6B2, rat IgG2a), NK1·1 (PK136, mouse IgG2a) and H-2Kd (SF1-1·1, mouse IgG2a). All FITC-, phycoerythrin (PE)-, or peridinin chlorophyll protein (PerCP)-conjugated MoAbs were obtained from BD-PharMingen (San Diego, CA, USA).
BMT
BM cells and T cell-depleted (TCD)-BM cells treated with anti-Thy1·2 (30-H-12, BD-PharMingen) MoAb and rabbit complement (Cedarlane, Ontario, Canada) were obtained as described previously [4]. Sublethally irradiated (6·5 Gy) BALB/c recipient mice received TCD-BM (2·5 × 107) and either whole or asialo GM1+ cell-depleted splenocytes (2·0 × 106) from wild-type B6 mice. For depletion of asialo GM1+ cells, splenocytes were treated with antiasialo GM1 antibody (Rabbit IgG, Yamasa, Choshi, Japan) and guinea pig complement (Cedarlane) and then used as asialo GM1− splenocytes. A control group of mice received TCD-BM cells alone. The day of BMT was designated as day 0. Recipients receiving TCD-BM and splenocytes from wild-type B6 mice were divided randomly into three groups and treated with either control reagent, a combination of anti-CD80 and CD86 (CD80/86) MoAbs or anti-CD154 MoAb. One hundred micrograms of each MoAb was injected i.p. every other day until day 21 post-BMT.
Engraftment of leukaemic cells
BCL1 was originally derived as a spontaneous murine B-cell leukaemia from BALB/c mouse [20] and is maintained in vivo by serial passages in a syngeneic BALB/c strain. Leukaemia was introduced by injection of 1 × 105 BCL1 tumour cells at the time of BMT. Recipient mice were killed and spleen weight was measured on day 28. A20 is a B cell lymphoma of BALB/c origin and cultured in RPMI-1640 medium supplemented with l-glutamine, gentamicin and 10% fetal bovine serum (FBS). BCL1 cells express high levels of MHC class I, class II, CD40, CD80 and CD86 on their surface, but A20 cells express reduced levels of MHC class I and CD80, and do not express CD86 (not shown).
Multicolor staining for intracellular IFNγ and cell-surface antigens
Multicolour staining for intracellular IFNγ and T cell surface antigens were performed as described previously [21]. Briefly, single cell suspensions of splenocytes were stimulated with PMA and ionomycin in the presence of brefeldin A for 4 h. After washing, cells were stained with MoAb against cell surface antigens, and then intracellular staining was performed after fixation and permeabilization. Samples were analysed by flow cytometry. Flow cytometry and data analysis were performed using a FACSort and CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
Measurement of cytotoxicity against host allo-antigens
Single cell suspensions of whole splenocytes from recipients at 14 days post-BMT were used as effector cells. Cytotoxicity against BCL1, A20 and BALB/c PHA blasts was measured by a 6-h 51Cr-release assay. BALB/c PHA blasts were prepared by the culture of BALB/c splenocytes with 5 μg/ml of PHA (Dako-Japan, Tokyo, Japan) for 4 days.
RT-PCR for IL-12
For determination of mRNA for IL-12, B6 mice received irradiated (20 Gy) 2 × 107 cells of BALB/c splenocytes. Recipient mice were treated with anti-CD80/86 MoAbs, anti-CD154 MoAb or control reagent. Two hundred micrograms per mouse of each MoAb was administrated i.p. at the time of splenocyte transfer. After the indicated periods, total RNA was isolated using ISOGEN (Nippon Gene, Toyama, Japan). First-strand cDNA was synthesized using 0·5 μg oligo(dT) primer (Pharmacia Biotech, Uppsala, Sweden) and SuperScript II-reverse transcriptase (Gibco-BRL, Gaithersburg, MD, USA) from 5 μg of each RNA sample. For quantification of PCR products, the amounts of cDNA were preliminarily normalized to produce the same amount of PCR products for β2-microgloblin based on the intensity of the ethidium-bromide fluorescence of each band measured by the charge-coupled device (CCD) imaging system (Densitograph AE-6920 m, Atto, Tokyo, Japan). The cDNA samples were amplified in a reaction mixture containing 2·5 U of Taq DNA polymerase (Takara, Shiga, Japan), 200 μm each of dNTP, 0·5 μm each of 5′ and 3′ primers and 1 × PCR buffer with 15 mm MgCl2 (Takara). PCR was performed on a DNA thermal cycler (Perkin Elmer) for 22 and 32 cycles (94°C for 1 min, 58°C for 1 min, 72°C for 1 min) for β2-microgloblin and IL-12 p40, respectively, followed by a 5-min extension at 72°C. Oligonucleotide primers used for mouse IL-12 p40 and β2-microgloblin were follows: IL-12 p40, forward, CTGGCCAGTACACCTGCCAC; reverse, CTGGCCAGTACACCTGCCAC and β2- microgloblin, forward, TGACCGGCTTGTATGCTATC; reverse, CAGTGTGAGCCA GGATATAG. The PCR products were electrophoresed on 2·5% agarose gel with ethidium bromide and the image was acquired using a densitograph.
Statistical analyses
Significant differences between experimental groups were analysed by the Mann–Whitney U-test, the Mantel–Pete–Cox χ2, and two-way analysis of variance (anova).
RESULTS
Treatment with anti-CD154 and anti-CD80/86 MoAbs comparably reduced the clinical manifestation of acute GVHD
Previously we and others have shown that the in vivo administration of anti-CD154 [3,5] or anti-CD80/86 MoAbs [4] ameliorated the lethality of murine acute GVHD. In addition, donor T cells lacking either CD154 [22] or CD28 [23] reduced the GVHD-associated mortality. Thus, the blockade of CD40 or CD28 or the lack of CD154 or CD28 on donor T cells comparably inhibited the lethality of GVHD. Here we have established a B6 into BALB/c fully MHC-mismatched allogeneic BMT model to investigate simultaneously both GVHD prophylaxis and the GVL effect. We transferred 2 × 106 splenocytes and 2·5 × 107 TCD-BM from the wild-type B6 donor mice into sublethally (6·5 Gy) irradiated BALB/c mice and monitored the survival and body weight. The mice that received TCD-BM alone (referred to as BM mice) showed no evidence of clinical GVHD and graft rejection, and over 85% of splenocytes were of donor origin at 28 days post-transfer (data not shown). In this BMT model, more than 70% of the mice that received TCD-BM and splenocytes (referred to as BMS mice) could survive without treatment and over 90% of mice treated with either anti-CD154 or anti-CD80/86 MoAbs survived at 28 days post-BMT (Fig. 1a). The mean body weight was comparable at 28 days post-BMT (Fig. 1b). All groups of BMS mice showed a severe lymphopenia in the peripheral blood and a clear atrophy of the spleen demonstrated by a reduction in the total cell number of splenocytes (Fig. 1c). Although the reduction in lymphocytes was milder in the anti-CD154 MoAb-treated mice, a significant difference was not observed.
Fig. 1.
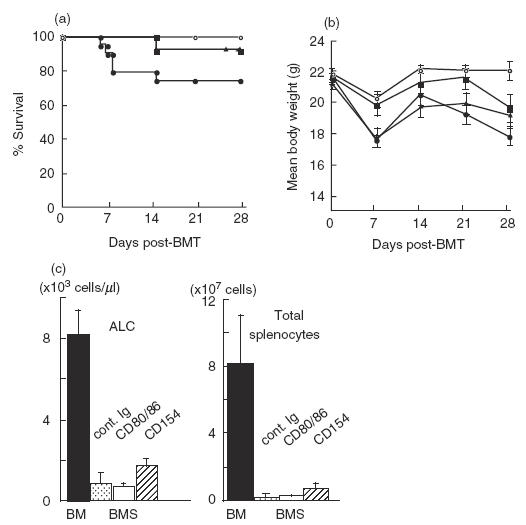
Effects of treatment with either anti-CD154 or anti-CD80/86 MoAbs in a complete allogeneic GVHD model. Each group of sublethally (6·5Gy) irradiated BALB/c mice received 2·0 × 106 splenocytes and 2·5 × 107 TCD-BM cells from wild-type B6 mice, and treated with either control reagents (BMS, •) anti-CD80/86 (RM80/PO3) MoAbs (BMS CD80/86, ▪), or anti-CD154 (MR1) MoAb (BMS CD154, ▴). One group of mice received TCD-BM cells alone (BM, ○). Each group consists of 8–19 mice. Survival rates (a) and mean body weight (b) are plotted. Data shown are representative of three experiments. BM versus BMS, P = 0·013, BMS CD80/86 versus BMS, P = 0·043, BMS CD154 versus BMS, P = 0·043. (c) Peripheral blood cells and splenocytes were analysed at day 28 post-BMT. ALC; absolute lymphocyte count. Splenocytes were stained with FITC-anti-CD4, PE-anti-CD8 and PerCP-anti-CD3 MoAbs. CD3+, CD3+4+ and CD3+8+ cells were counted as donor T, CD4+ T, CD8+ T cells, respectively. More than 99% of splenocytes were H-2d− donor-derived cells in all groups. Data represent the mean ± s.d. from five mice in each group. *Statistically different (P < 0·05).
GVL effect was significantly reduced by the anti-CD154 MoAb treatment, but not by the anti-CD80/86 MoAb treatment
BCL1 is a spontaneous murine B-cell leukaemia derived from a BALB/c mouse [20]. Inoculation of 105 BCL1 cells into BALB/c mice results in a typical B cell leukaemia characterized by extreme splenomegaly with subsequent peripheral blood lymphocytosis exceeding 20000/mm3 and 100% mortality occurring within 4–5 weeks. In this model, we transferred 1 × 105 of BCL1 tumour cells at the time of BMT. The individual spleen weight at day 28 is shown in Fig. 2a. All BM mice inoculated with BCL1 showed an extreme splenomegaly by growth of the leukaemia. In contrast, both BCL1 inoculated and non-inoculated BMS mice showed a slight splenic atrophy associated with GVHD. Splenomegaly in half of the anti-CD154 MoAb-treated BMS mice was suggestive of BCL1 cell growth whereas, except for one mouse, no indications of splenomegaly were observed in the anti-CD80/86-treated BMS mice. To evaluate a minor leukaemia infiltration which is visually undetectable, 1 × 106 cells of individual recipient splenocytes were transferred into immunocompetent BALB/c secondary host mice and the tumour growth was assessed by the survival of the secondary host. All secondary host mice receiving splenocytes from the BM and anti-CD154-treated BMS mice died by day 120, while 20% of secondary host mice that received splenocytes from the control BMS and 45% of those that received cells from anti-CD80/86-treated BMS mice were still alive. These results indicate that the treatment with anti-CD154 MoAb significantly reduced antileukaemia immune responses, whereas the treatment with anti-CD80/86 MoAbs did not clearly affect those responses.
Fig. 2.
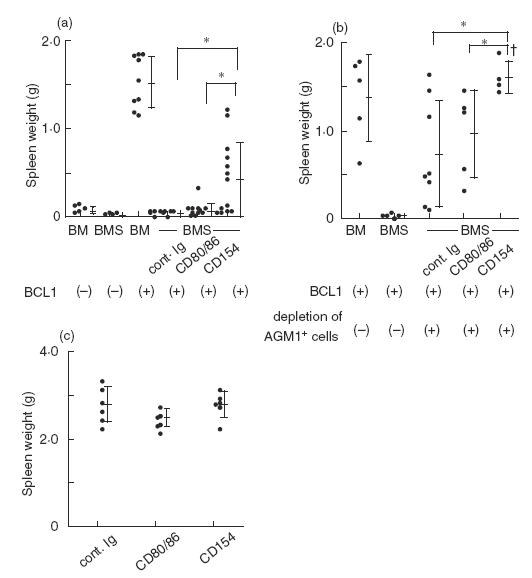
Treatment with anti-CD154 MoAb permits growth of BCL1. Sublethally irradiated BALB/c recipient mice received TCD-BM (2·5 × 107) and either whole (a) or asialo GM1+ cell-depleted (b) splenocytes (2·0 × 106) from B6 mice with 1 × 105BCL1 cells. In (c), BALB/c nude mice were inoculated with 1 × 105 BCL1 cells. In all experiments, MoAb treatment was performed as described in Fig. 1. Recipient mice were killed on day 28 and each spleen weight was measured. †One mouse treated with anti-CD154 MoAb died by leukaemia before day 28 in (b). The bars represent the mean ± s.d. *Statistically different (P < 0·05).
Depletion of donor asialo GM1+ cells reduces the GVL effect
It has been reported that asialo GM1+ cells are involved in the GVL response against BCL1 [24]. To see the involvement of asialo GM1+ cells in the differential effect of both treatments, we depleted asialo GM1+ cells from donor splenocytes. This treatment eliminated most conventional NK (CD3−NK1·1+), NKT (CD3+NK1·1+), and a part of CD8+ T cells from 4·4 to 0·9%, 2·2 to 0·4% and 12·5 to 8·0%, respectively. Asialo GM1− splenocytes, TCD-BM cells and BCL1 tumour cells were co-transferred, and then treated with MoAbs. The individual spleen weight was evaluated at 28 days post-BMT. Depletion of asialo-GM1+ cells significantly enhanced the enlargement of spleens in all groups of mice (Fig. 2b) compared with the corresponding experimental mice retaining asialo-GM1+ cells (Fig. 2a). Treatment with anti-CD154 MoAb enhanced the growth of BCL1 as assessed by the spleen weight. Two-way analysis of variance between populations with and without depletion of donor asialo GM1+ cells (a and b) indicates that the depletion of donor asialo GM1+ cells did not significantly interact with the differential effects of BCL1 tumour growth observed between the anti-CD80/86 and anti-CD154 MoAb treatments. To account for the possibility that anti-CD80/86 or anti-CD154 MoAb treatment might allow for host NK or antibody dependent cell-mediated cytotoxicity (ADCC) against BCL1, BALB/c nude mice were infused with the same number of BCL1 cells and underwent MoAb treatments as described previously. At 28 days after BCL1 transfer, a clear difference was not observed between the mean spleen weights of control, anti-CD80/CD86 and anti-CD154 treated groups (Fig. 2c), suggesting that host ADCC and NK cells may not be involved in the differential effects observed between anti-CD80/86 and anti-CD154 MoAb treatments. These results suggest that CD40 blockade may inhibit the GVHD-related immune responses mediated by asialo GM1− cells and the additional responses which are independent of GVHD. Our results demonstrated that donor asialo GM1+ cells were critical effectors for immune responses against BCL1, but CD40 blockade may preferentially inhibit the GVL responses that are independent of asialo GM1+ cells.
Effector function by T cells is comparably inhibited by both co-stimulatory blockades
To investigate the reason why the anti-CD154 MoAb treatment dramatically reduced the GVL effect, we examined T cell effector function. Because our preliminary kinetic analysis showed that donor T cell expansion and IFNγ expression in the BMS mice appears to be maximum during 12–14 days post-BMT, we compared IFNγ expression in splenocytes at 14 days post-BMT. IFNγ expression by CD4+ T cells was significantly inhibited by either treatment with anti-CD80/86 or anti-CD154 MoAb (Fig. 3a). No statistically significant difference was observed between both treatments. IFNγ expression by CD8+ T cells was suppressed to similar levels by both treatments. Cytotoxicity of recipient splenocytes against BCL1, A20 and BALB/c PHA blasts was observed in an E/T ratio dependent manner (Fig. 3b). However, no clear differences were observed between the three groups. In the recipient splenocytes at 28 days post-BMT, the percentages of T cells, CD4+ T cells and CD8+ T cells were comparably inhibited by both treatments (Fig. 3c). These results indicate that T cell effector function at 28 days post-BMT was similar between both co-stimulatory blockades.
Fig. 3.
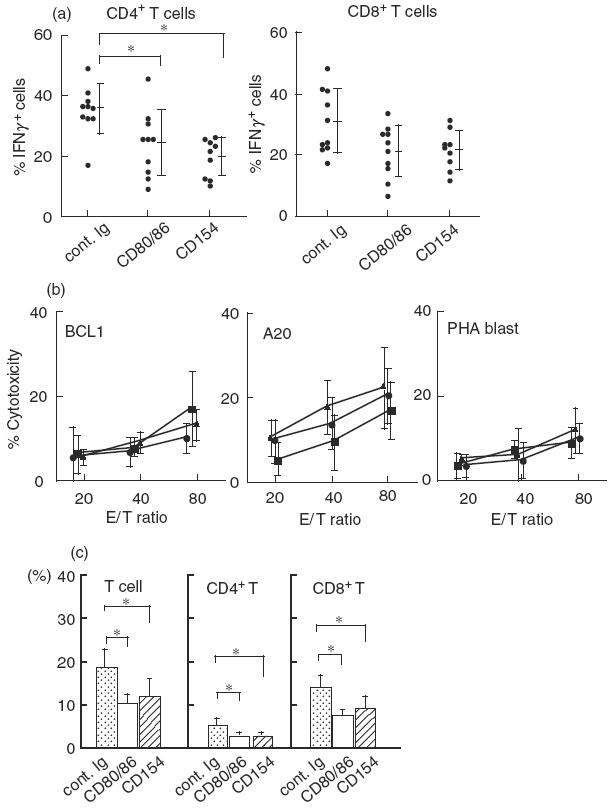
IFNγ expression by CD4+T cells and T cell expansion are inhibited comparably by both treatments. (a) Expression of IFNγ in individual splenocytes from each group of mice at 14 days post-BMT was assessed by immunofluorescent staining of intracellular cytokines by flow cytometry. Cells were stained with either FITC-anti-CD4 or anti-CD8, PerCP-CD3 and PE-anti-INFγ or with appropriated fluorochrome-conjugated control Ig. Samples were analysed by flow cytometry. An electronic gate was set on either CD3+4+ or CD3+8+ lymphocytes, and the expression of IFNγ was analysed. (b) Splenocytes from each recipient at 14 days were used as effector cells. Cytotoxicity against BCL1, A20 and BALB/c PHA blasts was measured. Each bar represents the mean ± s.d. from five mice; •, cont. Ig; ▪, CD80/86; ▴, CD154. (c) Cells were stained with FITC-anti-CD4, PE-anti-CD8 and PerCP-anti-CD3 MoAb or with appropriated fluorochrome-conjugated control Ig. Samples were analysed by flow cytometry. CD3+, CD3+4+ or CD3+8+ lymphocytes were counted as T, CD4+ T and CD8+ T cells, respectively. Over 98% of splenocytes were H-2d− donor origin in all groups (not shown). *Statistically different (P < 0·05). Data are representative of two experiments.
Treatment with anti-CD154 MoAb preferentially reduces IL-12 mRNA expression
We next focused on APC function. IL-12 produced from APC just after antigen stimulation may be critical for Th1, CTL and NK cell differentiation and activation. We examined IL-12 p40 mRNA expression in splenocytes after allo-antigen stimulation by RT-PCR. Expression of IL-12 mRNA was induced rapidly at 3 h after transfer of allogeneic splenocytes and reached maximum at 6–12h (Fig. 4a). We therefore compared the expression of IL-12 mRNA at 6h in each group. The treatment with anti-CD154 MoAb significantly reduced IL-12 mRNA expression compared with the anti-CD80/86 MoAb treatment (Fig. 4b). These results suggest the preferential suppression of IL-12 production from APC by CD40 blockade.
Fig. 4.
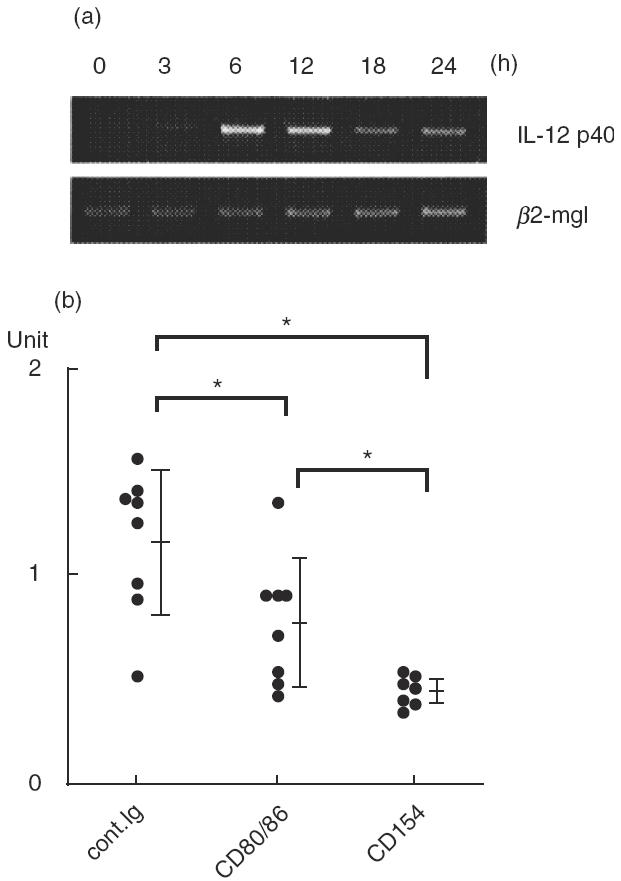
Treatment with anti-CD154 MoAb significantly inhibits IL-12 mRNA. B6 mice received 2 × 107 irradiated BALB/c splenocytes and MoAb treatment as described in the Methods. Total RNA from splenocytes at the indicated time after transfer was isolated and expression of IL-12 p40 mRNA was assessed by RT-PCR as described in the Methods. (a) Kinetic change in IL-12 p40 mRNA expression after transfer. The predicted product sizes for IL-12 p40 and β2-microglublin were 384 and 222 bp, respectively. (b) Comparison of IL-12 p40 mRNA at 6 h after transfer. The amount of mRNA for IL-12 p40 and β2-microglobulin in individual samples at 6 h after transfer was measured by densitograph and the unit values were normalized to the amount of mRNA for β2-microglobulin. The bars represent the mean ± s.d. from each group of seven to eight mice. *Statistically different (P < 0·05).
DISCUSSION
In this study we have demonstrated that, despite the comparable inhibition in clinical manifestation of acute GVHD assessed by survival, body weight and lymphocyte reconstitution, CD40 blockade clearly reduced the GVL effect, while CD28 blockade preserved the GVL effect in an allogeneic BMT model using BCL1 leukaemia.
BCL1 has been commonly used for assessment of GVL effect in a murine model of BMT. In earlier studies, the dominant involvement of CD8+ T cells rather than NK cells for GVL activity has been reported in F1 hosts after allogeneic BMT [25,26]. On the one hand, the elimination of asialo GM1+ cells [24] from the graft reduced antileukaemic activity, while the GVL effect can be developed even after T cell depletion in GVHD-free recipients [27]. These observations suggest that asialo GM1+ NK and T cells may be major effectors for eliciting GVL effect in a BCL1 model. Consistent with these observations, our results also revealed that asialo GM1+ cells are potent mediators of the GVL effect.
It should be noted that even after the depletion of asialo GM1+ cells, a preferential abrogation of antileukaemic responses was seen consistently by CD40 blockade. This suggests that CD40 blockade preferentially affected a reduction of the GVL effect in an asialo GM1+ cell-independent mechanism. However, in our analysis using splenocytes at 14 days post-BMT, we failed to observe obvious differences in IFNγ expression by both CD4+ and CD8+ T cells and cytotoxicity against host alloantigen, suggesting that T cell function at the effector phase was not preferentially reduced. The only single difference that we could observe in this study was IL-12 mRNA expression just after transfer. Ligation of CD40 is crucial in conditioning immunogenic versus tolerogenic presentation of antigen to T cells by DC [28–31]. CD40 ligation via CD154 activates APC leading to up-regulation of a number of co-stimulatory molecules and cytokines. IL-12 produced by CD40 ligation on APC is a crucial cytokine for CTL priming and CD4+ T cell help for generation of CTL [28,32,33]. At present, we cannot clarify the discrepancy of our results between the in vivo GVL effect and the in vitro analysis. A mechanism of differential tolerance induction between the CD28 and CD40 blockades has been suggested [31]. Recent accumulating reports suggested a regulatory function induced by CD40 blockade. The regulatory property of linked suppression [34] and the involvement of CD4+CD25+ regulatory cells [35,36] in CD40 blockade were demonstrated. CD40 blockade caused a shift from Th1 to Th2 in murine allergic contact dermatitis [37], and high IL-10 production in allogeneic responses in vitro[38]. Thus, it is likely that regulatory cytokines or regulatory cells may be involved in the in vivo suppression of GVL by CD40 blockade, although we failed to detect such suppression in the analysis in vitro. Further studies will be required to clarify this issue.
In contrast to a broad range of immune suppressive effects elicited by CD40 blockade, CD28 blockade does not directly inactivate APC. Therefore, the conditioned APC activated by CD40 signalling could present antigen and prime CTL in a B7-independent manner. A considerable number of CD28−8+ T cells exist, and they possess less proliferative ability but a high level of cytolytic activity [39]. Furthermore, less dependence on CD28 co-stimulation in naive CD8 T cells has been suggested in antitumour [40,41] and antiviral responses [42]. CD28-independent activation of naive CD8+ T cells was reported in 2C TCR transgenic mice [43] or in an assay using peptide/MHC tetramers [44]. CD28 blockade was less effective in CD8+ T cell-mediated allograft rejection [45]. Reverse of CTLA-4 mediated regulatory function by anti-CD80/86 MoAb treatment may be involved in the augmentation of antileukaemia immune responses. These accumulating reports suggest that naive CD8+ T cells are not readily tolerized by blockade of CD28 co-stimulation. This may be the reason why CD28 blockade retained the GVL effect.
In this study, we first demonstrated the differential effect on GVL between CD28 and CD40 co-stimulatory blockades in a comparative study using BCL1 leukaemia. Our results provide a warning against CD40 blockade for tolerance induction, although further studies using a panel of leukaemias are required. In leukaemia patients undergoing BMT, blockade of CD28 signal may be favourable for clinical application.
Acknowledgments
We thank Dr T. A. Johnson for critical reading of this manuscript. This work was supported, in part, by grants from Science and Technology Promotion Bureau for Organized Research Combination System, and by grants from the Ministry of Health, Labor, and Welfare and the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Blazar BR, Taylor PA, Linsley PS, et al. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–25. [PubMed] [Google Scholar]
- 2.Wallace PM, Johnson JS, MacMaster JF, et al. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–10. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Durie FH, Aruffo A, Ledbetter J, et al. Antibody to the ligand of CD40, gp39, blocks the occurrence of the acute and chronic forms of graft-vs-host disease. J Clin Invest. 1994;94:1333–8. doi: 10.1172/JCI117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito K, Yagita H, Hashimoto H, et al. Effect of CD80 and CD86 blockade and anti- interleukin-12 treatment on mouse acute graft-versus-host disease. Eur J Immunol. 1996;26:3098–106. doi: 10.1002/eji.1830261241. [DOI] [PubMed] [Google Scholar]
- 5.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Blockade of CD40 ligand–CD40 interaction impairs CD4+ T cell-mediated alloreactivity by inhibiting mature donor T cell expansion and function after bone marrow transplantation. J Immunol. 1997;158:29–39. [PubMed] [Google Scholar]
- 6.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 7.Wekerle T, Sayegh MH, Hill J, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with co-stimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–44. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Li XC, Zheng XX, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Med. 1999;5:1298–302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 9.Matulonis U, Dosiou C, Freeman G, et al. B7-1 is superior to B7-2 co-stimulation in the induction and maintenance of T cell-mediated autileukemia immunity. J Immunol. 1996;156:1126–31. [PubMed] [Google Scholar]
- 10.Dilloo D, Brown M, Roskrow M, et al. CD40 ligand induces an antileukemia immune response in vivo. Blood. 1997;90:1927–33. [PubMed] [Google Scholar]
- 11.Boyer MW, Vallera DA, Taylor PA, et al. The role of B7 co-stimulation by murine acute myeloid leukemia in the generation and function of a CD8+ T-cell line with potent in vivo graft-versus-leukemia properties. Blood. 1997;89:3477–85. [PubMed] [Google Scholar]
- 12.Nakajima A, Kodama T, Morimoto S, et al. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumour responses by IL-12 and B7. J Immunol. 1998;161:1901–7. [PubMed] [Google Scholar]
- 13.Kato K, Cantwell MJ, Sharma S, et al. Gene transfer of CD40-ligand induces autologous immune recognition of chronic lymphocytic leukemia B cells. J Clin Invest. 1998;101:1133–41. doi: 10.1172/JCI1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blazar BR, Taylor PA, Boyer MW, et al. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J Immunol. 1997;159:3460–73. [PubMed] [Google Scholar]
- 15.Sykes M, Harty MW, Szot GL, et al. Interleukin-2 inhibits graft-versus-host disease-promoting activity of CD4+ cells while preserving CD4- and CD8-mediated graft-versus-leukemia effects. Blood. 1994;83:2560–9. [PubMed] [Google Scholar]
- 16.Hattori KH, Hirano T, Miyajima H, et al. A metalloproteinase inhibitor prevents acute graft-versus-host disease while preserving the graft-versus-leukemia effect of allogeneic bone marrow transplantation. Br J Haematol. 1999;105:303–12. [PubMed] [Google Scholar]
- 17.Teshima T, Hill GR, Pan L, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest. 1999;104:317–25. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima A, Azuma M, Kodera S, et al. Preferential dependence of autoantibody production in murine lupus on CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:3060–9. doi: 10.1002/eji.1830251112. [DOI] [PubMed] [Google Scholar]
- 19.Nuriya S, Yagita H, Okumura K, et al. The differential role of CD86 and CD80 co-stimulatory molecules in the induction and the effector phases of contact hypersensitivity. Int Immunol. 1996;8:917–26. doi: 10.1093/intimm/8.6.917. [DOI] [PubMed] [Google Scholar]
- 20.Slavin S, Strober S. Spontaneous murine B-cell leikemia. Nature. 1978;272:624–6. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai J, Ohata J, Saito K, et al. Blockade of CTLA-4 signals inhibits Th2-mediated murine chronic graft-versus-host disease by an enhanced expansion of regulatory CD8+ T cells. J Immunol. 2000;164:664–9. doi: 10.4049/jimmunol.164.2.664. [DOI] [PubMed] [Google Scholar]
- 22.Buhlmann JE, Gonzalez M, Ginther B, et al. Sustained expansion of CD8+ T cells requires CD154 expression by Th cells in acute graft versus host disease. J Immunol. 1999;162:4373–6. [PubMed] [Google Scholar]
- 23.Saito K, Sakurai J, Ohata J, et al. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J Immunol. 1998;160:4225–31. [PubMed] [Google Scholar]
- 24.Weiss L, Reich S, Slavin S. The role of antibodies to IL-2 receptor and asialo GM1 on graft-versus-leukemia effects induced by bone marrow allografts in murine B cell leukemia. Bone Marrow Transplant. 1995;16:457–61. [PubMed] [Google Scholar]
- 25.Weiss L, Weigensberg M, Morecki S, et al. Characterization of effector cells of graft vs leukemia following allogeneic bone marrow transplantation in mice inoculated with murine B-cell leukemia. Cancer Immunol Immunother. 1990;31:236–42. doi: 10.1007/BF01789175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen P, Vourka-Karussis U, Weiss L, et al. Spontaneous and IL-2-induced anti-leukemic and anti-host effects against tumor- and host-specific alloantigens. J Immunol. 1993;151:4803–10. [PubMed] [Google Scholar]
- 27.Weiss L, Lubin I, Factorowich I, et al. Effective graft-versus-leukemia effects independent of graft-versus-host disease after T cell-depleted allogeneic bone marrow transplantation in a murine model of B cell leukemia/lymphoma. Role of cell therapy and recombinant IL-2. J Immunol. 1994;153:2562–7. [PubMed] [Google Scholar]
- 28.Garza KM, Chan SM, Suri R, et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–7. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diehl L, Den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nature Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 30.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 31.Howland KC, Ausubel LJ, London CA, et al. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465–70. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- 32.Lefrancois L, Altman JD, Williams K, et al. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–32. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 33.Schuurhuis DH, Laban S, Toes RE, et al. Immature dendritic cells acquire CD8 (+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. 2000;192:145–50. doi: 10.1084/jem.192.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–10. [PubMed] [Google Scholar]
- 35.Taylor PA, Noelle RJ, Blazar BR. CD4 (+) CD25 (+) immune regulatory cells are required for induction of tolerance to alloantigen via co-stimulatory blockade. J Exp Med. 2001;193:1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor PA, Lees CJ, Waldmann H, et al. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood. 2001;98:467–74. doi: 10.1182/blood.v98.2.467. [DOI] [PubMed] [Google Scholar]
- 37.Tang A, Judge TA, Turka LA. Blockade of CD40-CD40 ligand pathway induces tolerance in murine contact hypersensitivity. Eur J Immunol. 1997;27:3143–50. doi: 10.1002/eji.1830271210. [DOI] [PubMed] [Google Scholar]
- 38.Van Gool SW, Vermeiren J, Rafiq K, et al. Blocking CD40-CD154 and CD80/CD86–CD28 interactions during primary allogeneic stimulation results in T cell anergy and high IL-10 production. Eur J Immunol. 1999;29:2367–75. doi: 10.1002/(SICI)1521-4141(199908)29:08<2367::AID-IMMU2367>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Azuma M, Phillips JH, Lanier LL. CD28− T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–59. [PubMed] [Google Scholar]
- 40.Ramarathinam L, Castle M, Wu Y, et al. T cell co-stimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection. an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells. J Exp Med. 1994;179:1205–14. doi: 10.1084/jem.179.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, McGowan P, Ashe S, et al. Tumor immunogenicity determinants the effect of B7 co-stimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523–32. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell co-stimulatory requirements in CD28-deficient mice. Science. 1993;261:609–12. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein JS, Chen T, Brunswick M, et al. Purified MHC class I and peptide complexes activate naive CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 co-stimulatory interactions. J Immunol. 1998;160:3180–7. [PubMed] [Google Scholar]
- 44.Wang B, Maile R, Greenwood R, et al. Naive CD8+ T cells do not require co-stimulation for proliferation and differentiation into cytotoxic effector cells. J Immunol. 2000;164:1216–22. doi: 10.4049/jimmunol.164.3.1216. [DOI] [PubMed] [Google Scholar]
- 45.Newell KA, He G, Guo Z, et al. Blockade of the CD28/B7 costimualtory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163:2358–62. [PubMed] [Google Scholar]


