Abstract
This study was undertaken to investigate the phenotypic and functional status of T lymphocytes of human fetuses from early second- to third-trimester. Cord blood samples were obtained from 19 healthy human fetuses (gestation weeks: 18–36), by cordocentesis, and 16 term newborns (gestation weeks 37–42). Maternal and unrelated male blood samples were also taken as controls. Percentage of lymphocytes in fetal white blood cells was 79·3%, reducing to 40% by term birth, much higher than that of adults. Cord blood mononuclear cells (CBMC), prepared by density gradient centrifugation followed by lysis of erythrocytes, were stained using PE- or FITC-labelled monoclonal Abs and analysed by flow cytometry. The frequencies of CD3+ T cells in fetal (40·1%) and neonatal (42·4%) CBMC were significantly lower than that of men (59·6%) and pregnant women (53·6%). Proportions of CD8+ T cells (9·5%), γδ-T cells (0·5%) and NK cells (4·8%) in fetal CBMC were also lower than that of neonates (except γδ-T cells) and adults. A negative linear correlation (r = −0·609) between the ratio of CD4+/CD8+ T cells in fetal blood and gestation age could also be established. Fetal CBMC showed vigorous spontaneous proliferation but failed to respond to mitogen (PHA) or allogeneic stimulation in vitro. The fetal mononuclear cells were unable to produce IL-2, IL-4 or IFN-γ, but spontaneously secreted IL-10, IL-6 and TNF-α in vitro. Stimulation with PHA up-regulated the production of IL-10, IL-6 and TNF-α substantially.
Keywords: fetal T lymphocytes, phenotype, cytokine, proliferation
INTRODUCTION
The phenotypic and functional characteristics of fetal lymphocytes are of considerable interest for our understanding of the development of the immune system. Recent advances demonstrate that prenatal contact of allergens and other antigens is possible and also that the fetal T and B cells might be able to response to various stimuli during gestation [1–6]. A potential link between prenatal contact with allergens and allergic disease in future life has also been proposed [1]. Mononuclear cells in the human umbilical cord blood collected after delivery at term have been thoroughly studied in the last two decades [7–14] and it is generally accepted that T cells from the neonates are phenotypically and functionally immature. However, currently little is known about the maturational process of fetal T cells during human gestation, due to the difficulties in obtaining, and also ethical dilemmas of working with, human fetal tissues. So far, only a handful of reports documented the phenotypes of T lymphocytes in human fetuses [15–21], few covered the functional status of these cells [1,2,6].
T cells are identified by monoclonal Ab against CD3 and are further divided into CD4+ (helper) and CD8+ (cytotoxic) subsets. The majority of peripheral T cells express αβ-TCR, while a small proportion (about 10%) express γδ-TCR. CD16 and CD56 are commonly used for the identification of NK cells that do not have T cell surface markers. In the present study, we collected blood samples by ultrasound-guided cordocentesis from umbilical cord of human fetuses at early second- and third-trimester. Phenotypes of the T cell subsets were quantitatively determined by flow cytometry and their functional status analysed in proliferation and cytokine (IL-2, IL-4, IL-16, IL-10, TNF-α and IFN-γ) assays. Term cord blood, maternal and healthy unrelated male adult peripheral blood samples were also included as controls.
MATERIALS AND METHODS
Subjects and blood samples
Blood samples (3–5 mls, less than 0.5% of expecting weight in all cases) were obtained after written consent by ultrasound-guided cordocentesis from pregnancies under going prenatal diagnosis at People's Hospital, Beijing, China between January 1999 and March 2002. Gestation age in each case was calculated by last menstrual period and also confirmed by ultrasound measurement of fetal length in the 16th and 23rd week of pregnancy. After exclusion of fetuses with intrauterine restriction, bacterial infection, abnormal karyotype or maternal complications, 19 fetuses remained for analysis in this study (median gestation time: 26 weeks, range 18–36 weeks. In addition, a total of 16 healthy neonates (gestation time: 37–42 weeks) were included in the study. Of these, 12 were delivered by spontaneous labour and the other four by elective caesarean section. Umbilical cord blood samples (10–15 ml) were collected immediately after delivery of healthy newborns, avoiding contamination by the maternal blood. Maternal venous blood (20 mls) was collected, before labour, after informed consent from 16 subjects (median age 26, range 22–35 years). Blood samples were also obtained from 7 healthy unrelated male volunteers (23–45 years) as controls. Blood collection for research purposes in this study was approved by Beijing Medical Bureau, China.
WBC count and mononuclear cell separation
All blood samples were collected in heparin tubes and analysed within 3 h of sampling. Full blood counts, including white cell differential counts, were performed using an automatic blood cell counter (STKS, San Diego, CA, USA). Ficoll-Hypaque (Sigma, St Louis, MO, USA) density gradient (d = 1·077) centrifugation was performed for isolation of mononuclear cell. In the cases of fetal and neonatal cord blood samples, lysis buffer (Gibco, New York, NY, USA) was used to lyse the remaining red blood cells (RBC) in the cord blood mononuclear cell (CBMC) preparation.
Fluorescence staining and flow cytometric analysis
Mononuclear cells (106 cells/100 μ l PBS) were stained with FITC- or PE-conjugated mAbs against CD3, CD4, CD8, CD34, CD45, CD16, TCR-αβ or TCR-γδ (all from Immunotech, USA) in different two colour combinations for 45 min on ice. After 3 washes in PBS containing 1% BSA, the cells were analysed on a FACScan 440 machine (BD, Franklin Lakes, NJ, USA), collecting 10 000 evens for each sample. Fluorescent mAb against CD45 (common leucocyte antigen) was used to identify and gate the leucocytes, thereby minimizing the interference of contaminating erythrocytes (CD45−) in CBMC preparations. PE-labelled anti-CD71 mAb was used, in selected experiments, to quantify CD71+/CD45− erythrocytes in CMBC. Lysis II-VerC software (Becton-Dickson, Rutherford, NJ, USA) was used for data analysis.
Proliferation assays
Mononuclear cells (4 × 105) were incubated in 96-well round-bottomed plates (Nunc, Roskilde, Denmark) in the presence, or absence, of PHA or irradiated stimulator cells (e.g. allogeneic PBMC) in a total volume of 200 μl RPMI-1640 (Sigma) supplemented with human AB serum (5%), glutamate, penicillin/streptomycin (Gibco). Triplicate wells were dispensed for each stimulator group. The cultures were incubated at 37°C and 5% CO2 for 3–4 days. In the last 8 h of incubation, 0·5 μ Ci 3H-thymidine (3H-TdR, BRM Inc., Beijing, China) was added into each well. The cells were harvested, using a 96-well plate harvester (Tomtec, USA), onto fiberglass filters and radioactivity on the filters counted in a MicroBeta Trilux LSC counter (EG & G Wallac, Turku, Finland).
Cytokine analysis
Mononuclear cells (2 × 106) were incubated in RPMI-1640 supplemented with AB serum (5%), glutamate, penicillin/streptomycin (Gibco), in the presence of PHA (5 μg/ml) on 24-well Costar plates for 48 h days before the supernatant was harvested. Concentration of IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ in the samples were quantified using ELISA kits purchased from Endogene (Woburn, MA, USA).
Statistical analysis
Cord blood samples were stratified into 2 groups representing fetuses (gestation weeks 18–36) and neonates. All data are given as mean ± SEM. Statistical comparisons were performed using statistical software SPSS-10·0. P-value <0·05 by 2-sided test was considered significant.
RESULTS
High percentage of lymphocytes in fetal WBC
Absolute counts of fetal and neonatal WBC were lower than that of pregnant women (Table 1). However, percentage of lymphocytes in fetal WBC was approximately 79·3%, much higher than that of the neonates (40%) and pregnant women (21·2%). When percentages of lymphocytes in WBC were plotted against gestation ages of the fetuses and newborn babies, an inverse linear correlation was clearly demonstrable (r = −0·798, Fig. 1). Progenitor cells are similar to lymphocytes in size and the two populations of cells are not easily distinguishable in WBC counting, raising the possibility that progenitor cells in fetal blood might cause significant distortion to the lymphocyte counting results in our experiments. To address this question, mononuclear cells were prepared from fetal blood samples (median gestation weeks: 23, n = 10) and analysed for frequency of CD34+ cells by flow cytometry. CD34+ cells accounted for about 6·2% in fetal CBMC, similar to that (5%) in fetuses of 7–17 weeks of gestation, as reported by Compagnoli et al. [22].
Table 1.
Phenotype analysis of T lymphocytes in fetal, neonatal and maternal blood
| Fetuses (n = 19) | Neonates (n = 16) | Preg. women (n = 16) | ||
|---|---|---|---|---|
| WBC ( × 109/l)‡ | 4·8 ± 1·1 | 3·0 ± 3·2 | 8·8 ± 0·5 | <0·003*,** |
| Percent lymphocyte§ | 79·3 ± 14·2 | 40 ± 8·1 | 21·2 ± 5·4 | <0·001*,**,*** |
| Surface markers¶ | ||||
| CD71+ (n = 4) | 4·8 ± 1·9 | 0·9 ± 0·6 | ND † | <0·05*** |
| CD34+ | 6·2 ± 0·8 (n = 10) | 2·8 ± 0·3 (n = 4) | ND | |
| CD3+ | 40·1 ± 4·9 | 42·4 ± 7·5 | 53·6 ± 12·2 | <0·05*,** |
| CD4+ | 28·9 ± 3·7 | 25·9 ± 5·5 | 28·2 ± 9·3 | |
| CD8+ | 9·5 ± 3·7 | 15·7 ± 2·2 | 23·2 ± 9·2 | <0·005*,**,*** |
| CD4+ CD8+ | 0·8 ± 0·1 | 0·8 ± 0·1 | 0·6 ± 0·5 | |
| CD4/CD8 | 3·4 ± 0·4 | 2·4 ± 0·2 | 1·2 ± 0·3 | <0·05*,** |
| TCR-αβ+ | 29·9 ± 6·4 (n = 15) | 30·7 ± 6 (n = 12) | 42·7 ± 3·8 | <0·05*,** |
| TCR-γδ+ | 0·5 ± 0·3 (n = 15) | 0·7 ± 0·3 (n = 12) | 7·0 ± 2·3 | <0·05*,** |
| CD16+ (n = 10) | 4·8 ± 1·3 | 12·0 ± 2·9 | 19·3 ± 1·3 | <0·05*,** |
(a) Statistical differences between groups.
: Fetuses compared with pregnant women (PW);
: Neonates compared with PW;
: Fetuses compared with neonates. Only statistically significant differences are indicated.
(b) Absolute leucocyte counts.(c) Percentage of lymphocytes in WBC with nucleated red blood cells corrected.(d) Mononuclear cells were prepared and analysed by flow cytometry. The means of percentage of cells positive for each or combination of two of the indicated surface antigens are shown, as well as SEM for each population of cells. Total numbers of subjects included were 19 fetuses, 16 neonates and 16 pregnant women. Because of limited blood volumes, the number of samples analysed for individual markers may be less than the total number and these are indicated in brackets. Seven unrelated male adults were also included in the study, although the data are not shown in the table.(e) ND: Not determined.
Fig. 1.
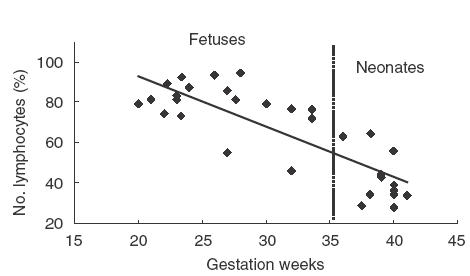
Correlation between percentage of lymphocytes in WBC and gestation age Absolute WBC counts were performed on cord blood samples from 19 healthy fetuses and 16 term newborns. Percentages of lymphocytes in WBC are plotted against gestation weeks. First-degree regression shows an inverse linear correlation between the two, r = −0·798.
T cell subsets in fetal blood
The frequency of CD3+ cells in CBMC of fetuses (40·1%) and neonates (42·4%) were significantly lower than that of pregnant women (53·6%) (Table 1) and male adults (59·6 ± 14·6%, n = 7). Fetuses appeared to have fewer CD8+ T cells (9·5%) compared to neonates (15·7%) and a negative correlation between the ratio of CD4+/CD8+ T cells in CBMC and gestation age could also be established (r = −0·609, Fig. 2). Percentage of CD4 and CD8 double positive cells was less than 1% in all three groups. Proportion of TCR-γδ+ cells in fetal (0·5%) and neonatal (0·7%) CBMC was only about a tenth of that in pregnant women (7%, Table 1). Percentage of CD16+ cells (mainly NK) in fetal CBMC (4·8%) appeared to be lower than that of the neonates (12%) and adults (19·3%) (Table 1).
Fig. 2.
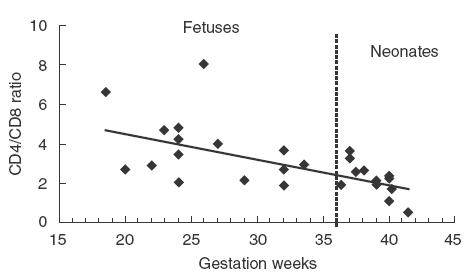
Correlation between the ratio of CD4+/CD8+ T cells and gestation age Percentage of CD4+ and CD8+ T cells in CBMC from 19 fetuses and 16 healthy newborn babies were determined by flow cytometry. CD4/CD8 ratios are plotted against gestational weeks. First-degree regression shows an inverse linear correlation between the two, r = −0·609.
Proliferative responses of fetal CBMC in vitro
To investigate the functional properties of fetal T lymphocytes, fetal, neonatal and adult mononuclear cells were stimulated with, or without, irradiated allogeneic cells or PHA (a useful T cell mitogen) in proliferation assays. The spontaneous proliferation of fetal and neonatal CBMC in vitro was vigorous, while that of the peripheral blood mononuclear cells (PBMC) from pregnant women and male adults was minimal (Fig. 3 and Table 2). However, fetal and neonatal T cells responded poorly, in comparison to adult cells, to stimulation with either PHA (Fig. 3) or allogeneic stimulator cells (Table 2). Fetal CBMC contained efficient antigen presenting cells (APC) as they induced vigorous proliferation of adult T cells in one-way mixed lymphocyte response (MLR) (Table 2).
Fig. 3.
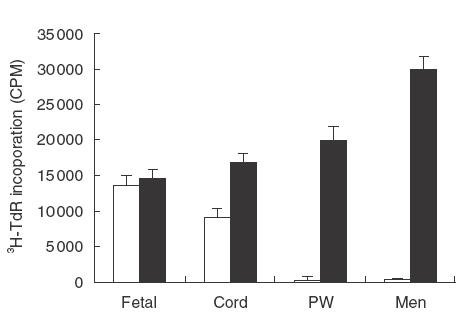
Proliferative responses of mononuclear cells from fetuses, neonates and adults. Mononuclear cells (2 × 105 cells/well) from a fetus (Fetal), a term newborn (Cord), delivered by caesarean section, a pregnant woman (PW) and an unrelated male adult (Men) were cultured in the presence (▪), or absence (□), of PHA on 96-well plates for 72 h. 3H-TdR was added to the cultures for the last 8 h of the incubation before harvesting. The results are expressed as 3H-TdR incorporation (CPM). This is a representative of 5 experiments using blood samples from different subjects. Four out of the 5 neonates were delivered by elective caesarean section.
Table 2.
Proliferative responses of fetal, neonatal and maternal mononuclear cells in MLR†
| Irradiated stimulator cells | ||||
|---|---|---|---|---|
| Responders | Fetal CBMC | Neonatal CBMC | Mother-F PBMC | Mother-N PBMC |
| Fetal CBMC | 1·0 (ALR) | 0·8 | 1·6 | 2·6 |
| Neonatal CBMC | 2·2 | 1·0 (ALR) | 3·1 | 3·9 |
| Mother-F PBMC | 3·1 | 6·2 | 1·0 (ALR) | 5·9 |
| Mother-N PBMC | 5·6 | 12·2 | ND | 1·0 (ALR) |
Mononuclear cells (Responders) from a fetus-mother (Mother-F) and a neonate-mother (Mother-N) pair were cultured either alone (autologous lymphocyte response, ALR) or in the presence of irradiated mononuclear cells (Stimulators) from the other three donors (mixed lymphocyte response, MLR) for 4 days. 3H-TdR was added to the cultures for the last 8 h of the incubation before harvesting and then 3H-TdR incorporation (CPM) determined. The readouts (3H-TdR incorporation) of spontaneous proliferation (i.e. ALR) of fetal, neonatal, mother-F and mother-N mononuclear cells were 12 004 ± 1432CPM, 7568 ± 634CPM, 504 ± 98CPM and 489 ± 102CPM, respectively (also see Fig. 3). The proliferation results of the responder cells are expressed as stimulation index (SI). SI = MLR-readout (CPM)/responder-alone-readout (CPM). SI of ALR is 1. Note that the spontaneous proliferation of fetal CBMC was over 20 times higher than that of adults. This is a representative of 6 experiments, using blood samples from different subjects.
Cytokine production
Ability of making cytokines reflects the maturational status of T lymphocytes. As illustrated in Fig. 4, fetal T cells appeared unable to make any IL-2, IL-4 and IFN-γ, which is in contrast to neonate T cells that secreted modest amount of these cytokines, especially after stimulation with PHA. CBMC from both fetuses and neonates spontaneously secreted IL-10, IL-6 and TNF-α in vitro. Stimulation with PHA up-regulated the production of these cytokines substantially.
Fig. 4.
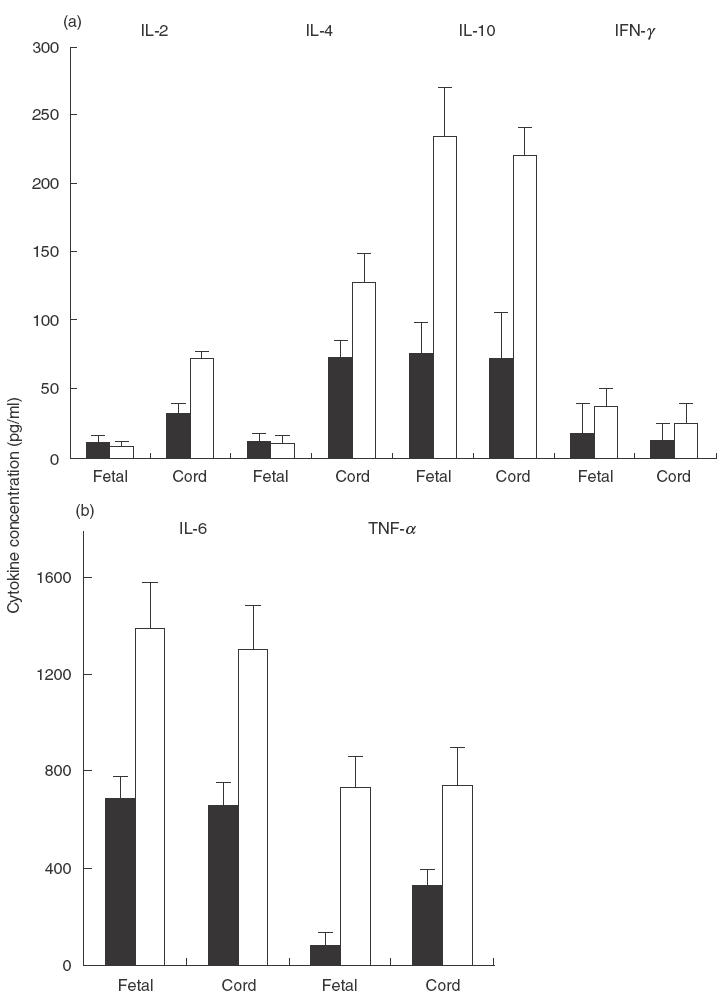
Cytokine production by fetal and neonatal CBMC CBMC from a fetus (Fetal) and a term neonate (Cord), delivered by caesarean section, were cultured in the presence (PHA), or absence (Medium), of PHA in 24-well Costar plates for 48 h. The culture supernatant was harvested and concentration of (a) IL-2, IL-4 and IFN-γ and IL-10 and (b) TNF-α and IL-6 determined by ELISA. The results are expressed as pictograms of cytokine in 1 ml supernatant. This is a representative of 5 experiments using blood samples from different subjects. Four out of the 5 neonates were delivered by elective caesarean section.
DISCUSSION
Leucocyte counting results showed that percentage of lymphocytes in fetal WBC was about 79·3%, reducing to 40% by the end of the pregnancy (Table 1; Fig. 1). CD34+ progenitor cells accounted for 6·2% in fetal CBMC, they were therefore unlikely to cause significant distortion to the lymphocyte counting results. Furthermore, the lymphocyte counts of cord blood samples were corrected for nucleated RBC. The high percentage of lymphocytes in fetal blood is therefore more likely the results of lymphocytes gaining populational homeostasis ahead of myeloid cells (granulocytes and megakaryocytes) during prenatal development.
Separation of mononuclear cells from cord blood faces the problem of contamination by nucleated RBC, which may interfere with the phenotypic analysis of lymphocyte subsets in flow cytometry. We combined the density gradient centrifugation and blood lysis methods when preparing CBMC. The results were satisfactory because that percentage of CD71+ cells (mostly RBC) in fetal CBMC prepared this way was less than 5% (Table 1). The use of fluorescent mAb against CD45 to identify and gate the leucocytes also helped to minimize the influence of contaminating RBC in our flow cytometric analysis.
Different proportions of lymphocyte subsets in fetal [15–17,19–21, and neonatal [7,10,11,14] CBMC have been reported by previous investigators. We confirmed the previous reports that neonatal [7,10,14,20] CBMC contained 40–55% CD3+ T cells, vs. 60–75% in adults. Our data and that of the others [14–20] suggest a gradual expansion, from early fetal life to time birth to adults, of γδ-T-cells, NK and CD8+ T cells in the periphery. We also demonstrated that there is an inverse linear correlation between fetal CD4/CD8 ratio and gestation age, which is in agreement with previous studies [16–20]. Kutvirt and colleagues [23] argued that density gradient centrifugation could result in higher CD4/CD8 ratio. However, our results on CD4/CD8 ratio are comparable to that reported by previous workers who used the whole blood lysis technique alone [17,19,20].
The assay of cytokine production by T cells during gestation may provide valuable insights into the developmental mechanisms underlying T cell maturation and also fetal–maternal interaction. It has been demonstrated that CBMC of term newborns have a reduced capacity to secret IL-10 [8], IL-4, IFN-γ [12,13] and TNF-α [7], although they produced significant amount of GM-CSF [7]. Williams et al. [1] have shown that fetal T cells spontaneously produced IL-13 in vitro. In our study, fetal T cells were unable to make IL-2, IL-4 and IFN-γ, but they secreted modest amount of IL-6 and IL-10 (Fig. 4). These results are in line with the notion that Th2 cytokines (including IL-10 and IL-13) might play an important role in fetal–maternal interaction during pregnancy [24,25]. Production of IL-6, IL-10 and TNF-α by fetal and neonatal CBMC was even more impressive following PHA stimulation (Fig. 4). IL-6 and TNF-α are the so-called endogenous pyrogens (IL-1, IL-6 and TNF-α) involved in innate immune responses. More importantly, IL-6 induces an increase of circulating neutrophils that are summoned from the bone marrow [26,27]. Such a function of IL-6 may be of great importance in supporting the expansion of myeloid cells during development. The increased TNF-α, IL-10 and IL-6 responses of CBMC to PHA stimulation (Fig. 4) suggest that at least some of these cytokines were contributed by fetal T cells. However it must also be noted that IL-6, IL-10 and TNF-α can also be synthesized by activated monocytes and macrophages. Steinborn et al. [28] identified myolomonocytic cells as the main producers of IL-6 in neonatal cord blood. They also demonstrated that spontaneous labour at term was associated with fetal monocyte activation and IL-6 production [28]. In addition, Thilaganathan et al. [29] documented that term neonates delivered vaginally had significantly higher median values for neutrophils, monocytes, and natural killer cells in their umbilical cord blood, compared to that obtained from normal pregnancies delivered by elective caesarean section. In our study, four out of the 5 neonates included in cytokine assays were delivered by elective caesarean section. The cytokine production pattern of CBMC from the one delivered spontaneously via the vaginal route was not significantly different from the others (not shown). It should be emphasized that the leukocytosis and activation effect associated with spontaneous labour seemed selective for innate immunity. No evidence was found for activation of cord blood T cells in association with spontaneous term labour [28,29].
Fetal CBMC proliferated more vigorously in a spontaneous fashion compared to that of term newborns and adults (Fig. 3). It is highly unlikely that fetuses had plentiful autoreactive T cells that responded to autologous cells in the culture. We reckon that lack of effective regulatory (suppressor) T lymphocytes in fetal and neonatal CBMC is perhaps a more likely explanation, although proliferation of progenitor cells could not be ruled not completely. Fetal CMBC responded poorly to mitogen or allogeneic stimulation in vitro (Table 2,Fig. 3), however, they induced vigorous proliferation of adult PBMC (Table 2), suggesting that fetal CBMC contained very efficient APC. The main classes of APC in CBMC are dendritic cells and monocytes, it is of importance to investigate the phenotypic and functional status of these cells in future studies.
Maternal blood samples were used as controls through out this study, although samples from 7 male adults were also analysed for proportions of CD3+, CD4+, CD8+ and CD4+/CD8+ T cell subsets. It is possible that pregnancies may cause alterations in components of the maternal immune system. However, if one considers the fact that pregnancies may affect the immune systems of the mother and the fetus in a similar way, then maternal blood may be more favourable as controls in a study like ours. Furthermore, it is also evident that alterations in maternal cellular immunity during pregnancy are not very significant [30–36].
Acknowledgments
This work was supported by grants from the Natural Science Foundation and also National Key Basic Research Program of China (2001CB51000).
REFERENCES
- 1.Williams TJ, Jones CA, Miles E, Warner JO, Warner JA. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J Allergy Clin Immunol. 2000;105:951–9. doi: 10.1067/mai.2000.106211. [DOI] [PubMed] [Google Scholar]
- 2.Piccinni MP, Mecacci F, Sampognaro S, et al. Aeroallergen sensitization can occur during fetal life. Int Arch Allergy Immunol. 1993;102:301–3. doi: 10.1159/000236541. [DOI] [PubMed] [Google Scholar]
- 3.Szepfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R. Direct evidence for transplacental allergen transfer. Pediatr Res. 2000;48:404–7. doi: 10.1203/00006450-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Duran-Smith K, Pichter J, Ebner C, et al. Prenatal contact with inhalant antigens. Pediatr Res. 1997;41:128–31. doi: 10.1203/00006450-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Szepfalusi Z, Nentwich I, Gestmayr M, Jost E, Todoran L, Gratzl R, et al. Prenatal allergen contact with milk proteins. Clin Exp Allergy. 1997;27:28–35. [PubMed] [Google Scholar]
- 6.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996;7:109–16. doi: 10.1111/j.1399-3038.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 7.Harris DT, Schumacher MJ, Locascio J, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci USA. 1992;89:10006–10. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotiranta-Aminamo A, Rautonen J, Rautonen N. IL-10 production by cord blood mononuclear cells. Pediatr Res. 1997;41:110–3. doi: 10.1203/00006450-199701000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Liao SY, Liao TN, Chiang BL, et al. Decreased production of IFN-γ and increased production of IL-6 by cord blood mononuclear cells of newborns with high risk of allergy. Exp Allergy. 1996;26:397–405. [PubMed] [Google Scholar]
- 10.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyere M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;3:215–8. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 11.Han P, Hodge G, Story C, Xu X. Phenotypic analysis of functional T-lymphocyte subtypes and natural killer cells in human cord blood: relevance to umbilical cord blood transplantation. Br J Haematol. 1995;89:733–40. doi: 10.1111/j.1365-2141.1995.tb08409.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis DB, Yu CC, Meyer J, English K, Kahn SJ, Wilson CB. Cellular and molecular mechanisms for reduced IL-4 and IFN-γ production by neonatal T cells. J Clin Invest. 1990;87:194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott ME, Kubin M, Khol S. High level IL-12 production, but not diminished IFN-γ production, by cord blood mononuclear cells. Blood. 1996;8:945–54. doi: 10.1203/00006450-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Griffith-Chu S, Patterson JAK, Berger CL, Edelson RL, Chu AC. Characterization of immature T cell subpopulations in neonatal blood. Blood. 1984;64:296–300. [PubMed] [Google Scholar]
- 15.Berry SM, Fine N, Bichalski JA, Cotton DB, Dombrowski MP, Kaplan J. Circulating lymphocyte subsets in second- and third-trimester fetuses: Comparison with newborns and adults. Am J Obestet Gynecol. 1992;167:895–900. doi: 10.1016/s0002-9378(12)80008-1. [DOI] [PubMed] [Google Scholar]
- 16.De Waele M, Foulon W, Renmans W, Segers E, Smet L, Jochmans K, Van Camp B. Hematological values and lymphocyte subsets in fetal blood. Am J Clin Pathol. 1988;89:742–6. doi: 10.1093/ajcp/89.6.742. [DOI] [PubMed] [Google Scholar]
- 17.Rainaut M, Pagniez M, Hercend T, Daffos F, Forestier F. Characterization of mononuclear cell sub-populations in normal fetal peripheral blood. Human Immunol. 1987;18:331–7. doi: 10.1016/0198-8859(87)90079-6. [DOI] [PubMed] [Google Scholar]
- 18.Rukavina D, Podack ER, Robesa G, Spanjol-Pandelo S, Randic L. Down regulated expression of perforin-positive/CD16+ cells in the peripheral blood lymphocytes in the first trimester and up-regulation at the end of pregnancy. Am J Reprod Immunol. 1997;38:189–96. doi: 10.1111/j.1600-0897.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 19.Plebani A, Proserpio AR, Guarneri D, Buscaglia M, Cattoretti G. B and T lymphocyte subsets in fetal and cord blood: age-related modulation of CD1c expression. Biol Neonate. 1993;63:1–7. doi: 10.1159/000243901. [DOI] [PubMed] [Google Scholar]
- 20.Schultz C, Reiss I, Bucsky P, Gopel W, Gembruch U, Ziesenitz S, Gortner L. Maturational changes of lymphocyte surface antigens in human blood: comparison between fetuses, neonates and adults. Biol Neonate. 2000;78:77–82. doi: 10.1159/000014253. [DOI] [PubMed] [Google Scholar]
- 21.Thilaganathan B, Mansur CA, Morgan G, Nicolaides KH. Fetal T-lymphocyte subpopulations in normal pregnancies. Fetal Diagn Ther. 1992;7:53–61. doi: 10.1159/000263651. [DOI] [PubMed] [Google Scholar]
- 22.Campagnoli C, Fisk N, Overton T, Bennett P, Watts T, Roberts I. Circulating hematopoietic progenitor cells in first trimester fetal blood. Blood. 2000;95:1967–72. [PubMed] [Google Scholar]
- 23.Kutvirt SG, Lewis SL, Simon TL. Lymphocyte phenotypes in infants are altered by separation of blood on density gradients. Br J Biomed Sci. 1993;50:321–8. [PubMed] [Google Scholar]
- 24.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 25.Prescott SL, Macaubas C, Holt BJ, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell response toward the Th2 cytokine profile. J Immunol. 1998;160:4730–7. [PubMed] [Google Scholar]
- 26.Liechty KW, Christensen RD. In vivo effect of IL-6 on cycling status of hematopoietic progenitors from adults and neonates. Pediatr Res. 1990;28:323–6. doi: 10.1203/00006450-199010000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Suwa T, Hogg JC, English D, Van Eeden SF. IL-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am. J Physiol Heart Circ Physiol. 2000;279:H2954–60. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- 28.Steinborn A, Sohn C, Sayehli C, Baudendistel A, Huwelmeier D, Solbach C, Schmitt E, Kaufmann M. Spontaneous labour at term is associated with fetal monocyte activation. Clin Exp Immunol. 1999;117:147–52. doi: 10.1046/j.1365-2249.1999.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thilaganathan B, Meher-Homji N, Nicolaides KH. Labor an immunologically beneficial process for the neonate. Am J Obstet Gynecol. 1994;171:1271–2. doi: 10.1016/0002-9378(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 30.Moore MP, Carter NP, Redman CW. Lymphocyte subsets in normal and pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1983;90:326–31. doi: 10.1111/j.1471-0528.1983.tb08918.x. [DOI] [PubMed] [Google Scholar]
- 31.Bardeguex AD, McNerney R, Frieri M, et al. Cellular immunity in pre-eclampsia: alterations in T-lymphocyte subpopulations during early pregnancy. Obstet Gynecol. 1991;42:859–62. [PubMed] [Google Scholar]
- 32.Coulam C, Silverfield J, Kazmar RE, Fatham CG. T lymphocyte subsets during pregnancy and the menstrual cycle. Am J Reprod Immunol. 1983;4:88–90. doi: 10.1111/j.1600-0897.1983.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 33.Lucivero G, Selvaggi A, Dell'osso A, et al. Mononuclear cell subpopulations during normal pregnancy. I. Analysis of cell surface markers using conventional techniques and monoclonal antibodies. Am J Reprod Immunol. 1983;4:142–5. doi: 10.1111/j.1600-0897.1983.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 34.Tallon DF, Corcoran DJD, O'Dwyer EM, Greally JF. Circulating lymphocyte subpopulations in pregnancy: a longitudinal study. J Immunol. 1984;132:1784–7. [PubMed] [Google Scholar]
- 35.Gehrz RC, Christianson WR, Linner KM. A longitudinal analysis of lymphocyte proliferative responses to mitogens and antigens during human pregnancy. Am J Obstet Gynecol. 1981;140:665–70. doi: 10.1016/0002-9378(81)90201-5. [DOI] [PubMed] [Google Scholar]
- 36.Feinberg BB, Gonik B. General precepts of the immunology of pregnancy. Clin Obstet Gynecol. 1991;34:3–16. doi: 10.1097/00003081-199103000-00005. [DOI] [PubMed] [Google Scholar]


