Abstract
High levels of interleukin-6 (IL-6) and interleukin-8 (IL-8) have been demonstrated in the peritoneal fluid of benign and malignant gynaecological disease. Peritoneal monocytes and macrophages, endometrial cells, endometrial and peritoneal stromal cells and tumour cells produce these cytokines in vitro. To investigate whether normal human peritoneal mesothelial cells (HPMC) produce IL-6 and IL-8, HPMC were isolated from omental biopsies. Primary HPMC (P-HPMC) were transfected with pSV3-neo encoding SV40 large T antigen (T-HPMC) to generate sufficient cells. T-HPMC preserved the characteristics of P-HPMC as assessed by phase contrast microscopy, electron microscopy, immunocytochemistry and flow cytometry (FACS) analysis. T-HPMC retained a stable phenotype up to passage 14–19, whereas P-HPMC proliferated poorly and became senescent by passage 4–6. T-HPMC and P-HPMC constitutively expressed IL-6 and IL-8 at both protein and mRNA level. IL-6 and IL-8 production was stimulated by recombinant human interleukin-1β (hIL-1β) or human tumour necrosis factor-α (hTNF-α) alone in a dose-dependent manner. Moreover, hIL-1β or hTNF-α up-regulated IL-6 and IL-8 gene expression as determined by competitive PCR. In contrast, human interferon-γ (hIFN-γ) or lipopolysaccharide (LPS) showed no effect. These data indicate that (1) T-HPMC lines mimic the morphological and functional features of P-HPMC, (2) P-HPMC and T-HPMC constituitively produce IL-6 and IL-8, which is enhanced by hIL-1β and hTNF-α and (3) HPMC in vivo may participate in the pathogenesis of benign and malignant gynaecological disease.
Keywords: gynaecological disease, IL-6, IL-8, mesothelium, SV40
INTRODUCTION
Endometriosis and epithelial ovarian cancer (EOC) are important gynaecological diseases characterized by intraperitoneal spread, peritoneal deposits and free peritoneal fluid. Despite their clinical importance, little is known about the local intraperitoneal immune response in these diseases, and the pathogenesis of both diseases is unclear. Proinflammatory cytokines, including IL-6 and IL-8, have been detected at high levels in the peritoneal fluid of patients with endometriosis [1,2] and in ovarian cancer ascites [3]. IL-6 is a multi-functional cytokine involved in numerous diverse biological functions, including induction of B-cell differentiation, regulation of haematopoiesis and acute-phase reactions [4,5]. It has also been reported to be a putative autocrine and paracrine growth factor in endometriosis [6,7] and in ovarian cancer [8,9]. IL-8, which functions mainly as a neutrophil chemoattractant and activating factor, is also a potent angiogenic factor and an autocrine growth factor for tumour cells [10–13]. Monocytes and macrophages in peritoneal fluid, endometrial glandular cells, endometrial stromal cells, subserosal (stromal) peritoneal cells and tumour cells have been reported to produce IL-6 and IL-8 in vitro [14,15].
Human peritoneal mesothelial cells (HPMC), the largest resident cell population in the peritoneal cavity, comprise the flat monolayer of cells lining the peritoneal surfaces and viscera. This monolayer functions as a semipermeable membrane [16] and plays a role in adhesion formation in response to infection and surgery [17]. Previous studies have shown that HPMC expressed cytokines in vitro, either constitutively or in response to stimuli such as epidermal growth factor (EGF), tumour necrosis factor (TNF) or lipopolysaccharide (LPS) [18–20]. However, in most of these studies, mesothelial cells were isolated from human malignant and cirrhotic ascites and cultured in monocyte or macrophage conditioned medium, and it is not clear whether patients were treatment-naive. It is possible the cytokine expression of these cells was premodulated by the intra-abdominal disease conditions, by the previous treatments or by the culture conditions. In addition, mesothelial cells can take on a wide range of ‘atypical’ and/or ‘reactive’ morphological changes in response to different stimuli [21–23]. To define better how normal HPMC participate in intra-abdominal immune reactions, we isolated and characterized HPMC from patients with benign gynaecological conditions [22].
We set out to determine whether HPMC are immunologically active, in particular, whether HPMC contribute to the high levels of intraperitoneal IL-6 and IL-8 reported in benign and malignant gynaecological diseases. Studies on HPMC have been hampered by the limited proliferative capacity and senescence of these cells in early passages in vitro. We therefore established HPMC lines by introducing the early region of SV40 into normal P-HPMC using poly l-ornithine (PLO)-mediated transfection. The phenotypic characteristics, growth patterns and the expression of IL-6 and IL-8 were studied first in T-HPMC and then compared with normal P-HPMC. The modulation of IL-6 and IL-8 gene and protein expression by other cytokines was also studied.
MATERIALS AND METHODS
Isolation and culture of HPMC
HPMC were isolated from fresh omental biopsies from consenting, treatment-naive patients undergoing selective surgery for benign gynaecological conditions (excluding endometriosis) [22]. The study was approved by the local research ethics committee. For transfection experiments, HPMC were plated out in 6-well flat-bottomed plates (NUNC, Gibco, UK) and maintained in complete growth medium (CM) consisting of M199 supplemented with 10% heat-inactivated fetal calf serum (FCS), 20 mm Hepes, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mml-glutamine. Non-adherent cells were removed and the medium was changed after 24 h incubation in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Thereafter the growth medium was replaced every 3 days. This method yields HPMC with a purity of over 90%, as assessed by simultaneous expression of cytokeratin 8/18 and vimentin (data not shown). To prepare P-HPMC, isolated HPMC were plated out on tissue culture flasks and maintained [22]. Cultures initiated in this fashion were serially passaged by treating with 0·05% trypsin/0·02% EDTA for 1–2 min. All experiments on P-HPMC were performed between passages 2 and 4.
Cell transfection with pSV3-neo
HPMC were transfected with the pSV3-neo (ATCC, 37150) encoding SV40 large T antigen and the selectable marker neomycin phosphotransferase which confers resistance to the antibiotic G418 [24]. A previously described method of combining PLO with a dimethylsulphoxide (DMSO) shock [25] was followed with minor modification. Briefly, P-HPMC were incubated with transfection solution containing 5 μg of pSV3-neo DNA and 10 μg/ml of PLO for 6 h at 37°C. The transfection solution was removed, and cells were subjected to DMSO shock (30% v/v in CM) for 4 min at room temperature. Fresh CM was added and the cells were allowed to grow for 48–72 h at 37°C. Transfected cell cultures were then selected in medium containing the neomycin analogue G418 (400 μg/ml, Gibco, UK). Single cell colonies were isolated by trypsinization using cloning rings and seeded into 48-well plates and later to progressively larger vessels in G418 containing medium and repeated three times. Cells were returned to CM for all subsequent steps. All experiments on T-HPMC were performed between passages 6 and 10.
Transmission electron microscopy
For electron microscopy, cells cultured on cover slips were fixed overnight at 4°C in 2·5% gluteraldehyde in 0·1m Sö rensen's phosphate buffer (pH 7·4), postfixed for 2 h at room temperature in 1% osmium tetroxide and dehydrated with increasing concentration of ethanol before embedding in Araldite II resin. Transversally cut sections were stained with uranyl acetate and lead citrate, and viewed with a Phillips 410 electron microscope.
Growth assays
T-HPMC and P-HPMC were seeded in 96-well plates at a density of 6400 cells/well and incubated for 24 h. Cells were pulsed with 0·5 μCi/well of [3H]thymidine (ICN Biomedicals, Inc., Irvine, CA, USA) for 16 h, and harvested using the Micromate 196 harvester (Canberra Packard, Berks, UK). Thymidine incorporation was measured by the Matrix 96 direct β-counter (Canberra Packard). To determine the population doubling time (PDT), exponentially growing cells were plated in 25 cm2 culture flasks containing 5 ml of CM at an adjusted concentration of 1 × 105 cells/ml and cultured for 7 days. At 24-h intervals, cells were detached using 0·05% trypsin and cell numbers in triplicate 0·5 ml aliquots of each sample were determined by a haemocytometer. PDT was calculated at the time of exponential growth (log phase), i.e. between 48 h and 96 h after initial plating, using the formula: PDT = ln2 × t × [ln (Nt/ No)]−1, where t is time interval (48 h), Nt is number of cells at 96 h and No is number of cells at 48 h.
Immunocytochemistry
HPMC were resuspended with CM, seeded on eight-chamber glass slides (Laboratory Tek, Nunc Inc., Naperville, IL, USA) and grown to subconfluence. The cells were then washed three times with PBS and fixed in absolute methanol. ABC immunostaining method was carried out [22] using the following monoclonal antibodies: anticytokeratin 8/18 (Clone 5D3, Novocastra Laboratories, Newcastle upon Tyne, UK), antivimentin (Clone VIM 3B4, Novocastra Laboratories), antimesothelioma cell antigen (Clone HBME-1, DAKO, Carpinteria, CA, USA), anti-E-Cadherin (Clone DECMA-1, Sigma-Aldrich), and anti-SV40 large T antigen (Clone PAb416, Oncogene Research Products, Cambridge, MA, USA) antibodies. Human breast adenocarcinoma, reactive lymph node, mesothelioma and ovarian adenocarcinoma sections were used as positive controls for anti-cytokeratin, vimentin, mesothelioma cell antigen and E-cadherin antibodies, respectively. Human endothelial cell line, SGHEC-7 (kindly provided by Dr G Whitley, St George's Hospital Medical School, London, UK) was used as a positive control for anti-SV40 large T antigen antibody. Negative controls consisted of substituting normal mouse IgG for the primary antibodies.
FACS analysis
FACS was performed as described previously [22] using FACScan (Becton Dickinson Immunocytometry Systems [BDIS], MountainView, CA, USA). Briefly, 1 × 105 cells were stained directly with PE-conjugated mouse antihuman CD16 (clone DJ130c, Dako) and CD14 (clone TUK4, Dako) antibodies, respectively. The indirect immunofluorescence method was used to stain HPMC with anti-CD34 antibody (clone HPCA-2, BDIS, San Jose, CA, USA) followed by the addition of FITC-conjugated rabbit antimouse immunoglobulin (BDIS, San Jose, CA, USA); 5 × 103 events were collected for analysis by Cellquest™ software (BDIS, San Jose, CA, USA). For the negative controls isotype matched normal mouse IgG were substituted for the primary antibodies.
Enzyme linked immunosorbent assay (ELISA)
Cells, 5 × 104, were incubated in CM alone or in the presence of varying concentrations of recombinant hIL-1β (10 ng/ml, 10-fold serial dilution to 0·001 ng/ml; R&D Systems, Oxon, UK), or recombinant hTNF-α (1000 units/ml, 10-fold serial dilution to 0·1 units/ml; R&D), or recombinant hIFN-γ (1000 units/ml, 10-fold serial dilution to 0·1 units/ml; R&D) or LPS (Escherichia coli serotype 0127 : B8; 50 μg/ml, 10-fold serial dilution to 0·005 μg/ml; Sigma, St Louis, MO, USA) for 24 h. Cell-free culture supernatants were collected and stored at −70°C for further analysis. Samples of medium containing hIL-1β, hTNF-α, hIFN-γ or LPS were used as controls. Supernatants were measured for IL-6 and IL-8 using IL-6 OptEIA™ Set and IL-8 OptEIA™ Set (Pharmingen, San Diego, CA, USA). The manufacture's instructions were followed exactly.
RT-PCR and competitive PCR
P-HPMC and T-HPMC were incubated in CM with or without hIL-1β (1 ng/ml) or hTNF-α (100 units/ml) for 4 h or 24 h. Total RNA was isolated from approximately 2 × 106 cells using SV total RNA isolation system (Promega, Madison, WI, USA). Total RNA was pretreated with DNase I at room temperature for 15 min to remove any contaminating genomic DNA. First-strand cDNA synthesis was carried out at 42°C for 1 h using a reverse transcription system (Promega). Control samples were run without the addition of total RNA. By performing the first competitive PCR, initial cDNA amount among samples was corrected based on the amount of expressed human β-actin (hβ-actin). Thereafter, equal amounts of cDNA samples were diluted 30-fold and assayed for cytokine mRNA by cytokine-specific competitive PCR [26]. For competitive PCR, each cDNA sample was co-amplified with a known amount of cytokine specific mimic (or competitor) DNA fragments preconstructed using a competitive DNA construction kit (Takara Shuzo Co. Ltd, Japan). The cytokine-specific mimic was designed to give a product different in size from the target product by 150 base pairs. For each sample cDNA was co-amplified in a series of five reactions with a fivefold dilution series of cytokine specific mimic concentrations. The following oligonucleotide primers were used: (1) hβ-actin forward, TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA; (2) hβ-actin reverse, CTA GAA GCA TTG CGG TGG ACG ATG GAG GG; (3) hIL-6 forward, ATG AAC TCC TTC TCC ACA AGC GC; (4) hIL-6 reverse, GAA GAG CCC TCA GGC TGG ACT G; (5) hIL-8 : forward, ATG ACT TCC AAG CTG GCC GTG GCT; (6) hIL-8 reverse, TCT CAG CCC TCT TCA AAA ACT TCT C. The amplification conditions were as follow: hβ-actin and hIL-6, 5 min at 96°C, then 40 cycles of 96°C for 45 s, 60°C for 45 s, 72°C for 45 s, and a final extension at 72°C for 5 min; hIL-8, 5 min at 94°C, then 35 cycles of 94°C for 45 s, 60°C for 45 s, 72°C for 2 min and a final extension at 72°C for 10 min The PCR products were separated on 1·5% agarose gels. The relative amount of target and mimic DNA products in each amplification was determined by densitometry (Ultra-Violet Products Ltd, Cambridge, UK) and plotted using Labworks Image Acquisition and Analysis Software (Ultra-Violet Products) to enable the determination of equimolarity of target cDNA and mimic DNA and, thus, the amount of target mRNA in each sample.
All reagents were purchased from Sigma-Aldrich, Dorset, England, unless stated otherwise.
Statistical analysis
Results were expressed as mean ± s.d. of three triplicate experiments. Significance was assessed either by one-way analysis of variance (anova) followed by Tamhane procedure to adjust for multiple comparisons among groups, or by Student's t-test (two-tailed) to compare differences between two groups. A computer-based statistical package (SPSS, Chicago, IL, USA) was used for the analysis. P-values ≤0·05 were regarded as significant.
RESULTS
Phenotypic characteristics of pSV3-neo transfected HPMC
Central to the study of established lines was the characterization of transfected cells. Eleven individual G418-resistant colonies were obtained and all were expanded for subsequent characterization, including daily morphology check by phase contrast microscopy and screening for characteristics associated with human mesothelial cells by immunocytochemistry and FACS. Co-expression of intermediate filaments cytokeratin 8/18 and vimentin; typical characteristics of mesothelial cells [22,23] were found in eight of 11 clones of T-HPMC with similar staining pattern and intensity as P-HPMC (Fig. 1a–d). These cells failed to express the epithelial lineage marker, E-cadherin; the endothelial cell marker, CD34; and leucocyte markers, CD14 and CD16 (data not shown). Furthermore, T-HPMC from these eight clones did not show detectable expression of the mesothelioma cell antigen. These findings are consistent with our previous observation of undetectable expression of mesothelioma cell antigen in P-HPMC after the second passage [22]. T-HPMC from the eight clones therefore preserved the phenotypic characteristics of mesothelial cells. The similar staining patterns persisted in precrisis T-HPMC (data not shown), suggesting that the T-HPMC were phenotypically stable.
Fig. 1.
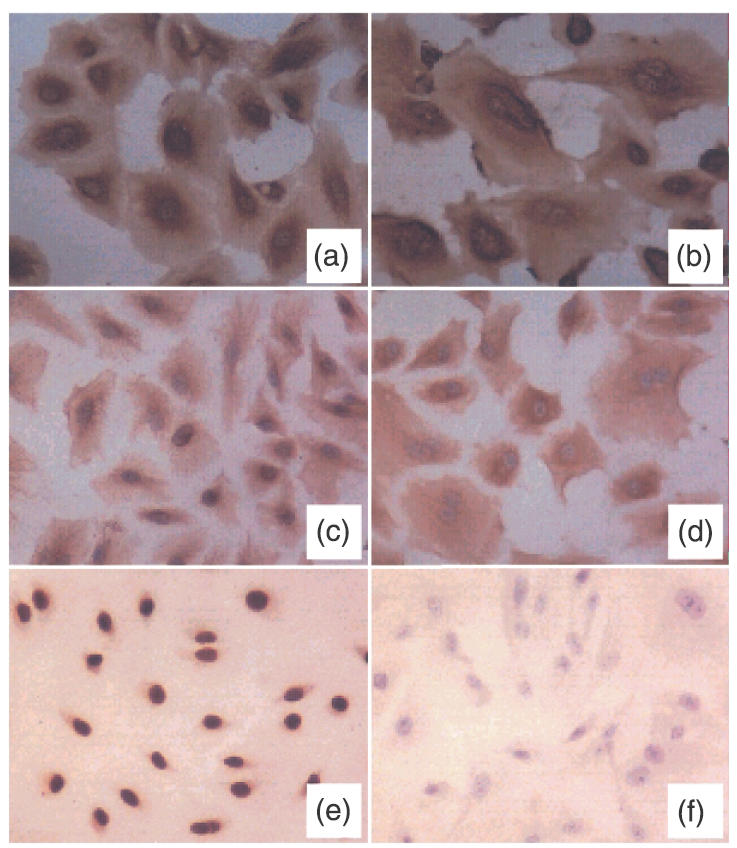
Comparative study of cytokeratin 8/18, vimentin and SV40 large T antigen expression in HPMC culture. Monolayer T-HPMC (passage 8) (a, c, e) and P-HPMC (passage 3) (b, d, f) were incubated with anticytokeratin 8/18, vimentin and SV40 large T antigen antibody, respectively, detected by Vectastain® UNIVERSAL Elite ABC KIT. Co-expression of cytokeratin 8/18 (a, b) and vimentin (c, d) was observed on both T-HPMC and P-HPMC culture. Nearly all T-HPMC exhibit an intense nuclear expression of SV40 large T antigen (e), whereas P-HPMC show no detectable expression of this antigen (f).
Morphological characteristics and growth patterns of T-HPMC
Cultures of T-HPMC and P-HPMC displayed similar morphological characteristics by phase contrast microscopy. Neither T-HPMC nor P-HPMC formed multi-layers but displayed a homogeneous, monolayer of polygonal cells with prominent and centrally located, round nuclei and prominent nucleoli when cultured in CM and possessed a tight cobblestone morphology with no overlapping of neighbouring cells when confluent (data not shown). With T-HPMC this morphology persisted to passages 14–19 when the cells became enlarged, sometimes vacuolated, resembling morphologically senescent P-HPMC which in contrast occurred at passages 4–5. By transmission electron microscope, T-HPMC (Fig. 2a) exhibited ultrastructural features similar to that of P-HPMC (Fig. 2b), apart from partial loss of microvillous projections and junction formation. Both T-HPMC and P-HPMC had ovoid nuclei with smooth contours and finely granular chromatin with several small nucleoli. The cytoplasm contained endoplasmic reticulum, lysozomes, mitochondria and aggregates of intermediate filaments in places resembling tonofilaments.
Fig. 2.
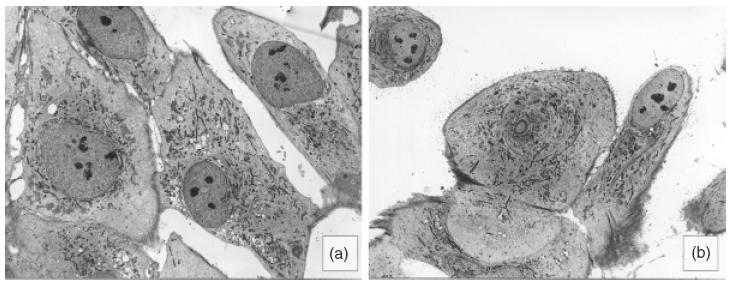
Ultrastructure of T-HPMC in comparison to P-HPMC. By transmission electron microscopy T-HPMC (a, ×725) and P-HPMC (b, × 725) display nuclei and nucleoli with similar sizes and shapes and cytoplasms with similar tinctorial patterns, although microvilli are abundant in P-HPMC and few in T-HPMC.
T-HPMC lines grew more rapidly in CM without growth factor supplementation. Population doubling time (PDT) was 30–46 h, compared to 56–98 h – for P-HPMC. Mean [3H]thymidine uptake of T-HPMC was six to 10 times greater than that of P-HPMC (P <0·001) (data not shown). The proliferation rate of T-HPMC was greater than that of P-HPMC from day 1 onwards, and the density of T-HPMC was constantly higher than that of primary cells, regardless of cell sources (data not shown). T-HPMC had an extended life span, exhibiting non-proliferating or senescent cells when they were beyond the 14th to 19th passages. Subsequently there was a loss in proliferative capacity of all cell cultures.
SV40 large T antigen expression by T-HPMC
As determined by immunocytochemistry, virtually all T-HPMC that survived repeated G418 selection expressed SV40 large T antigen with a similar pattern and intensity of nuclear staining (Fig. 1e). In contrast, P-HPMC failed to stain with the same antibody (Fig. 1f). The SV40 large T antigen expression in T-HPMC was consistent during cell culture, even in cells entered into crisis (data not shown), indicating stable integration of SV40 large T antigen into the host genome.
Constitutive expression of IL-6 and IL-8 by HPMC
First, we examined the constitutive expression of IL-6 and IL-8 protein and genes in T-HPMC and then repeated the experiments in P-HPMC. As assessed by ELISA, IL-6 and IL-8 protein levels were 3246·25 ± 413·62 and 6157·15 ± 658·13 pg/ml/5 × 104 cells, respectively, in T-HPMC supernatants, and 4537·45 ± 406·05 and 4959·46 ± 344·912 pg/ml/5 × 104 cells, respectively, in P-HPMC supernatants. To analyse further the constitutive IL-6 and IL-8 gene expression, RT-PCR was performed. A specific IL-6 single band of 628 base pairs and a specific IL-8 single band of 289 base pairs were demonstrated in both non-stimulated T-HPMC and P-HPMC cultures (Fig. 3). These data indicate that normal HPMC constitutively expressed IL-6 and IL-8 genes, which were transcribed, resulting in constitutive production and secretion of IL-6 and IL-8 in the cell cultures. The above properties were well preserved in T-HPMC.
Fig. 3.
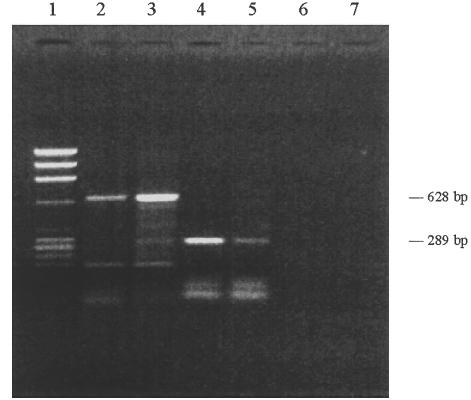
Constitutive expression of IL-6 and IL-8 mRNA. mRNA was extracted from T-HPMC and P-HPMC cultured in CM alone. RT-PCR was performed using primers specific for IL-6 or IL-8 (see Materials and methods). Samples were resolved in 1·5% agarose gel. Lane 1, DNA marker (φX174/ Hae III); Lane 2, IL-6 mRNA of T-HPMC; Lane 3, IL-6 mRNA of P-HPMC; Lane 4, IL-8 mRNA of T-HPMC; Lane 5, IL-8 mRNA of P-HPMC; Lane 6, negative control for IL-6; Lane 7, negative control for IL-8.
Modulation of IL-6 and IL-8 expression by HPMC
IL-1, TNF-α and IFN-γ, referred to as proinflammatory cytokines, and LPS, the major constituent of the cell wall of Gram-negative bacteria, are potent inducers of cytokine expressions. We first studied the effects of these cytokines/LPS on T-HPMC using two transfected cell lines designated Om-40T3 and Om-64T1 throughout the study. As shown in Fig. 4a and 4c, hIL-1β and hTNF-α stimulated IL-6 secretion significantly in a dose-dependant manner. No obvious changes in IL-6 protein production were found in cells exposed to LPS or hIFN-γ (data not shown). The minimum concentration of each cytokine required to stimulate significantly higher levels of IL-6 compared to control was 0·1 ng/ml for hIL-1β (P = 0·001) and 10 U/ml for hTNF-α (P <0·05). Maximal stimulation occurred with 1 ng/ml of hIL-1 (P <0·001) and 100 U/ml of hTNF-α (P <0·001). Similar to IL-6, the protein production of IL-8 was found to be significantly enhanced in the presence of either hIL-1β or hTNF-α in a dose-dependent fashion (Fig. 5a,c) and, similar to IL-6, was unaffected by LPS or hIFN-γ (data not shown). The minimum dose of hIL-1β and hTNF-α required to significantly up-regulate the release of IL-8 was 0·1 ng/ml for hIL-1β (P = 0·001) and 10 U/ml for hTNF-α (P = 0·001). Maximum enhancement of IL-8 secretion by T-HPMC was induced by 1 ng/ml of hIL-1β (P <0·001) and 100 U/ml of hTNF-α (P <0·001). Furthermore, both IL-6 and IL-8 mRNA increased as assessed by semiquantitative competitive PCR (Fig. 6a,c), indicating that hIL-1β and hTNF-α increased IL-6 and IL-8 gene expression in T-HPMC.
Fig. 4.
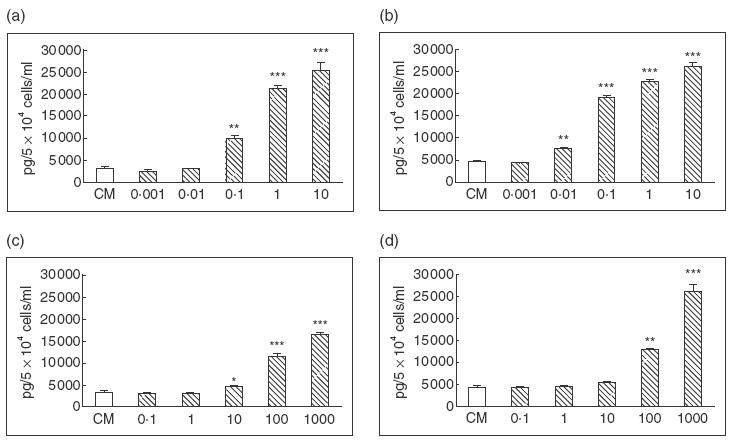
Constitutive IL-6 production and its modulation by cytokines. 5 × 104 of T-HPMC (a, c) and P-HPMC (b, d) were incubated at 37°C for 24 h in CM alone or in medium contain different doses of hIL-1β (a, b) and hTNF-α (c, d). The results were representatives of three similar experiments of two T-HPMC lines and two P-HPMC cultures, and were expressed as mean ± s.d. tested in quadruplicate. Doses of hIL-1β≥ 0·1 ng/ml (T-HPMC) and 0·01 ng/ml (P-HPMC), hTNF-α≥ 10 U/ml (T-HPMC) and 100 U/ml (P-HPMC) resulted in significantly increased IL-6 release compared to control medium. * P <0·05, ** P = 0·001, *** P <0·001.
Fig. 5.
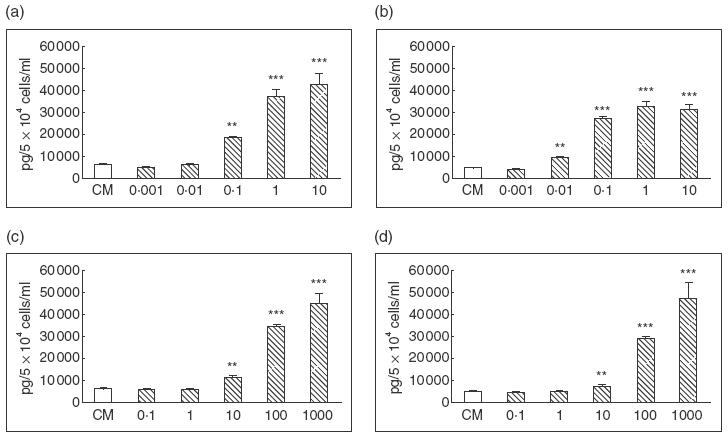
Constitutive IL-8 production and its modulation by cytokines. 5 × 104 of T-HPMC (a, c) and P-HPMC (b, d) were incubated at 37°C for 24 h in CM alone or in medium contain different doses of hIL-1β (a, b) and hTNF-α (c, d). The results were representatives of three similar experiments of two T-HPMC lines and two P-HPMC cultures, and were expressed as mean ± s.d. tested in quadruplicates. Doses of hIL-1β≥ 0·1 ng/ml (T-HPMC) and 0·01 ng/ml (P-HPMC), hTNF-α≥10 U/ml (T-HPMC and P-HPMC) resulted in significantly increased IL-8 release compared to control medium. ** P = 0·001, *** P <0·001.
Fig. 6.
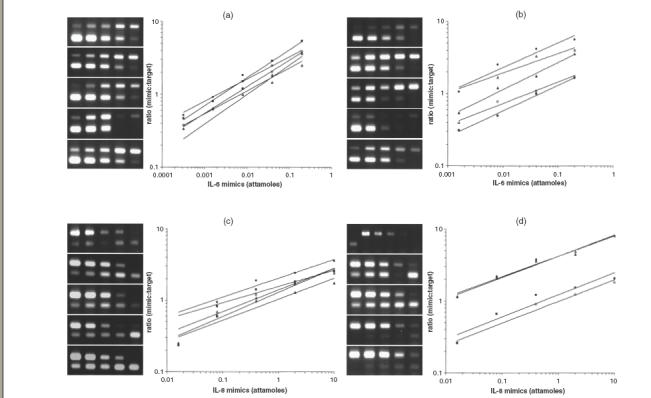
The effect of hIL-1β and hTNF-α on the expression of IL-6 (a, b) and IL-8 (c, d) mRNA by competitive PCR. A series of RT-PCR plots from ethidium bromide-stained gels shows the ratio of mimic fragment to target (y-axis) versus mimic fragment concentration (x-axis). Each gel (and each line in the graph) represents one stimulation condition (from top to bottom): no stimulation (filled circle); 1 ng/ml of hIL-1β for 4 h (open square); 1 ng/ml of hIL-1β for 24 h (filled square); 100 units/ml of hTNF-α for 4 h (open triangle); 100 units/ml of hTNF-α for 24 h (filled triangle). Each gel lane represents one PCR reaction with the highest mimic concentration at the left descending in a fivefold series to the right (cDNA amount is constant in each reaction). Target band is the upper band for IL-6, while it is the lower band for IL-8. For each cytokine, initial amount of target cDNA (in attamoles) corresponds to the mimic's amount (x-value), where target/mimic ratio (y) = 1. (a) and (c) are representatives of two experiments for T-HPMC, (b) and (d) are representatives of two experiments for P-HPMC. Note that, on the first gel lane in (d), specific IL-8 band presents when un-diluted cDNA from non-stimulated P-HPMC is amplified in the absence of IL-8 mimic; whereas no band exists when 30-fold diluted cDNA of the sample is amplified in the presence of IL-8 mimic.
As the SV40 gene is integrated randomly into the chromosome DNA, this may cause some functional changes in the resulting transfected cells. To exclude a specific effect of SV40 large-T antigen on the cytokine modulated IL-6 and IL-8 production, we repeated the experiments on P-HPMC (passage 3, from two different benign cases). Similar stimulatory effects of hIL-1β and hTNF-α on IL-6 and IL-8 expression were observed at both protein (Figs 4b,d, 5b,d) and mRNA (Fig. 6b,d) levels, indicating that IL-6 and IL-8 protein production and gene expression by P-HPMC were up-regulated by hIL-1β and hTNF-α. The minimum dose of each cytokine required to significantly stimulate the release of IL-6 and IL-8 was 0·01 ng/ml hIL-1β (P = 0·001) and 100 U/ml hTNF-α (P = 0·001) for IL-6; and 0·01 ng/ml hIL-1β (P = 0·001) and 10 U/ml hTNF-α (P = 0·001) for IL-8. Maximal stimulation occurred with 0·1 ng/ml hIL-1β and 1000 U/ml hTNF-α for IL-6 production and 0·1 ng/ml hIL-1β and 100 U/ml hTNF-α for IL-8 production. Again, LPS and hIFN-γ did not alter IL-6 or IL-8 production by P-HPMC (data not shown). The similar profiles of constitutive and cytokine modulated IL-6 and IL-8 gene expression and protein production in both T-HPMC and P-HPMC suggest strongly that SV40 transfection and gene integration had negligible effects on the cytokine encoding gene function in HPMC.
DISCUSSION
IL-6 and IL-8 production has been documented in a variety of human cell types, including haemopoietic cells [11,14], fibroblasts [27], vascular endothelial cells [28], epithelial cell lines [8,9,12,13] and endometrial glandular and endometrial stromal cells [6,15,29,30]. HPMC are a constituent of the cell suspension in peritoneal fluid associated with endometriosis and EOC-ascites but there are limited data on their immunological activity. Studies on HPMC have been hindered by their slow rate of proliferation, early senescence and the lack of established cell lines. Our results show that HPMC can be transfected by SV40 large T antigen and that these cell lines (T-HPMC) and their normal parental cells (P-HPMC) constitutively expressed IL-6 and IL-8 mRNA and protein at similar levels. These data indicate that the effect of SV40 gene integration on IL-6 and IL-8 gene expression was minimal. The P-HPMC and T-HPMC cultures had no detectable contamination by other cells, had typical morphological characteristics, co-expressed cytokeratin 8/18 and vimentin, did not express endothelial cell or monocyte/macrophage markers, confirming the cells as mesothelial cells [22].
Goodman et al. reported that mesothelial cells, obtained from the pleural effusions of patients with congestive heart failure, constitutively expressed IL-8 [18]. Lanfrancone reported that HPMC cultured in monocyte-conditioned medium spontaneously released IL-6 [19]. In our studies, and in contrast to other studies, HPMC (and T-HPMC) were cultured from normal omental tissue and maintained in unconditioned medium and without added growth factors and arguably these conditions resemble in vivo conditions more closely. We also reported that the morphology of mesothelial cells from EOC peritoneal biopsies changed, and HPMC derived from benign and malignant conditions had different morphology and growth patterns in vitro [22]. These data indicate that the source of mesothelial cells and the in vitro conditions need to be considered in the interpretation of in vitro results on mesothelial cells.
This study describes the successful establishment of HPMC lines by introduction of SV40 large T antigen into normal primary HPMC. Introduction of SV40 into primary cultures of human cells with either whole virus or plasmid DNA results in enhanced cell division and prolonged life span, and in a few cases will lead to the development of an immortalized cell line [31]. Very few mesothelial cell lines have been established and to our knowledge the method we used has not been described previously for the transfection of HPMC. Similar to reports on other human cell lines established with SV40, T-HPMC lines eventually became senescent. The growth pattern of non-proliferation after 14th to 19th passages together with the observed contact growth inhibition indicate that T-HPMC retained the differentiating characteristics of normal HPMC.
As the SV40 gene is integrated randomly into the host genome [32], it is possible that the phenotypic characteristics and endogenous functions of the transfected cells could be different from those of parental cells. We therefore compared the phenotypic and functional characteristics of T-HPMC and normal HPMC. Our data show that T-HPMC (1) stably maintained the morphologic and phenotypic features of normal mesothelial (HPMC) cells until they entered crisis; (2) proliferated more rapidly, had an extended life span and could be propagated in large numbers. Cells from three of the 11 clones did not express detectable levels of cytokeratin 8/18 and were not studied further; cells from eight clones simultaneously expressed cytokeratin 8/18 and vimentin, and preserved the typical phenotypic characteristics of normal P-HPMC (Figs 1 and 2). The expression of IL-6 and IL-8 by HPMC was then determined. All experiments were performed initially on T-HPMC and then repeated in normal P-HPMC to exclude the possible effects of SV40 large T antigen on cytokine expression. We established that normal and SV40 transfected HPMC grew in unconditioned media and without added growth factors and constituitively expressed IL-6 and IL-8.
Some proinflammatory cytokines have been shown to stimulate IL-6 and IL-8 production in many human cells in vitro [33–38]. IL-6 and IL-8 production by T-HPMC and P-HPMC was stimulated by both hIL-1β and hTNF-α. Furthermore, IL-6 and IL-8 mRNA expression was up-regulated by hIL-1β and hTNF-α as assessed by semiquantitative competitive PCR. In previous reports LPS stimulated haemopoietic cells and vascular endothelial cells to produce IL-6 and IL-8, but it showed no effect on HPMC [28,35]. Similarly, LPS did not increase IL-8 production by pleural mesothelial cells, fibroblasts and epithelial cells [18,36]. There are variable results reporting response to IFN-γ. In our study, IFN-γ did not stimulate IL-6 and IL-8 production, similar to a study on monocytes [37]. In contrast, Goodman et al. reported up-regulation of IL-8 production in human pleural mesothelial cells [18]. These varying results may reflect the culture conditions, and the functional diversity of peritoneal mesothelial cells and pleural mesothelial cells.
Endometriosis and epithelial ovarian cancer share some common clinical features. The pathogenesis of these diseases is unknown and they are characterized by a predominantly intraperitoneal spread of disease, by peritoneal deposits and by vascularization of deposits. Rarely, some patients with endometriosis will develop an uncommon type of epithelial ovarian cancer. In both diseases there are increased peritoneal fluid levels of IL-6 and IL-8, the fluids contain angiogenic factors and have angiogenic properties [33,34]. Furthermore, both IL-6 and IL-8 are expressed and released by endometrial cells and ovarian cancer cells. Our findings suggest that HPMC may represent a significant intraperitoneal source of both IL-6 and IL-8 in vivo. The observed increased peritoneal levels of IL-6 and IL-8 may be a response to implantation of endometrial glandular or stromal cells or of EOC cells, and HPMC-derived cytokines may participate in pathogenetic pathways of these diseases.
HPMC are not the sole source of the high levels of IL-6 and IL-8 in peritoneal fluids or in ascites. The other major resident cells, i.e. peritoneal monocytes or macrophages or lymphocytes and, in the case of endometriosis and ovarian cancer, endometrial glandular and stromal cells, subserosal peritoneal cells and tumour cells have also been reported to produce IL-6 and IL-8 in vitro. However, the relatively large number of HPMC compared to other resident cells in the peritoneal cavity suggests that HPMC are an important source of IL-6 and IL-8. Furthermore, the expression of IL-6 and IL-8 by HPMC increased 5–10-fold in response to IL-1β and TNF-α, two proinflammatory cytokines that have been found at high levels in the peritoneal fluid of patients with endometriosis and in ovarian cancer ascites [1–3,38. IL-1α, IL-1β and TNF-α are also variously expressed by endometrium (and by the endometrial glands and stromal cells of endometriosis) [39,40]. IL-6 has been demonstrated to be a growth factor for many tumour cell lines, including melanoma, prostate and ovarian cancer [8,41]. IL-8 also stimulates the proliferation of tumour cells [42]. Interestingly, both cytokines have been shown to regulate angiogenesis [43], which is important for the growth and dissemination of cancer. Our observations provide evidence that HPMC responded to proinflammatory cytokines in vitro and we postulate that in vivo HPMC may have an immunological function in endometriosis and EOC.
In conclusion, we established SV40 transfected HPMC cell lines which preserved the characteristics of normal HPMC, differentiated in culture, had an increased growth capacity and retained a stable phenotype up to passage 14–19. The constitutive production of IL-6 and IL-8 by normal HPMC was demonstrated and is likely to be of physiological importance. The increased production of IL-6 and IL-8 by HPMC in response to proinflammatory cytokines probably contributes to the high in vivo peritoneal levels of IL-6 and IL-8 in endometriosis and ovarian cancer, disease also characterized by high intraperitoneal levels of proinflammatory cytokines. The pathological features of endometriosis and EOC may reflect, in part, responses from HPMC.
Acknowledgments
We thank Dr J. Blake Marriott (Department of Cellular and Molecular Sciences, St George's Hospital Medical School, London, UK) for the excellent technical assistance in competitive RT-PCR analysis. We are grateful to Dr Cyril Fisher (Department of Pathology, Royal Marsden Hospital, London, UK) for his expertise in electron microscopy.
REFERENCES
- 1.Harada T, Yoshioka H, Iwabe T, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176:593–7. doi: 10.1016/s0002-9378(97)70553-2. [DOI] [PubMed] [Google Scholar]
- 2.Iwabe T, Harada T, Tsudo T, et al. Pathogenetic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1998;69:924–30. doi: 10.1016/s0015-0282(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 3.Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNF alpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10:33–41. doi: 10.1046/j.1525-1438.2000.00003.x. 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 4.Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–5. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 5.Miles SA, Rezai AR, Salazar-Gonzalez JF, et al. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci USA. 1990;87:4068–72. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rier SE, Zarmakoupis PN, Hu X, Becker JL. Dysregulation of interleukin-6 responses in ectopic endometrial stromal cells: correlation with decreased soluble receptor levels in peritoneal fluids of women with endometriosis. J Clil Endocrinol Metab. 1995;80:1431–7. doi: 10.1210/jcem.80.4.7714120. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RN, Ryan IP, Moore ES, et al. Angiogenesis and macrophage activation in endometriosis. Ann NY Acad Sci. 1997;828:194–207. doi: 10.1111/j.1749-6632.1997.tb48540.x. [DOI] [PubMed] [Google Scholar]
- 8.Watson JM, Sensintaffar JL, Berek JS, Martinez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res. 1990;50:6959–65. [PubMed] [Google Scholar]
- 9.Asschert JG, Vellenga E, Ruiters MH, de Vries EG. Regulation of spontaneous and TNF/IFN-induced IL-6 expression in two human ovarian-carcinoma cell lines. Int J Cancer. 1999;82:244–9. doi: 10.1002/(sici)1097-0215(19990719)82:2<244::aid-ijc15>3.0.co;2-n. 10.1002/(SICI)1097-0215(19990719)82:2244::AID-IJC153.3.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis [see comments] Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 12.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 13.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 14.Navarro S, Debili N, Bernaudin JF, Vainchenker W, Doly J. Regulation of the expression of IL-6 in human monocytes. J Immunol. 1989;142:4339–45. [PubMed] [Google Scholar]
- 15.Piva M, Horowitz GM, Sharpe-Timms KL. Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotis cells in vitro: a model for endometrial–peritoneal interaction in endometriosis. J Clin Endocrinol Metab. 2001;86:2553–61. doi: 10.1210/jcem.86.6.7613. [DOI] [PubMed] [Google Scholar]
- 16.Shostak A, Chakrabarti E, Hirszel P, Maher JF. Effects of histamine and its receptor antagonists on peritoneal permeability. Kidney Int. 1988;34:786–90. doi: 10.1038/ki.1988.250. [DOI] [PubMed] [Google Scholar]
- 17.Muijsken MA, Heezius HJ, Verhoef J, Verbrugh HA. Role of mesothelial cells in peritoneal antibacterial defence. J Clin Pathol. 1991;44:600–4. doi: 10.1136/jcp.44.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman RB, Wood RG, Martin TR, Hanson-Painton O, Kinasewitz GT. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol. 1992;148:457–65. [PubMed] [Google Scholar]
- 19.Lanfrancone L, Boraschi D, Ghiara P, et al. Human peritoneal mesothelial cells produce many cytokines (granulocyte colony-stimulating factor [CSF], granulocyte-monocyte-CSF, macrophage- CSF, interleukin-1 [IL-1], and IL-6) and are activated and stimulated to grow by IL-1. Blood. 1992;80:2835–42. [PubMed] [Google Scholar]
- 20.Douvdevani A, Rapoport J, Konforty A, Argov S, Ovnat A, Chaimovitz C. Human peritoneal mesothelial cells synthesize IL-1 alpha and beta. Kidney Int. 1994;46:993–1001. doi: 10.1038/ki.1994.359. [DOI] [PubMed] [Google Scholar]
- 21.Kimura A, Koga S, Kudoh H, Iitsuka Y. Peritoneal mesothelial cell injury factors in rat cancerous ascites. Cancer Res. 1985;45:4330–3. [PubMed] [Google Scholar]
- 22.Zhang XY, Pettengell R, Nasiri N, Kalia V, Dalgleish AG, Barton DPJ. Characteristics and growth patterns of human peritoneal mesothelial cells: comparison between advanced epithelial ovarian cancer and non- ovarian cancer sources. J Soc Gynecol Invest. 1999;6:333–40. doi: 10.1016/s1071-5576(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 23.Connell ND, Rheinwald JG. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983;34:245–53. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- 24.Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1:327–41. [PubMed] [Google Scholar]
- 25.Dong Y, Skoultchi AI, Pollard JW. Efficient DNA transfection of quiescent mammalian cells using poly-L-ornithine. Nucl Acids Res. 1993;21:771–2. doi: 10.1093/nar/21.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marriott JB, Westby M, Cookson S, et al. CC-3052 : a water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-alpha production. J Immunol. 1998;161:4236–43. [PubMed] [Google Scholar]
- 27.Zhang YH, Lin JX, Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol. 1990;10:3818–23. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strieter RM, Kunkel SL, Showell HJ, et al. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989;243:1467–9. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- 29.Akoum A, Lawson C, McColl S, Villeneuve M. Ectopic endometrial cells express high concentrations of interleukin (IL)-8 in vivo regardless of the menstrual cycle phase and respond to oestradiol by up-regualting IL-1-induced IL-8 expression in vitro. Mol Hum Reprod. 2001;7:859–66. doi: 10.1093/molehr/7.9.859. [DOI] [PubMed] [Google Scholar]
- 30.Bergqvist A, Bruse C, Carlberg M, Carlstrom K. Interleukin 1β, interleukin-6 and tumor necrosis factor-α in endometriotic tissue and in endometrium. Fertil Steril. 2001;75:489–95. doi: 10.1016/s0015-0282(00)01752-0. [DOI] [PubMed] [Google Scholar]
- 31.Shay JW, Van der Haegen BA, Ying Y, Wright WE. The frequency of immortalisation of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52. doi: 10.1006/excr.1993.1283. [DOI] [PubMed] [Google Scholar]
- 32.Hara H, Kaji H. Random integration of SV40 in SV40-transformed, immortalized human fibroblasts. Exp Cell Res. 1987;168:531–8. doi: 10.1016/0014-4827(87)90025-5. [DOI] [PubMed] [Google Scholar]
- 33.Maas JWM, Calhaz-Jorge C, ter Riet G, et al. Tumor necrosis factor-α but not interleukin-1β or interleukin-8 concentrations correlate with angiogenic activity of peritoneal fluid from patients with minimal to mild endometriosis. Fertil Steril. 2001;75:180–5. doi: 10.1016/s0015-0282(00)01656-3. [DOI] [PubMed] [Google Scholar]
- 34.Barton DPJ, Cai A, Wendt K, Young M, Gamero A, DeCesare S. Angiogenic protein expression in advanced epithelial ovarian cancer. Clin Cancer Res. 1997;3:1579–86. [PubMed] [Google Scholar]
- 35.Sylvester I, Rankin JA, Yoshimura T, Tanaka S, Leonard EJ. Secretion of neutrophil attractant/activation protein by lipopolysaccharide-stimulated lung macrophages determined by both enzyme-linked immunosorbent assay and N-terminal sequence analysis. Am Rev Respir Dis. 1990;141:683–8. doi: 10.1164/ajrccm/141.3.683. [DOI] [PubMed] [Google Scholar]
- 36.Thornton AJ, Strieter RM, Lindley I, Baggiolini M, Kunkel SL. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL- 8 in human hepatocytes. J Immunol. 1990;144:2609–13. [PubMed] [Google Scholar]
- 37.Cluitmans FH, Esendam BH, Landegent JE, Willemze R, Falkenburg JH. Regulatory effects of T cell lymphokines on cytokine gene expression in monocytes. Lymph Cytokine Res. 1993;12:457–64. [PubMed] [Google Scholar]
- 38.Mori H, Sawairi M, Makagawa M, et al. Peritoneal fluid interleukin 1-beta and tumor necrosis factor in patients with benign gynecologic disease. Am J Reprod Immunol. 1991;26:62–7. doi: 10.1111/j.1600-0897.1991.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 39.Hunt JS, Chen H-L, Hu X-L, Tabibzadeh S. Tumor necrosis factor messenger ribonucleic acid and protein in human endometrium. Biol Reprod. 1992;47:141–7. doi: 10.1095/biolreprod47.1.141. [DOI] [PubMed] [Google Scholar]
- 40.Giudice LC. Growth factors and growth modulators in human uterine endometrium: their potential relavance to reproductive medicine. Fertil Steril. 1994;61:1–17. doi: 10.1016/s0015-0282(16)56447-4. [DOI] [PubMed] [Google Scholar]
- 41.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42:239–42. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. 10.1002/(SICI)1097-0045(20000215)42:3239::AID-PROS103.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor [published erratum appears in J Immunol 1994 October 1; 153(7): 3360] J Immunol. 1993;151:2667–75. [PubMed] [Google Scholar]
- 43.Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA. 1990;87:3092–6. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]


