Abstract
It is established that veterans of the 1991 Gulf War have an increased frequency of experiencing multiple symptoms. The underlying mechanism of these ailments is unclear, although they do not correspond to any clearly defined syndrome. The most common symptoms overlap with those of chronic fatigue syndrome (CFS). CFS was recently associated with a novel subtype of antinuclear autoantibody (ANA) that reacts with nuclear envelope (NE) antigens. NE autoantibodies are not known to be linked with any distinct clinical condition, but have been observed in patients with unusual mixed chronic autoimmune disorders and connective tissue diseases. In this study we examined whether NE ANAs are a feature of patients with CFS and symptomatic Gulf War veterans (sGWV). We studied the prevalence of ANA in 130 sGWV, 90 well Gulf War veterans (wGWV), 128 symptomatic Bosnia and Era veterans (sBEV), 100 CFS patients, and 111 healthy control subjects matching for age and sex. We found no significant difference in the prevalence of ANAs between any of the groups. None of the patients/or veterans we studied had ANA of the NE type. Our results show that multisymptom illness due to CFS or related to Gulf War service is not associated with antinuclear autoimmunity.
Keywords: antinuclear autoantibodies, Gulf War related illness, chronic fatigue syndrome, nuclear envelope autoantibodies
INTRODUCTION
Ill health in Gulf War veterans has attracted a great deal of attention, and has been termed ‘Gulf War Syndrome’ (GWS). There is no sound evidence for a new syndrome. Similar to veterans of previous conflicts, particularly the Vietnam War, where a detailed study of veterans was undertaken, Gulf veterans do report multiple symptoms more frequently than non-Gulf veterans [1]. The aetiology of ill health in Gulf veterans remains controversial. There are a number of proposed explanations for ill health in Gulf veterans: chemical weapons [2–4], post-traumatic stress disorder [5], or that the vaccination schedule designed to protect against exposure to biological weapons could have caused dysregulation in the immune system [6].
Some of the symptoms reported in veterans are similar to those found in various autoimmune conditions. These include joint and muscle aches, chronic fatigue, rashes and sleep disorders. Serological abnormalities have also been reported including hypergammaglobulinemia and abnormal serum proteins [7]. These symptoms overlap with those of chronic fatigue syndrome (CFS), and hence it is not surprising that some Gulf War veterans reporting illness fulfil criteria for symptom-based definition of chronic fatigue syndrome [8,9].
CFS is characterized by severe, disabling fatigue and other symptoms, including musculoskeletal pain, sleep disturbance, impaired concentration, and headaches [10–12]. The cause of CFS is poorly understood. Currently no diagnostic test exists for CFS, which can only be diagnosed after other medical and psychiatric causes have been excluded.
A recent report [13] has suggested that CFS is associated with a substantial increase in the prevalence of a subtype of antinuclear antibody (ANA), namely autoantibodies to nuclear envelope (NE) antigens. This is an uncommon form of ANA, which to date has only been associated, albeit rarely, with unusual mixed, chronic autoimmune disorders including primary biliary cirrhosis, chronic hepatitis, primary Sjö gren's syndrome, systemic rheumatic diseases, systemic lupus erythematosus (SLE) and variably with vasculitis, thrombocytopenia, and Raynaud's phenomen [14–19], in each of which it is a rare finding. It has been suggested that such autoantibodies might be one consequence of prolonged immune activation, claimed to be a feature of both CFS and Gulf War-related illness [20,21]. Equally, it has been proposed that environmental toxins may elicit NE ANAs.
In the present report, we study large cohorts comprising epidemiologically derived symptomatic and asymptomatic Gulf War veterans, appropriate military controls, symptomatic civilians fulfilling criteria for CFS, and healthy civilians, in order to test the hypothesis that autoantibodies to NE antigens are a feature of CFS and illness associated with Gulf War service.
MATERIALS AND METHODS
Study groups
The serum samples for this study were drawn from a subset of patients in a previously reported large Phase I epidemiological study of Gulf War veterans [1,22,23]. We now report a nested case control study randomly selected from the larger cohort, in which cases were symptomatic Gulf War veterans, compared to both well Gulf war veterans, and symptomatic military personnel who had served in either Bosnia or were nondeployed military personnel (Era) during the same time frame. The case definition for the symptomatic Gulf War veterans included reporting two or more symptoms and a score of less than 72·2 on the Short Form (SF)-36 Physical Functioning (PF) subscale (i.e. lower than the 10th centile of the SF-36 PF in the baseline control, group).
A total of 348 serum samples from servicemen and servicewomen (342 males, 6 females; age range 25–59 years) were tested blind to status. Amongst these were 130 symptomatic Gulf War Veterans (sGWV), 90 well Gulf War Veterans (wGWV) and 128 symptomatic military personnel, either veterans of the Bosnia conflict or nondeployed (Era) controls (sBEV). In addition, we studied 100 civilian-CFS patients (47 male, 53 female; age range 18–57 years) that were referred to our specialist clinic and satisfied the standard Center for Disease Control criteria for a diagnosis of CFS [10].
Finally we tested 111 serum samples from control subjects (blood donors of the South-east Thames Blood Transfusion Service); 49 were male and 62 female and the age range was 18–57 years. These control subjects were matched with the CFS sample for age and sex demographics. However, since veterans are predominantly male, the control group for this comparison comprised all male control subjects and one female selected at random.
Serum from a patient with SLE known to be positive for NE ANA was aliquoted and stored at −80°C and used as a positive control for indirect immunofluorescence screening.
In summary therefore, we report a unique study that includes epidemiologically derived symptomatic and asymptomatic Gulf War veterans, appropriate military controls, symptomatic civilians fulfilling criteria for CFS, and normal civilians. Ethical permission for this study was obtained from the King's NHS Trust Ethical Committee.
Indirect immunofluorescence
Anti-nuclear autoantibodies were detected by indirect immunofluorescent (IIF) microscopy using commercially available Hep-2 slides (Binding Site, Birmingham, UK). IIF was performed according to the protocol suggested by the manufacturer. In brief, the slides were incubated with the human sera at 1 : 40 dilution in PBS, pH 7·2 for 30 min in a humid chamber at room temperature. After washing (3 times) with PBS, the autoantibodies were revealed using rabbit antihuman IgG conjugated with FITC (Dako Ltd, Ely, UK) in 1 : 50 dilution and incubation was carried for another 30 min at room temperature in the dark. The slides were then washed 3 times and examined under a fluorescence microscope (Olympus BX40, Olympus, Southall, UK). In each assay negative control sera as well as positive control sera were always included. Slides were examined independently by three of the authors (AS, ES and ETD, of which ETD has in excess of 20 years experience of diagnostic IIF assays) who were blinded to the source of the samples. Samples testing positive for ANA were titrated in doubling dilutions to end-point extinction.
Statistical analysis
The distribution of ANA amongst the different study groups was compared by Fisher exact probability test using 2 × 2 tables.
Results
Antinuclear antibodies detected using indirect immunofluorescence (IIF) microscopy
We detected ANA in all of the study groups, with several different patterns of staining being observed (Table 1). The sGWV group had 8% positives (10/130), amongst which the ANA patterns were mainly homogeneous and speckled (7/10), or nuclear/nuclear dots (3/10). wGWV had 3% positives (3/90) of whom two out of three had a nuclear staining pattern and one speckled. The sBEV group had 9% positives (11/128) of which nine were homogeneous and speckled, and two had nuclear/nuclear dots. The results were compared with 51 age and sex matched healthy controls. This group had 12% (6/51) positive results (various patterns).
Table 1.
Antibody staining patterns in study groups
| Patterns | Homogeneous | Speckled | Nucleolar | Nucleolar dots | Nucleolar membrane | Speckled/ nucleolar | Nucleolar/ homogeous | Total [sum of positives (%)] |
|---|---|---|---|---|---|---|---|---|
| Study groups | ||||||||
| sGWV (n = 130) | 4 | 3 | 2 | 1 | 10 (8%) | |||
| wGWV (n = 90) | 1 | 2 | 3 (3%) | |||||
| sBEV (n = 128) | 7 | 2 | 1 | 1 | 11 (9%) | |||
| HC group A (for GW group) | 2 | 2 | 2 | 6 (12%) | ||||
| (n = 51) | ||||||||
| CFS (n = 100) | 9 | 7 | 1 | 1 | 18 (18%) | |||
| HC group B (for CFS) (n = 111) | 7 | 6 | 4 | 1 | 18 (16%) | |||
sGWV, sick Gulf War veterans; wGWV, well Gulf War veterans; sBEV, sick Bosnia and Era veterans; CFS, Chronic Fatigue Syndrome patients; HC, Healthy controls.
We found no statistical difference in the frequency and prevalence of ANA amongst the veterans study groups when comparing with each other or with matching healthy control subjects (Table 2).
Table 2.
Results for statistical comparison (Fisher exact probability test)all study groups and appropriate control groups (all P > 0·05)
| P-value | Unadjasted odds ratio | 95% CI | |
|---|---|---|---|
| sGWV versus wGWV | 0·25 | 2·41 | 0·6, 9·0 |
| sGWV versus sBEV | 0·82 | 0·89 | 0·4, 2·2 |
| sGWV versus HC | 0·39 | 0·62 | 0·2, 1·8 |
| CFS versus HC | 0·86 | 1·01 | 0·5, 2·3 |
CFS patients had a similar prevalence of ANA (18%, 18/100) to the matched healthy control subjects (16%, 18/111). In the CFS group the positive samples were mainly of speckled pattern (9/18); some had the nuclear pattern (7/18) and the rest had other patterns (2/18). 59% of the positive results were found in female CFS patients.
In the matching healthy control subjects, amongst various patterns, we found one individual with a pattern characteristic of NE antigens (Fig. 1) and 67% of the positive results were female subjects.
Fig. 1.
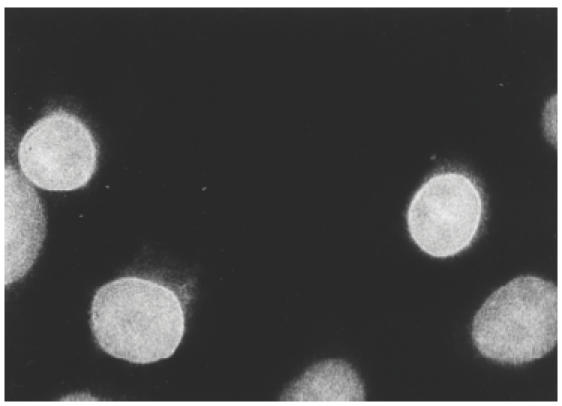
Photomicrograph of indirect immunofluorescence staining pattern of autoantibodies to nuclear envelope (NE) antigens, demonstrated by characteristic peri-nuclear ring pattern. Substrate, Hep2 cells. Original magnification × 40.
To examine whether the strength of positivity of ANA differed between study groups, positive samples were titrated to extinction. None of the ANAs tested were positive beyond a dilution of 1/160. In each of the study groups, approximately half of the positive samples had titres of 1/80 and half of 1/160. There was no significant difference in the distribution of high and low ANA titres between any of the study groups.
DISCUSSION
Since the Persian Gulf conflict ended in 1991 there has been considerable interest in the increased number of Persian Gulf War veterans from coalition forces reporting unexplained illness. Many hypotheses have been proposed to explain this phenomenon [2–7,24, but no single explanation accounts satisfactorily for the symptoms. In the present study, we sought evidence for the presence of ANAs in patients with Gulf War-related illness. Our study was prompted by three considerations. First, many of the symptoms experienced by Gulf War veterans are similar to those experienced by patients with CFS [24]. The aetiology of CFS is also unknown, but a recent report has suggested that CFS patients have a high prevalence of a rare ANA, reactive with nuclear envelope antigens and giving a characteristic ring pattern on immunofluorescence with Hep-2 cells as substrate [13]. For this reason we sought evidence of a serological link between CFS and Gulf-War related illness.
Our second consideration was that many of the symptoms reported by Gulf-War veterans are reminiscent of those seen in the nonorgan-specific autoimmune diseases. These disorders are characterized by a high prevalence and wide variety of different autoantibodies to nuclear antigens, giving rise to numerous patterns of immunofluorescence on Hep-2 cells and numerous molecularly defined nuclear antigens [14,16–18,25]. In the present study we sought evidence of a similar, widespread manifestation of antinuclear autoimmunity.
Our third reason for carrying out the study was to examine whether there was serological evidence, in the form of multiple autoantibodies, to support the proposal that both CFS and Gulf-War related illness exhibit features of a T helper-2 (Th2) type of polarized immune response. It has been suggested that Gulf War veterans were exposed to stimuli that would strongly favour a T helper 2-biased (Th2) cellular immune response [6], such as:
multiple vaccinations received in a short space of time (large antigen load can deviate the immune response towards Th2 [26,27];
vaccines, including those against biological warfare agents, given in stressful active service conditions (high levels of stress have been shown to deviate the immune response towards Th2 [28–31]);
pertussis toxin used as an adjuvant by United Kingdom forces in the Gulf (which causes Th2 deviation [32,33]).
Antibody production by B lymphocytes is mainly promoted and supported by the Th2 cytokines IL-4, IL-6 and IL-10 [34], and in the context of an autoimmune response, production of autoantibodies may be considered to represent evidence of a shift towards Th2 type immunity [35–37].
Our results demonstrate that there is no increase in prevalence of ANA in either patients with CFS or those with Gulf War-related illness compared with control subjects. None of the patterns of ANA seen were especially characteristic of either condition. In addition, we did not detect any NE patterns of ANA.
These results differ substantially from those reported by Konstantinov et al. who found the sera of 52% of the CFS patients' reacted with nuclear envelope antigens by IIF [13]. As far as could be ascertained the protocols were identical other than that Konstantinov et al. [13] used a different source of Hep-2 slides to those employed in our study. It is conceivable that the results could have arisen as a consequence of differences in sensitivity, although we were able to detect other ANAs and one NE pattern in a control subject. Another difference is the higher percentage of female CFS subjects used in that study (85%), which is greater than in our study (53%). It is known that the general prevalence of autoantibodies is greater in female population, as shown in the healthy control subjects in the present study (67%), and this could account for the discrepancy, but not for the lack of association with either CFS or Gulf War related illness.
Nishikai et al. [38] also failed to detect autoantibodies reactive with nuclear membrane components by IIF amongst Japanese CFS patients, seeing instead a predominant ‘diffuse/speckled’ pattern on Hep-2 cells.
Another study [39] detected 15% ANA positive cases amongst CFS patients. In that paper they combined the results from two different chronic fatigue clinics (Boston and Seattle) in which there were differences in means of recruitment of CFS patients (different method of prescreening) as well as significantly varying results for ANA (Boston −8%; Seattle −20%).
Two additional studies found some evidence of ANA prevalence (50%), in Japanese children with nonspecific chronic symptoms similar to CFS (diagnosed as ‘autoimmune fatigue syndrome’ in these studies) [40,41]. Although no studies have been carried out previously on autoimmune phenomena in war veterans, extensive analysis of white blood cell number and phenotype, and serum immunoglobulin levels, revealed no abnormality in veterans of the Vietnam War [42].
We conclude that patients' multisymptom illness due to CFS (seen in our specialist clinic) and illness related to Gulf War service are not associated with increased frequency of antinuclear autoimmunity.
Acknowledgments
We are grateful to the Linbury Trust, which supported this study. Specimen collection and storage from Gulf War veterans was supported by the Medical Research Council (MRC). The US Department of Defense and MRC funded the epidemiological study of Gulf War-related illness at GKT School of Medicine. We also thank Dorothy Blair from the Gulf War Illness Research Unit, Guy's, King's & St Thomas' School of Medicine, London, for help in sample collection and Dedra Buchwald for valuable comments on the manuscript.
REFERENCES
- 1.Unwin C, Blatchley N, Coker W, et al. Health of UK servicemen who served in the Persian Gulf War. Lancet. 1999;353:169–78. doi: 10.1016/S0140-6736(98)11338-7. [DOI] [PubMed] [Google Scholar]
- 2.Persian Gulf Veterans Coordinating Board. Unexplained illnesses among Desert Storm veterans. A search for causes, treatment, and cooperation. Arch Inter Med. 1995;155:262–8. doi: 10.1001/archinte.155.3.262. [DOI] [PubMed] [Google Scholar]
- 3.Haley R, Kurt TL. Self reported exposure to neurotoxic chemical combinations in the Gulf War. A cross-sectional epidemiological study. J Am Assoc. 1997;277:231–7. [PubMed] [Google Scholar]
- 4.Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL. Neurotoxicity resulting from coexposure to pyridostygmine bromide, deet and permethrine: Implications of Gulf War chemical exposure. J Toxicol Environ Health. 1996;48:35–56. doi: 10.1080/009841096161456. [DOI] [PubMed] [Google Scholar]
- 5.Hyams KC, Wignall S, Roswell R. War syndromes and their evaluation: From the U.S. Civil War to the Persian Gulf War. Ann Inter Med. 1996;125:398–405. doi: 10.7326/0003-4819-125-5-199609010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Rook G, Zumla A. Gulf war syndrome: is it due to a systemic shift in cytokine balance towards a Th2 profile? Lancet. 1997;349:1831–3. doi: 10.1016/S0140-6736(97)01164-1. [DOI] [PubMed] [Google Scholar]
- 7.Grady EP, Carpenter M, Koening C, Older S, Battafarano DF. Rheumatic findings in Gulf War veterans. Arch Intern Med. 1998;158:367–71. doi: 10.1001/archinte.158.4.367. [DOI] [PubMed] [Google Scholar]
- 8.Black D, Doebbeling B, Voelker M, et al. Multiple chemical sensitivity syndrome. symptom prevalence and risk factors in a military population. Arch Intern Med. 2000;160:1169–76. doi: 10.1001/archinte.160.8.1169. [DOI] [PubMed] [Google Scholar]
- 9.Reid S, Hotopf M, Hull L, Ismail K, Unwin C, Wessely S. Multiple chemical sensitivity and chronc fatigue syndrome in UK Gulf War veterans. Am J Epidemiol. 2001;153:604–9. doi: 10.1093/aje/153.6.604. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins J, Komaroff A The International Chronic Fatigue Syndrome Study Group. The chronic fatigue syndrome. a comprehensive approach to its definition and study. Ann Inter Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe M, Archard LC, Banatvala JE, et al. A report – chronic fatigue syndrome: guidelines for research. J R Soc Med. 1991;84:118–21. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessely S, Chalder T, Hirsch S, Wallace P, Wright D. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health. 1997;87:1449–55. doi: 10.2105/ajph.87.9.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinov K, von Mikecz A, Buchwald D, Jones J, Gerace L, Tan EM. Autoantibodies to Nuclear Envelope Antigens in Chronic Fatigue Syndrome. J Clin Invest. 1996;98:1888–96. doi: 10.1172/JCI118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesher G, Margalit R, Ashkenazi YJ. Anti-Nuclear Envelope Antibodies. Clin Assocs Sem Arth Rheum. 2001;30:313–20. doi: 10.1053/sarh.2001.20266. [DOI] [PubMed] [Google Scholar]
- 15.McKeon FD, Tuffanelli DL, Fukuyama K, Kirschner MW. Autoimmune response directed against conserved determinants of nuclear envelope proteins in a patient with linear scleroderma. Proc Natl Acad Sci USA. 1983;80:4374–8. doi: 10.1073/pnas.80.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassoued K, Guilly M-N, Danon F, et al. Antinuclear autoantibodies specific for lamins. Ann Int Med. 1988;108:829–33. doi: 10.7326/0003-4819-108-6-829. [DOI] [PubMed] [Google Scholar]
- 17.Worman HJ, Courvalin J-C. Autoantibodies against nuclear envelope proteins in liver disease. Hepatology. 1991;14:1269–79. [PubMed] [Google Scholar]
- 18.Reeves WH, Chaudhary N, Salerno A, Blobel G. Lamin B autoantibodies in sera of certain patients with systemic lupus erythermatosus. J Exp Med. 1987;165:750–62. doi: 10.1084/jem.165.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyachi K, Shibata M, Onozuka Y, Kikuchi F, Imai N, Horigome T. Primary biliary cirrhosis sera recognize not only gp210 but also proteins of the p62 complex bearing N-acelylglucosamine residues from rat liver nuclear envelope. Mol Biol Rep. 1996;23:227–34. doi: 10.1007/BF00351173. [DOI] [PubMed] [Google Scholar]
- 20.Landay A, Jessop C, Lennette E, Levy J. Chronic fatigue syndrome: clinical condition associated with immune activation. Lancet. 1991;338:707–12. doi: 10.1016/0140-6736(91)91440-6. [DOI] [PubMed] [Google Scholar]
- 21.Patarca R, Mark T, Fletcher M, Klimas N. Review. Immunology of chronic fatigue syndrome. J Chronic Fatigue Syndrome. 2000;6:69–107. [Google Scholar]
- 22.Hotopf M, David A, Hull L, Ismail K, Unwin C, Wessely S. Role of vaccinations as risk factors for ill health in veterans of the Gulf war: cross sectional study. B M J. 2000;320:1363–7. doi: 10.1136/bmj.320.7246.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail K, Everitt B, Blatchley N, Hull L, Unwin C, David A, Wessely S. Is there a Gulf war syndrome? Lancet. 1999;353:179–82. doi: 10.1016/S0140-6736(98)11339-9. [DOI] [PubMed] [Google Scholar]
- 24.Wessely S. Ten Years On, What Do We Know About the Gulf War Syndrome? Clin Med (JRCPL) 2001;1:28–37. doi: 10.7861/clinmedicine.1-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstantinov K, Halberg P, Wiik A, Hoier-Madsen M, Wantzin P, Ullman S, Galcheva-Gargova Z. Clinical manifestation in patients with autoantibodies specific for nuclear lamin proteins. Clin Immunol Immunopathol. 1992;62:112–8. doi: 10.1016/0090-1229(92)90030-r. [DOI] [PubMed] [Google Scholar]
- 26.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes ‘susceptible’ mice resistant to Leishmania major. Science. 1992;257:539–42. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Pando R, Rook GA. The role of TNFα in T cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CY, Sarfati M, Heusser C, Fournier S, Rubio-Trujillo M, Peleman R, Delespesse G. Glucocorticoids increase the synthesis of immunoglobulin E by interleukin-4 stimulate human lymphocytes. J Clin Invest. 1991;87:870–7. doi: 10.1172/JCI115092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkman V, Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J Immunol. 1995;155:3322–8. [PubMed] [Google Scholar]
- 30.Padgett DA, Sheridan JF, Loria R. Steroid hormone regulation of a polyclonal Th2 immune response. Ann NY Acad Sci. 1995;774:323–5. doi: 10.1111/j.1749-6632.1995.tb17398.x-i1. [DOI] [PubMed] [Google Scholar]
- 31.Ramierz F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406–12. [PubMed] [Google Scholar]
- 32.Munoz JJ, Peacock MG. Action of pertussigen (pertussis toxin) on serum IgE and on Fce receptors on lymphocytes. Cell Immunol. 1990;127:327–36. doi: 10.1016/0008-8749(90)90136-f. [DOI] [PubMed] [Google Scholar]
- 33.Mu HH, Sewell WA. Enhancement of interleukin-4 production by pertussis toxin. Infect Immun. 1993;61:2834–40. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker DC. T cell-dependent B cell activation. Ann Rev Immunol. 1993;11:331–60. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 35.Tomer Y, Barak V, Gilburd B, Shoenfield Y. Cytokines in experimental autoimmune vasculitis: evidence for a Th2 type response. Clin Exp Rheumatol. 1999;17:521–6. [PubMed] [Google Scholar]
- 36.Ramanathan S, de Kozak Y, Saoudi A, Goureau O, Van der Meide PH, Druet P, Bellon B. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J Immunol. 1996;157:2209–15. [PubMed] [Google Scholar]
- 37.Florquin S, Amraoui Z, Goldman M. Persistent production of Th2-type cytokines and polyclonal B cell activation after chronic administration of staphylococcal enterotoxin B in mice. J Autoimmun. 1996;9:609–15. doi: 10.1006/jaut.1996.0080. 10.1006/jaut.1996.0080. [DOI] [PubMed] [Google Scholar]
- 38.Nishikai M, Tomomatsu S, Hankins RW, Takagi S, Miyachi K, Kosaka S, Akiya K. Autoantibodies to a 68/48 kDa protein in chronic fatigue syndrome and primary fibromyalgia: a possible marker for hypersomnia and cognitive disorders. Reumathol. 2001;40:806–10. doi: 10.1093/rheumatology/40.7.806. [DOI] [PubMed] [Google Scholar]
- 39.Bates DW, Buchwald D, Lee J, et al. Clinical Laboratory Test Findings in Patients With Chronic Fatigue Syndrome. Arch Inter Med. 1995;155:97–103. [PubMed] [Google Scholar]
- 40.Itoh Y, Hamada H, Imai T, Seki T, Igarashi T, Yuge K, Fukunaga Y, Yamamoto M. Antinuclear antibodies in children with chronic nonspecific complaints. Autoimmunity. 1997;25:243–50. doi: 10.3109/08916939708994733. [DOI] [PubMed] [Google Scholar]
- 41.Itoh Y, Igarashi T, Tatsuma N, Imai T, Yoshida J, Tsuchiya M, Murakami M, Fukunaga Y. Immunogenetic background of patients with autoimmune fatigue syndrome. Autoimmunity. 2000;32:193–7. doi: 10.3109/08916930008994092. [DOI] [PubMed] [Google Scholar]
- 42.Centres for Disease Control Vietnam Experience Study. Health status of Vietnam veterans. II. Physical health. J Am Med Assoc. 1988;259:2708–14. [PubMed] [Google Scholar]


