Abstract
Metallothionein is a low molecular weight, cysteine-rich, stress response protein that can act as an antioxidant and as an immunosuppressive agent in instances of antigen-dependent adaptive immunity. In this context, we assessed the therapeutic potential and mechanisms of action of metallothionein in a collagen-induced arthritis model. Repeated administration of metallothionein-I + II during the course of disease dramatically reduced the incidence and severity of the disease. Joint tissues isolated from boostered paws of metallothionein-I + II-treated mice expressed significantly reduced levels of proinflammatory mediators, such as tumour necrosis factor (TNF)-α and cyclooxygenase-2, when compared with those of control-treated mice. Lymph node cells obtained from metallothionein-I + II -injected mice exhibited a significant decrease in the proliferative response and a remarkable increase in tumour growth factor (TGF)-β production in response to type II collagen. Taken together, these results suggest that metallothionein-I + II promote the development of type II collagen-specific, TGF-β-producing cells to antagonize the expansion of arthritogenic cells. This could lead to local suppression of inflammatory responses by inhibiting the expression of proinflammatory molecules. Thus, this study demonstrates the suppressive effects of metallothionein on collagen-induced arthritis, and indicates that there may be a potential therapeutic application for manipulation of metallothionein during the treatment of autoimmune disorders.
Keywords: metallothionein, collagen-induced arthritis, autoimmune disease, TGF-β
INTRODUCTION
Metallothioneins are a family of low molecular weight (about 7 kD), cysteine-rich, stress response proteins that are associated with transition metal cations including Zn, Cd, Cu, and Hg [1]. Among four isoforms of these proteins identified to date, metallothionein-I and -II are readily inducible in most tissues by exposure to a variety of cellular stressors, such as heavy metal cations, reactive oxygen species, bacterial toxins, and some of the acute phase cytokines [2–4]. Metallothionein-I and -II have been implicated in the protection of essential cellular components from a wide variety of toxicants by binding and sequestering these toxicants [5,6]. Following induction, metallothionein can be released to the extracellular environment via unknown mechanisms, as metallothionein has been found at significant levels in extracellular compartments, such as blood, urine, liver sinusoids, renal tubule lumina, bronchoalveolar spaces, and pancreatic ducts [7–10]. Identification of a metallothionein-specific receptor on astrocytes suggests that one way in which extracellular metallothionein may evoke physiologic changes is by ligand–like interactions with target cells [11]. Although the significance of extracellular metallothionein is not fully understood, several lines of evidence suggest that extracellular metallothionein is intimately involved in the regulation of the immune response. Metallothionein can interact with cells of the immune system and can modify the functional activities of macrophages and T and B lymphocytes [12–15]. An observation complementary to the reports of exogenous metallothionein-mediated suppression of immune function is the finding that animals with targeted gene disruptions of the adjacent Mt1 and Mt2 genes display a significant improvement in the humoral response to antigen challenge [16], and that monoclonal antibody to metallothionein can enhance those same responses [13].
Elevated levels of metallothionein have been found to be associated with certain pathogenic stages of several immune disorders, including rheumatoid arthritis (RA) [17,18], experimental autoimmune encephalomyelitis (EAE) [19], and an animal model of severe inflammatory autoimmune disease (viable motheaten, Hcphmev) [20]. Intriguingly, the serum concentrations of metallothionein have been reported to be depleted as RA progresses, and could be significantly enhanced by cortisone treatment which subsequently results in significant clinical improvement [21]. Targeted gene disruptions of the Mt1 and Mt2 genes have been found to elicit much more severe autoimmune diseases in viable motheaten mice [20] and EAE-established mice [22]. These observations allow us to speculate that metallothionein may play a critical regulatory role in the pathologic status of these diseases.
RA is a chronic inflammatory autoimmune disease, characterized by the development of pathogenic type 1-helper T (Th1) cells and subsequent inflammatory responses evident in multiple joints [23]. To assess capacity of metallothionein to affect development of RA in vivo, we used an extensively studied collagen-induced arthritis (CIA) model to elucidate pathogenic and therapeutic mechanisms that may be relevant to human RA [24]. Murine CIA is induced in genetically susceptible DBA/1 mice by immunization with bovine type II collagen (CII) and resembles RA in that both cellular and humoral mechanisms are involved in the pathogenic process. Leukocytes that migrate into joints in conjunction with activated synovial cells produce inflammatory and type 1 cytokines together with degradative enzymes that lead to the progressive destruction of cartilage and bone.
Although efforts to develop safer and more effective treatments for RA have been made, most antirheumatic drugs have limited efficacy, many side-effects, and fail to improve the long-term prognosis of this disease [25,26]. Effective treatment of rheumatoid arthritis requires the suppression of arthritogenic lymphocytes that initiate and perpetuate joint inflammation and damage. Metallothionein-I + II action as an immunosuppressant and free radical scavenger could offer a new therapeutic approach using a naturally occurring small protein to suppress the autoimmune Th1 cell attack and to interrupt the amplification loop of the inflammatory process. In this present study, we investigated a potential therapeutic role for metallothionein in autoantigen-dependent immunoregulation.
MATERIALS AND METHODS
Mice and induction of arthritis
All mice used in the experiments were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and kept under specific pathogen-free conditions. The mouse work was conducted in accordance with the laboratory animal care guidelines provided by Hanyang University. Male DBA/1 J mice (7–9 weeks old) were immunized with 100 μg of bovine CII (Chondrex, Seattle, USA) emulsified in complete Freund's adjuvant (CFA) (Chondrex) by intradermal injection at the base of the tail essentially as described [27]. Two weeks later mice were subjected to booster immunization with 50 μg CII emulsified in incomplete Freund's adjuvant (IFA) in the footpad of the left hind paw. The day of the disease onset in our study varied between individual mice started on day 21 after the initial CII immunization. A mixture of rabbit liver metallothionein I and II (Sigma, St. Louis, USA) (100 μg dissolved in 100 μl of 0·85% saline solution) was intraperitoneally injected daily except days 24 and 28 in DBA/1 J mice over a 12-day period starting on day 21.
Clinical evaluation of arthritis
Starting on day 21 after primary immunization, mice were inspected for disease progression in a blinded manner. The clinical severity of disease was scored on a daily basis using a scoring system as previously described [27]: 0, normal; 1, slight erythema and mild swelling confined to the mid-foot (tarsals) or ankle joint; 2, erythema and mild swelling extending from the ankle to the mid-foot; 3, erythema and moderate swelling extending from the ankle to the metatarsal joints; 4, intensive erythema and severe swelling encompassing the ankle, foot, and digits. Each limb except the footpad-injected left hindpaw was graded, resulting in a maximal clinical score of 12 per animal and expressed as the mean arthritic index on a given day. Mice were scored as arthritic following more than one paw of greater than score 2.
Histopathological assessment
Right hindpaws (that were not subjected to footpad injection) were removed post mortem on day 34 after primary immunization, fixed in 10% (w/v) phosphate-buffered formalin, and decalcified in 5·5% EDTA in phospahate-buffered formalin. Decalcified paws were embedded in paraffin, sectioned, and stained with haematoxylin and eosin. Microscopic evaluation of arthritic paws was performed in a blinded manner. Arthritic changes in the ankle and foot were classified as normal, modest, and severe, based on the following criteria: normal, nonarthritic joint; moderate, modest leucocyte infiltration and erosion but intact joint architecture; severe, marked synovitis and loss of bone integrity.
Proliferation assays
As mice were sacrificed, draining lymph nodes (inguinal, axillary, and popliteal) were excised and teased apart to make a single cell suspension. Cells were cultured in 96 well plates at a density of 1 × 106 cells/ml (200 μl/well) in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mml-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5 × 10−5 M 2-mercaptoethanol (Life Technologies, Rockville, MD, USA). Cells were cultured in medium alone, or in the presence of 250 μg/ml bovine CII (Chondrex) or 5 μg/ml phytohemagglutinin (PHA) (Sigma) for 4 days, and 1 μCi/well of 3H-thymidine was added to the culture for the last 16 h. Cells were harvested on glass fibre filters and 3H-thymidine incorporation was measured using a liquid scintillation counter.
Tumour growth factor (TGF)-β assay by sandwich enzyme-linked immunosorbent assay (ELISA)
Draining lymph nodes were removed post mortem on day 34 after immunization. Single cell suspensions were prepared in RPMI 1640 containing 1·25% FBS and cultured in the presence or absence of 250 μg/ml CII. After 3 days of stimulation, culture supernatants were collected and assayed by standard sandwich ELISA according to the manufacturer's instructions (R & D Systems, Minneapolis, USA) and standardized to recombinant TGF-β (Peprotech EC, London, UK). Aliquots of culture supernatants were acidified and neutralized prior to measurement according to the manufacturer's instructions (R & D Systems).
Reverse transcription-polymerase chain reaction (RT-PCR)
After sacrificing mice on day 34, joints of booster-injected (left) and uninjected (right) hindpaws were separately collected, dissected free of soft tissue and bones, snap frozen in liquid nitrogen, and ground into fine pieces. Total RNA was isolated using the Trizol reagent (Life Technologies) and assayed for tumour necrosis factor (TNF)-α and cyclooxygenase (Cox)-2 expression by RT-PCR methods. In brief, cDNAs were synthesized by extension of random hexamer primers with 200 units of SuperScript II reverse transcriptase (Life Technologies) in a mixture containing 2 μg of total RNA for 1 h at 37°C. PCR of the cDNA was performed in a final 25 μl containing all four dNTPs, 1·5 mm MgCl2, 1·5 U of Taq polymerase (Takara Shuzo Co., Shiga, Japan), and each primer at 0·4 μm concentration (Bionex Co. Seoul, Korea). The amplification cycles were 94°C for 45 s, 58°C (for Cox-2) or 55°C (for TNF-α) for 60 s, and 72°C for 30 s. The PCR products were separated on a 1·5% agarose gel after 35 cycles for Cox-2 or 30 cycles for TNF-α. Primer sequences used for the PCR were as follows: Cox-2 sense, 5′ GCA CTA CAT CCT GAC CCA CT 3′; Cox-2 antisense, 5′GAA CCC AGG TCC TCG CTT AT 3′; TNF-α sense 5′ TCT CAT CAG TTC TAT GGC CC 3′; TNF-α antisense, 5′ GGG AGT AGA CAA GGT ACA AC 3′; β2 microglobulin (β2M) sense, 5′ TGA CCG GCT TGT ATG CTA TC 3′; β2M antisense, 5′ CAG TGT GAG CCA GGA TAT AG 3′. Cox-2 PCR products were further analysed by Southern blot hybridization with an internal probe (5′ CAA CTC CCA TGG GTG TGA AG 3′).
RESULTS
Repeated administration of metallothionein-I + II suppressed clinical and histopathological manifestations of CIA
The potential for metallothionein to ameliorate arthritis was examined by metallothionein protein therapy in CIA-established mice. Metallothionein-I + II proteins were administered daily (100 μg/mouse per day) to the experimental group from day 21 after primary immunization and the progression of disease was inspected on a daily basis. The control group was injected with vehicle (saline) alone. The administration of metallothionein-I + II markedly reduced clinical manifestations of established CIA, such as the arthritic index and disease incidence (Fig. 1). This suppression was evident from the day after the 2nd injection with metallothionein-I + II and sustained until the conclusion of the experiment following the 10th injection.
Fig. 1.
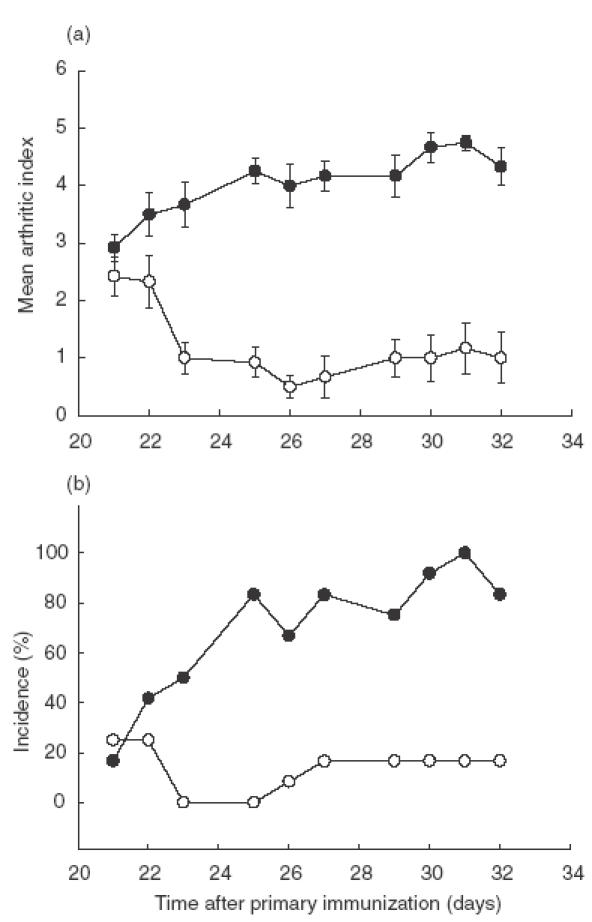
Metallothionein decreased the clinical manifestations of established CIA. DBA/1 J mice were immunized with CII plus CFA at day 0 and followed by booster immunization with CII plus IFA at day 14. From day 21–32, mice were injected intraperitoneally with 100 μg/day of metallothionein-I + II (○) or saline (•) everyday except days 24 and 28. These figures resulted from three independent sets of experiments and represent means ± SEMs (n = 15 per group). From day 23–32, arthritic indexes from metallothionein-injected mice were significantly different from those from vehicle-treated mice at each day (P <0·01, t-test).
Histopathologic observations at day 34 after primary immunization with type II collagen closely paralleled clinical data from individual mice. Microscopic analysis of haematoxylin and eosin-stained hindpaw sections showed that most of joints of metallothionein-treated mice were only mildly arthritic when compared to the control group (P <0·05, χ2) (Fig. 2). In metallothionein-treated mice, 58% of joints were normal and 34% modestly arthritic, consisting of small erosions limited to the cartilage-pannus junction (Fig. 2b,c,e). In contrast, 13% of control mice exhibited normal and 62% mildly affected joints (Fig. 2e). The percentage of severely arthritic joints accompanied by pannus invasion to bone and loss of bone integrity was significantly decreased in metallothionein-treated mice (8%), relative to that of control mice (25%) (Fig. 2d,e).
Fig. 2.
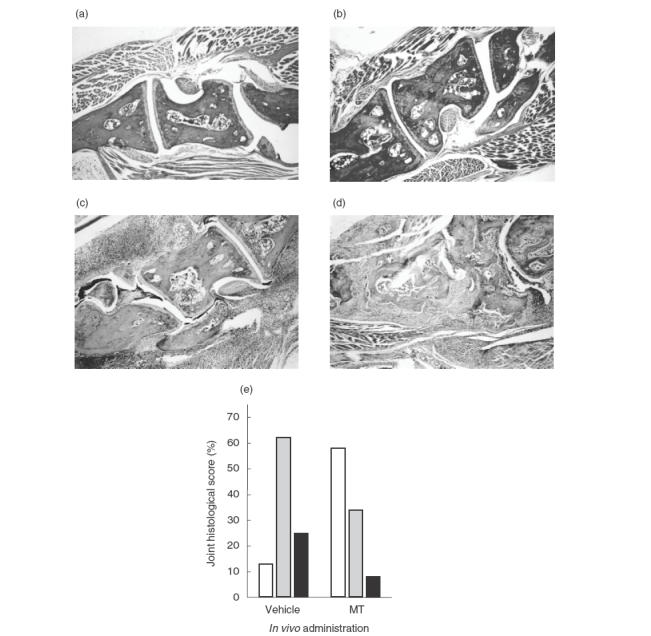
Metallothionein reduced histopathological manifestations of CIA. On day 34 postimmunization, mice (n = 15 per group) were sacrificed and right hindpaws were removed. The specimens were fixed, decalcified, embedded in paraffin, sectioned, and stained with haematoxylin and eosin. (a) Joints of normal mice; (b) mainly normal joints from metallothionein-treated mice; (c) moderately arthritic joints from metallothionein-treated mice; (d) severely arthritic joints from vehicle-treated mice. Original magnification: ×40. Percentage of histological score per group is shown in (e). □ Normal;  moderate; ▪ severe.
moderate; ▪ severe.
Proinflammatory mediators were decreased in boostered paws of metallothionein-I + II-injected mice
We assessed anti-inflammatory activity of exogenous metallothionein by measuring the expression levels of proinflammatory mediators such as TNF-α and Cox-2 in the affected joint tissues. Total RNA isolated from the hindpaws after removal of soft tissues was assayed using RT-PCR methods. TNF-α levels were higher in the left hindpaws that were subjected to the footpad injection with CII for the booster immunization than in the uninjected right hindpaws (Fig. 3a). Cox-2 was detectable only in the left hindpaws (booster immunized) (Fig. 3b). These results suggest that TNF-α and Cox-2 levels can be used to represent the inflammatory status of tissues subjected to the induction of the delayed-type hypersensitivity (DTH) reaction. RNA samples in booster-injected joints from metallothionein-injected mice contained significantly reduced levels of both TNF-α and Cox-2 transcripts, when compared with those from control mice.
Fig. 3.
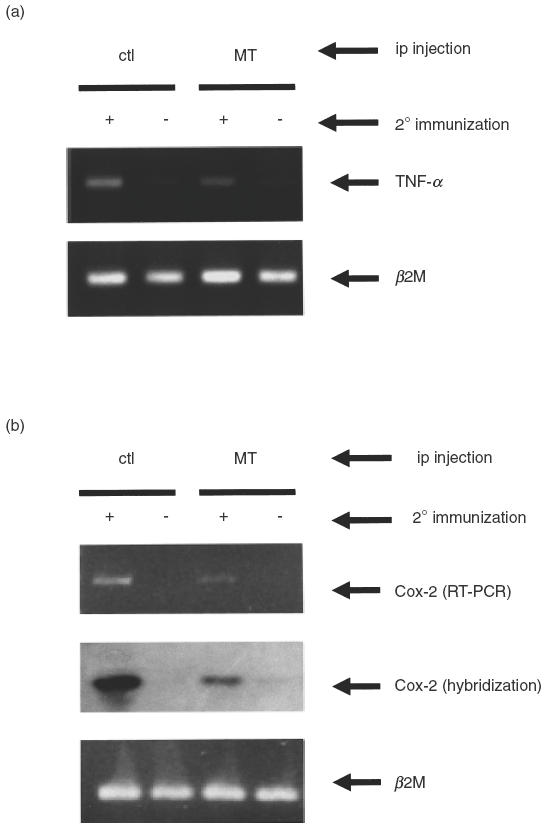
The expression of TNF-α and Cox-2 transcripts was reduced in joints of metallothionein-injected mice. On day 34, joints of booster injected left hindpaws (+) and uninjected right hindpaws (–) were separately removed and dissected free of soft tissue. Total RNAs were extracted from joints pooled 4–5 mice per group and expression levels of (a) TNF-α and (b) Cox-2 transcripts were determined by RT-PCR. PCR products of Cox-2 were further analysed by Southern blot hybridization with the Cox-2 internal probe. The data were reproducible in two independent experiments.
Metallothionein-I + II decreased the proliferative response of lymphocytes to CII
It has been previously demonstrated that CIA be mediated by pathogenic CII-specific T cells that develop following immunization with CII in adjuvant [28]. To determine whether metallothionein interferes with clonal expansion of CII-specific T cells, lymph node cells were isolated from all experimental groups at day 34 after primary immunization and were then cultured in the presence or absence of bovine CII or PHA in proliferation assays. As expected, in the vehicle control group, cells stimulated with CII exhibited a 2-fold increase in 3H-thymidine incorporation over unstimulated cells, indicating proliferation occurred in response to CII. However, cells obtained from metallothionein-administered mice were refractory to the stimulation with CII in terms of 3H-thymidine incorporation (Fig. 4). Cells from both groups proliferated in response to PHA at comparable levels. These results show that metallothionein suppresses the expansion of CII-reactive cells in mice primed with CII in vivo, while having no effect on the overall proliferative potential of the T lymphocyte population from these lymph nodes.
Fig. 4.
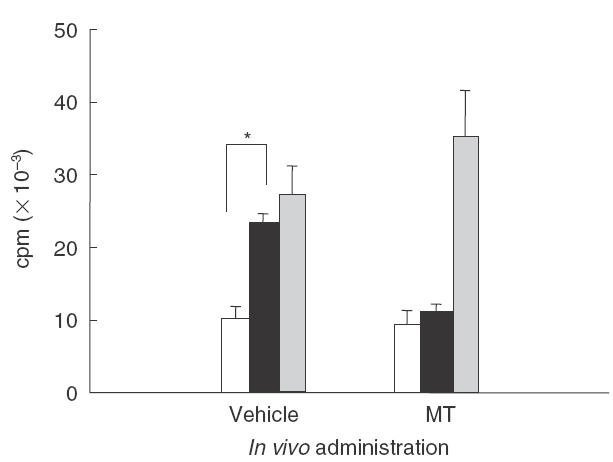
Metallothionein administration inhibited the proliferation of CII-specific lymphocytes upon in vitro restimulation with CII. Lymph node cells from mice sacrificed on day 34 postimmunization were stimulated in triplicate in the absence (□) or presence of 250 μg/ml CII (▪) or 5 μg/ml PHA (▪) for 72 h. The cells were cultured for another 16 h with 1 μCi/well 3H-thymidine, harvested, and counted using a scintillation counter. The data are representative of three independent experiments with similar results (* P <0·05, t-test).
Lymph node cells obtained from mice injected with metallothionein-I + II displayed higher TGF-β production upon stimulation with CII than those from control mice
TGF-β has been shown to suppress Th1-dominated autoimmune responses [29–31]. We speculated that the proliferative impairment of metallothionein-I + II-injected group to CII might be associated with TGF-β induction. Lymph node cells isolated from CIA-established mice at day 34 post immunization were cultured in the presence or absence of CII and the levels of active TGF-β in the culture supernatants were measured by sandwich ELISA. Following stimulation with CII, cells from mice administered with metallothionein-I + II produced significantly increased levels of TGF-β, as compared with those treated with vehicle alone (Fig. 5). This result demonstrates that metallothionein-I + II potentiate the development of CII-specific, TGF-β-producing cells.
Fig. 5.
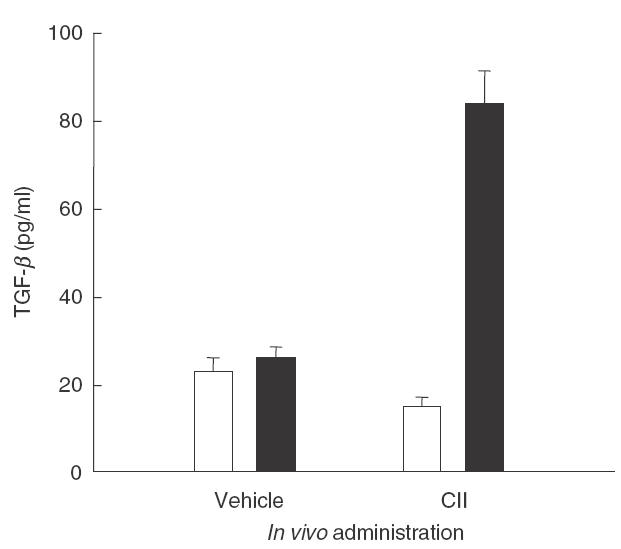
TGF-β production was increased in metallothionein-injected mice. Lymph node cells obtained on day 34 post mortem were cultured in RPMI medium supplemented with 1·25% FBS in the absence (□) or presence (▪) of 250 μg/ml bovine CII for 3 days. The culture supernatants were collected and analysed for TGF-β levels using cytokine sandwich ELISA. The result of a representative experiment out of three performed is shown.
DISCUSSION
In this study, we provide definitive experimental data supporting an in vivo therapeutic role for metallothionein-I + II in a murine experimental model of RA. Clinical and histopathological evaluations of arthritis-associated characteristics suggest that repeated administration of metallothionein-I + II during the course of disease can be sufficient to achieve a dramatic arrest in overall disease progression. This effect was reproduced by administration of ZnCl2 (which acts as a potent inducer of metallothionein; data not shown) at more broadly spaced intervals. Metallothionein-I + II also elicited an anti-inflammatory activity on the synovial tissue, as indicated by a reduction in the expression level of the proinflammatory mediators TNF-α and Cox-2. This data is compatible with observations by other groups that show the number of cells expressing proinflammatory cytokines, including TNF-α and IL-6, is increased in metallothionein-I + II knockout mice that have been subjected to a cortical cryo-injury [32]. In addition, injected metallothionein or metallothionein I-transgene expression can diminish the levels of inflammatory cytokines that are part of an experimental autoimmune encephalomyelitis or elicited by a cryoinjury to the cortex [33,34], suggesting metallothionein may employ a common mechanism to suppress diverse inflammatory autoimmune disorders.
We further investigated the mechanisms by which metallothionein might influence the disease progression of established CIA. One mechanism could be that metallothionein suppresses the development of pathogenic, CII-specific Th1 clones, as shown by the result of metallothionein treatment and the subsequent unresponsiveness to in vitro CII restimulation. CII-specific T cells have been previously characterized in the instances of both murine CIA and human RA [35,36]. CII-specific Th cell lines, which have phenotype characteristic of DTH cells in this CIA model, were able to elicit clinical arthritis after adoptive transfer to naïve DBA/1 J mice [35]. These cells can also provide useful therapeutic opportunities, as CII-specific T cell clones attenuated by X-ray irradiation have been employed in vaccinations against CIA [35]. T cell responses to CII and a CII peptide are higher in patients with RA, especially in early phase of disease, compared with in normal subjects [36]. These observations suggest that CII-specific T cells play an important role in the pathogenesis, especially in the initiation, of RA. In this context, impaired development of CII-reactive T lymphocytes by treatment with metallothionein could contribute to the suppression of this autoimmune disorder.
The suppression of CII-specific lymphocyte proliferation that metallothionein-I + II administration can produce is paralleled by enhancement of TGF-β production in response to CII stimulation. The finding that metallothionein knockout mice exhibit a reduction in the number of TGF-β-producing cells [32] complements our result. TGF-β is a pleiotrophic cytokine that controls cell growth and differentiation [37]. Within the immune system, TGF-β inhibits the growth of T cells, B cells, and other haematopoietic cells and suppresses IFN-γ production [38]. The multiorgan inflammation and excessive lymphocytic infiltration in TGF-β1 null mice further underscore the anti-inflammatory nature of TGF-β1 [39]. Production of endogenous TGF-β appears to be associated with remission of Th1-mediated experimental autoimmune neuritis [40], and systemic administration of TGF-β can ameliorate autoimmune diseases including EAE and CIA [29,30,41]. Regulatory CD4+ Th3 cells that predominantly secrete TGF-β1 mediate the oral tolerance induced by low dose myelin basic protein and proteolipid protein for EAE or insulin for lymphocytic choriomeningitis virus-induced autoimmune diabetes [31,42,43]. These observations indicate that TGF-β can play a central role in the suppression of inflammatory Th1-dominated autoimmunity. Our findings suggest that CII-specific, TGF-β-producing cells which can be induced by metallothionein-I + II may actively suppress the proliferation of arthritogenic Th1 cells, leading to blockade of the subsequent cascades that result in synovitis.
There are a number of other potential ways in which metallothionein may act to modify immune functions. Metallothionein binding to the plasma membrane could initiate inappropriate signal transduction cascades or block the effective interaction between the plasma membranes of different cells of the immune system as has been shown for Th:APC interactions [15]. Alternatively, metallothionein can cause redistribution of essential cations away from essential regulatory cells [44]. Metallothionein has also been shown to regulate transcription activators by regulating the available zinc [45], or by direct physical interactions with some transcription factors [46]. As a potent antioxidant, metallothionein protects against many agents known to act through a wide range of reactive oxygen or nitrogen species (ROS or RNS) including superoxide, hydrogen peroxide, hydroxyl radical, nitric oxide, and peroxynitrite, which are implicated in many pathogenesis [47,48]. The radical scavenging potential of metallothionein has been reported to be higher than that of other antioxidants such as glutathione, superoxide dismutase, and catalase for some species of oxidants [49,50]. Metallothionein may also affect oxidant-dependent signal transduction cascades or the activation of oxidant sensitive genes. In light of the importance of ROS and RNS in the progression of RA, this function of metallothionein may in part contribute to amelioration of synovitis.
Consistent with our investigation, metallothionein has been previously reported to inhibit the progression of other organ-specific autoimmune diseases. In an EAE model, intraperitoneal injection with exogenous metallothionein resulted in significantly reduced clinical manifestations of the disease [19] and metallothionein-I + II knockout mice were more susceptible to this disease than wild-type mice [22]. Other examples of metallothionein-mediated suppression of autoimmune disease include experiments which show the response of streptozotocin-induced diabetes to metallothionein therapy [51,52]. Metallothionein that is overexpressed following administration with zinc sulphate or after introduction of metallothionein-II transgene in pancreatic islets of mice protected against the onset of diabetes. In these reports, authors suggest that metallothionein functions as a potent hydroxyl radical scavenger to reduce the oxidative damage accompanied by the inflammatory autoimmune responses.
In conclusion, we demonstrated that metallothionein suppresses CIA by promoting TGF-β-producing cells and inhibiting the expression of proinflammatory mediators. The observation that there are individual differences between humans in the levels of inducible metallothionein that their cells can synthesize [53] suggests that metallothionein may be a regulatory factor that determines an individual's susceptibility to autoimmune disease or its severity. Administration with metallothionein-I + II may provide a beneficial therapeutic effect to treat RA in clinical settings.
Acknowledgments
We thank Chul Shik Lim, Won Gil Cho, and Kyung Hee Lee for excellent technical assistance. This work was supported by a Korea Research Foundation grant (KRF-2000–042-D00086).
REFERENCES
- 1.Hamer DH. Metallothionein. Annu Rev Biochem. 1986;55:913–51. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Bremner I. Oxygen-free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–37. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 3.Durnam DM, Hoffman JS, Quaife CJ, Benditt EP, Chen HY, Brinster RL, Palmiter RD. Induction of mouse metallothionein-I mRNA by bacterial endotoxin is independent of metals and glucocortocoid hormones. Proc Natl Acad Sci USA. 1984;81:1053–6. doi: 10.1073/pnas.81.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle P, Philcox JC, Rofe AM. Metallothionein induction in cultured rat hepatocytes by arthritic rat serum, activated macrophages, interleukin-6, interleukin-1 and leukaemia inhibitory factor. Inflamm Res. 1995;44:475–81. doi: 10.1007/BF01837913. [DOI] [PubMed] [Google Scholar]
- 5.Basu A, Lazo JS. A hypothesis regarding the protective role of metallothionein against the toxicity of DNA interactive anticancer drug. Toxicol Lett. 1990;50:123–35. doi: 10.1016/0378-4274(90)90002-4. [DOI] [PubMed] [Google Scholar]
- 6.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA. 1994;91:584–8. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner I, Mehra RK, Sato M. Metallothionein in blood, bile, and urine. Experientia. 1987;52(Suppl):507–17. doi: 10.1007/978-3-0348-6784-9_51. [DOI] [PubMed] [Google Scholar]
- 8.Hart BA, Garvey JS. Detection of metallothionein in bronchoalveolar cells and lavage fluid following repeated cadmium inhalation. Environ Res. 1986;40:391–8. doi: 10.1016/s0013-9351(86)80114-1. [DOI] [PubMed] [Google Scholar]
- 9.Danielson KG, Ohi S, Huang PC. Immunochemical localization of metallothionein in rat liver and kidney. J Histochem Cytochem. 1982;30:1033–9. doi: 10.1177/30.10.6752263. [DOI] [PubMed] [Google Scholar]
- 10.De Lisle RC, Sarras MP, Jr, Hidalgo J, Andrews GK. Metallothionein is a component of exocrine pancreas secretion: implications for zinc homeostasis. Am J Physiol. 1996;271:C1103–10. doi: 10.1152/ajpcell.1996.271.4.C1103. [DOI] [PubMed] [Google Scholar]
- 11.El Refaey H, Ebadi M, Kuszynski CA, Sweeney J, Hamada FM, Hamed A. Identification of metallothionein receptors in human astrocytes. Neurosci Lett. 1997;231:131–4. doi: 10.1016/s0304-3940(97)00548-x. [DOI] [PubMed] [Google Scholar]
- 12.Youn J, Lynes MA. Metallothionein-induced suppression of cytotoxic T lymphocyte function: An important immunoregulatory control. Toxicol Sci. 1999;52:199–208. doi: 10.1093/toxsci/52.2.199. [DOI] [PubMed] [Google Scholar]
- 13.Lynes MA, Borghesi LA, Youn J, Olson EA. Immunomodulatory activities of extracellular metallothionein. I. Metallothionein effects on antibody production. Toxicology. 1993;85:161–77. doi: 10.1016/0300-483x(93)90040-y. [DOI] [PubMed] [Google Scholar]
- 14.Youn J, Borghesi LA, Olson EA, Lynes MA. Immunomodulatory activities of extracellular metallothionein. II. Effects on macrophage functions. J Toxicol Environ Health. 1995;45:397–413. doi: 10.1080/15287399509532004. [DOI] [PubMed] [Google Scholar]
- 15.Borghesi LA, Youn J, Olson EA, Lynes MA. Interactions of metallothionein with murine lymphocytes: Plasma membrane binding and proliferation. Toxicology. 1996;108:129–40. doi: 10.1016/s0300-483x(95)03243-9. [DOI] [PubMed] [Google Scholar]
- 16.Crowthers KC, Kline V, Giardina C, Lynes MA. Augmented humoral immune function in metallothionein-null mice. Toxicol Appl Pharmacol. 2000;166:161–72. doi: 10.1006/taap.2000.8961. 10.1006/taap.2000.8961. [DOI] [PubMed] [Google Scholar]
- 17.Winters C, Jasani B, Marchant S, Morgan J. Immunocytochemical identification of metallothionein-positive cells in rheumatoid synovium and analysis of their cell lineage. Histochem J. 1997;29:301–7. doi: 10.1023/a:1026474531060. [DOI] [PubMed] [Google Scholar]
- 18.Backman JT, Siegle I, Fritz P. Immunohistochemical localization of metallothionein in synovial tissue of patients with chronic inflammatory and degenerative joint disease. Virchows Arch. 1998;433:153–60. doi: 10.1007/s004280050230. [DOI] [PubMed] [Google Scholar]
- 19.Penkowa M, Hidalgo J. Metallothionein I + II expression and their role in experimental autoimmune encephalomyelitis. Glia. 2000;32:247–63. doi: 10.1002/1098-1136(200012)32:3<247::AID-GLIA50>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Lynes MA, Richardson CA, McCabe R, Crowthers KC, Lee JC, Youn J, Schweitzer IB, Shultz LD. Metallothionein-mediated alterations in autoimmune disease processes. In: Klaassen C, editor. Metallothionein IV. Basel: Birkhauser; 1999. pp. 437–44. [Google Scholar]
- 21.Miesel R, Zuber M. Copper-dependent antioxidase defenses in inflammatory and autoimmune rheumatic diseases. Inflammation. 1993;17:283–94. doi: 10.1007/BF00918991. [DOI] [PubMed] [Google Scholar]
- 22.Penkowa M, Espejo C, Martinez-Caceres EM, Poulsen CB, Montalban X, Hidalgo J. Altered inflammatory response and increased neurodegeneration in metallothionein I + II deficient mice during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119:248–60. doi: 10.1016/s0165-5728(01)00357-5. [DOI] [PubMed] [Google Scholar]
- 23.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 24.Durie FH, Fava RA, Noelle RJ. Collagen-induced arthritis as a model of rheumatoid arthritis. Clin Immunol Immunopathol. 1994;73:11–8. doi: 10.1006/clin.1994.1164. [DOI] [PubMed] [Google Scholar]
- 25.Van Den Berg WB. Anti-cytokine therapy in chronic destructive arthritis. Arthritis Res. 2001;3:18–26. doi: 10.1186/ar136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman WJ. Prospects for autoimmune disease: Research advances in rheumatoid arthritis. JAMA. 2001;285:648–50. doi: 10.1001/jama.285.5.648. [DOI] [PubMed] [Google Scholar]
- 27.Rosloniec EF, Cremer M, Kang A, Myers LK. Collagen induced arthritis. In: Coligan JE, Kruisbeek AK, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: John Wiley & Sons, Inc; 1996. pp. 15.5.1–15.5.24. [Google Scholar]
- 28.Brand DD, Myers LK, Terato K, Whittington KB, Stuart JM, Kang AH, Rosloniec EF. Characterization of the T cell determinants in the induction of autoimmune arthritis by bovine α. J Immunol. 1994;152:3088–97. 1: (II) -CB11 in H-2q mice. [PubMed] [Google Scholar]
- 29.Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladino MA, Thorbecke GJ. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA. 1991;88:2918–21. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorbecke GJ, Shah R, Leu CH, Kuruvilla AP, Hardison AM, Palladino MA. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–9. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta 1-secreting Th3 cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penkowa M, Carrasco J, Giralt M, Molinero A, Hernandez J, Campbell IL, Hidalgo J. Altered central nervous system cytokine-growth factor expression profiles and angiogenesis in metallothionein-I+II deficient mice. J Cereb Blood Flow Metab. 2000;20:1174–89. doi: 10.1097/00004647-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Penkowa M, Hidalgo J. Metallothionein treatment reduces proinflammatory cytokines IL-6 and TNF-alpha and apoptotic cell death during experimental autoimmune encephalomyelitis (EAE) Exp Neurol. 2001;170:1–14. doi: 10.1006/exnr.2001.7675. [DOI] [PubMed] [Google Scholar]
- 34.Giralt M, Penkowa M, Lago N, Molinero A, Hidalgo J. Metallothionein-1+2 protect the CNS after a focal brain injury. Exp Neurol. 2002;173:114–28. doi: 10.1006/exnr.2001.7772. 10.1006/exnr.2001.7772. [DOI] [PubMed] [Google Scholar]
- 35.Kakimoto K, Katsuki M, Hirofuji T, Iwata H, Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988;140:78–83. [PubMed] [Google Scholar]
- 36.Kim H-Y, Kim W-U, Cho M-L, et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2085–93. doi: 10.1002/1529-0131(199910)42:10<2085::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 37.Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 38.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–4. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 39.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiefer R, Funa K, Schweitzer T, Jung S, Bourde O, Toyka KV, Hartung HP. Transforming growth factor-beta 1 in experimental autoimmune neuritis. Cellular localization and time course. Am J Physiol. 1996;148:211–23. [PMC free article] [PubMed] [Google Scholar]
- 41.Johns LD, Flanders KC, Ranges GE, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. J Immunol. 1991;147:1792–6. [PubMed] [Google Scholar]
- 42.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Herrath MG, Dyrberg T, Oldstone MB. Oral insulin treatment suppresses virus-induced antigen-specific destruction of beta cells and prevents autoimmune diabetes in transgenic mice. J Clin Invest. 1996;98:1324–31. doi: 10.1172/JCI118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daston GP, Overmann GJ, Taubeneck MW, Lehman-McKeeman LD, Rogers JM, Keen CL. The role of metallothionein induction and altered zinc status in maternally mediated developmental toxicity: comparison of the effects of urethane and styrene in rats. Toxicol Appl Pharmacol. 1991;110:450–63. doi: 10.1016/0041-008x(91)90046-h. [DOI] [PubMed] [Google Scholar]
- 45.Posewitz MC, Wilcox DE. Properties of the Sp1 zinc finger 3 peptide: coordination chemistry, redox reactions, and metal binding competition with metallothionein. Chem Res Toxicol. 1995;8:1020–8. doi: 10.1021/tx00050a005. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Mageed AB, Agrawal KC. Activation of nuclear factor κB: Potential role in metallothionein-mediated mitogenic response. Cancer Res. 1998;58:2335–8. [PubMed] [Google Scholar]
- 47.Cai L, Klein JB, Kang YJ. Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage. J Biol Chem. 2000;275:38957–60. doi: 10.1074/jbc.C000593200. [DOI] [PubMed] [Google Scholar]
- 48.Pearce LL, Gandley RE, Han W, et al. Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein. Proc Natl Acad Sci USA. 2000;97:477–82. doi: 10.1073/pnas.97.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura T, Muraoka S, Ogiso T. Antioxidant activity of metallothionein compared with reduced glutathione. Life Sci. 1997;60:301–9. doi: 10.1016/s0024-3205(97)00156-2. [DOI] [PubMed] [Google Scholar]
- 50.Thornalley PJ, Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 51.Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43:1020–30. doi: 10.1007/s001250050009. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Carlson EC, Pellet L, Moritz JT, Epstein PN. Overexpression of metallothionein in pancreatic β-cells reduces streptozotocin-induced DNA damage and diabetes. Diabetes. 2001;50:2040–6. doi: 10.2337/diabetes.50.9.2040. [DOI] [PubMed] [Google Scholar]
- 53.Yurkow EJ, Makhijani PR. Flow cytometric determination of metallothionein levels in human peripheral blood lymphocytes: utility in environmental exposure assessment. J Toxicol Environ Health. 1998;54:445–57. doi: 10.1080/009841098158737. [DOI] [PubMed] [Google Scholar]


