Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand dependent transcriptional factor known to be a regulator of adipogenesis. Recent studies have also shown that stimulation of PPARγ inhibits the transcriptional activities of other nuclear factors and down-regulates proinflammatory cytokine synthesis in T cells and monocytes. We examined, in the present study, the functional significance of PPARγ expressed in fibroblast-like synovial cells (FLS) isolated from patients with rheumatoid arthritis (RA). Incubation of FLS with a synthetic PPARγ ligand, troglitazone, inhibited endogenous production of TNF-α, IL-6 and IL-8, as well as matrix metalloprotease-3 (MMP-3), without inducing apoptosis of the cells. The gelatinase activity of FLS culture media was also inhibited by troglitazone. Electrophoretic mobility shift assay (EMSA) showed a significant reduction in the DNA binding activity of NF-κB in troglitazone-treated FLS in response to TNF-α or IL-1β. Moreover, long-term cultivation of FLS with troglitazone resulted in morphological changes with marked lipid accumulation in these cells. Our results show a negative regulatory function for PPARγ on cytokine and MMP production together with inhibition of cytokine-mediated inflammatory responses in rheumatoid synovial cells. Our results also suggest that FLS could differentiate into adipocyte-like cells in the presence of proper stimulatory signals including PPARγ.
Keywords: adipocyte, cytokine, matrix metalloproteinase, NF-κB fibroblast-like synovial cells, troglitazone
INTRODUCTION
Rheumatoid arthritis (RA) is a joint-affecting disease and threatens the daily physical activities of afflicted patients. Active proliferation of synovial cells is a central feature found in the rheumatoid synovial tissue, suggesting that activated synovial cells are a major source of proinflammatory cytokines and tissue-degrading proteases [1–3]. Treatment of RA, in turn, has focused on the inhibition of synovial cell function or induction of synovial cell death, including the modulation of activities of various nuclear transcriptional factors that are relevant to the process of inflammation [4–6].
The nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) has been implicated recently in the regulation of a variety of biological processes. PPARγ plays a crucial role in adipogenesis, where it functions in concert with members of the CAAT/enhancer-binding protein (C/EBP) family of transcriptional factors [7–11]. PPARγ is also expressed in other cell lineages, and stimulation of the PPARγ-mediated signalling pathway inhibits the synthesis of pro-inflammatory cytokines [12,13]. PPARγ-induced inhibition of cytokine gene expression is, in part, mediated by antagonizing the transcriptional activities of nuclear factor kappa B (NF-κB), nuclear factor of activated T cells (NF-AT), activating protein-1 (AP-1) and signal transducers and activators of transcription (STATs) [13–15]. Another function of PPARγ is the induction of apoptosis [16,17]. In this regard, Kawahito et al. have recently shown the potential clinical usefulness of PPARγ ligands in the treatment of RA through the induction of apoptosis of fibroblast-like synovial cells (FLS) [18].
In the present study, we examined the effects of PPARγ on the function and differentiation of FLS isolated from rheumatoid synovial tissues. The results show clearly the expression of PPARγ in cultured FLS. In short-term cultures of rheumatoid FLS with a relatively low concentration of a PPARγ ligand, PPARγ stimulation inhibited the production of tumour necrosis factor α (TNF-α), interleukin (IL)-6, IL-8 and matrix metalloprotease-3 (MMP-3) from the cells, together with the suppression of NF-κB nuclear activity, without inducing apoptosis. In addition, prolonged culture of FLS with the PPARγ ligand transformed the cells to an adipocyte-like phenotype. Because FLS are thought to arise locally as progeny of resident mesenchymal lineage cells [19], the latter change emphasizes the functional similarity between rheumatoid FLS and mesenchymal stem cells.
MATERIALS AND METHODS
Reagents
Troglitazone, a synthetic PPARγ ligand, was kindly provided by Sankyo Co. (Tokyo, Japan). Monoclonal antibodies (MoAbs) against human MMP-2 and MMP-3 were purchased from Fuji Pharmacology (Tokyo). Rabbit polyclonal antibody against human PPARγ was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Synovial cell preparation and culture
We obtained synovial tissue specimens from patients with RA who met the American College of Rheumatology criteria for the disease [20] at the time of orthopaedic surgery (all synovial samples were obtained during total knee replacement) in National Ureshino Hospital between April and October, 2000. We selected patients with RA who had active inflammation with elevated serum C-reactive protein (>2·0 mg/dL at the time of orthopaedic surgery, from a total of 12 RA patients). Informed consent was obtained from all participating subjects, and the study was conducted in accordance with the human experimental guidelines of our institution. FLS were isolated from the synovial tissues as described previously [21]. In all experiments, FLS were used after three passages following the removal of monocytes and lymphocytes. The cells were cultured with DMEM containing 10% fetal bovine serum (FBS) and 5 μg/ml insulin until the cell culture reached confluence. FLS were further cultured with DMEM containing 10% FBS, 5 μg/ml insulin and 10 pm dexamethasone for another 2 days. The above culture conditions were used based on a previous study designed to examine PPARγ-mediated adipogenesis [8, 10, 22]. After cultivation, the expression of PPARγ in FLS was examined, and the effect of troglitazone on synovial cell function in DMEM media containing insulin and dexamethasone was studied as described below.
Identification of PPARγ expression in FLS, and the effects of troglitazone on the production of cytokines and MMPs from FLS
The expression of PPARγ in FLS was examined by Western blot analysis. In brief, FLS were washed three times with PBS, and lysed by the addition of lysis buffer (50 mm Tris, pH 8·0, 150 mm NaCl, 0·1% SDS, 1% NP-40 and 100 μg/ml PMSF). The protein concentrations of the cell extracts were determined using a protein assay kit (Bio-Rad, Melville, NY, USA). An identical amount of protein for each lysate (5 μg/well) was subjected to 12% sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE). Proteins were transferred to a PVDF filter, which was subsequently blocked for 1 h using 5% nonfat dried milk in TBS containing 0·5% Tween 20 (TBS-T). The filter was then washed with 1% non-fat dried milk in TBS-T, and incubated at room temperature for 1 h with anti-PPARγ antibody at 4 μg/ml. The filter was washed with TBS-T and incubated with 1 : 1000 dilution of sheep antirabbit IgG coupled with horseradish peroxidase. The enhanced chemiluminescence (ECL) system (Amersham, Buckinghamshire, UK) was used for detection.
We also examined the effects of troglitazone on the production of cytokines and MMPs from FLS. FLS were cultured with varying concentrations of troglitazone for the indicated time intervals, and the culture supernatants were collected. Culture media for the analysis of MMP-2 and MMP-3 expression were FBS-free, as described previously [23], whereas those for IL-6, IL-8 and TNF-α contained 10% FBS. The concentrations of IL-6, IL-8 and TNF-α in the culture media were determined by respective specific enzyme-linked immunosorbent assays (ELISA) (Fujirebio Inc., Tokyo, Japan). The expression levels of MMP-2 and MMP-3 in FLS culture media were determined by Western blot analysis as described previously by our laboratory [23]. In brief, each culture medium was subjected to 12% SDS-PAGE. Proteins were transferred to a PVDF filter as described above and the expression of MMP-2 (1 : 200 dilution of MMP-2 mAb) and MMP-3 (1 : 200 dilution of MMP-3 MoAb) was detected by the ECL system with antimouse IgG coupled with HRP. The density of expression examined by Western blots analysis was quantified by image analysis software (NIH version 1·61).
Gelatin zymography
We investigated the gelatinase activity of FLS culture media by gelatin zymography as described previously [23]. FBS-free culture media were incubated at 37°C for 20 min in SDS sample buffer free of reducing agents, and then run on an 8% polyacrylamide gel containing 1 mg/ml of gelatin at 4°C. After electrophoresis, the gels were washed in 2·5% Triton-X 100 to remove SDS, and incubated with 50 mm Tris-HCl buffer (pH 7·5) containing 0·15m NaCl, 10 mm CaCl2 and 0·02% NaN3 for 16 h at 37°C and stained with 0·1% Coomassie Brilliant Blue R 250. Band intensities were quantified by NIH image analysis software.
Determination of nuclear NF- κB activity by electrophoretic mobility shift assay (EMSA)
NF-κB nuclear activity in FLS was examined by EMSA using the Gel Shift Assay System (Promega Co., Madison, WI, USA). For this purpose, FLS were incubated with or without 10 μm of troglitazone for 24 h, and then further incubated in the presence or absence of TNF-α (200 IU/ml, Upstate Biolab, Lake Placid, NY, USA) and IL-1β (20 IU/ml, Otsuka Pharmaceutical Co., Tokushima, Japan) for 30 min. After incubation, nuclear proteins extracted from the cells (7·5 μg of proteins from each cell culture) were mixed with 32P-radiolabelled double-stranded oligonucleotide containing the NF-κB binding sequence (5′-AGTTGAGGGGACTTTCCCAGGC-3′) and 0·25 mg/ml of poly (dI-dC) (Sigma) in 10 mm Tris (pH = 7·5), 50 mm NaCl, 0·5 mm EDTA, 1 mm MgCl2, 0·5 mm DTT and 4% glycerol. Reactions were incubated for 30 min at room temperature and analysed by 5% polyacrylamide gel electrophoresis. Nuclear lysates from MT-2 cells were used for positive controls, and cold competition was performed by adding excess unlabelled oligonucleotide.
Differentiation of FLS into adipocyte-like cells by long-term cell culture with troglitazone
To investigate the role of PPARγ stimulation in adipocyte-like cell differentiation of FLS, FLS were cultured with troglitazone for the indicated time intervals, and the morphological alterations, relative to lipid accumulation, which was examined by staining with Oil Red O, were studied as described previously [24].
Statistical analysis
Data were expressed as mean ± s.d. Differences between groups were examined for statistical significance using the Student's t-test. A P-value of less than 0·05 denoted the presence of a statistically significant difference.
RESULTS
PPARγ expression in FLS and PPARγ-induced inhibition of cytokine and MMP-3 production
We initially demonstrated the expression of PPARγ protein in FLS obtained from each patient with RA (Fig. 1). We next examined the effect of troglitazone on cytokine production from FLS. As shown in Fig. 2, troglitazone significantly inhibited the endogenous production of IL-6, IL-8 and TNF-α from the cells. Troglitazone also significantly inhibited the endogenous production of MMP-3 from synovial cells into the culture media (Fig. 3c). However, treatment of FLS with troglitazone did not alter the concentrations of MMP-2 in the culture media (Fig. 3a). Troglitazone tended to inhibit MMP-2 production, however, the data did not reach statistical difference. Gelatinase activity determined in the FLS culture media was significantly suppressed by 10 μm troglitazone (Fig. 3b).
Fig. 1.
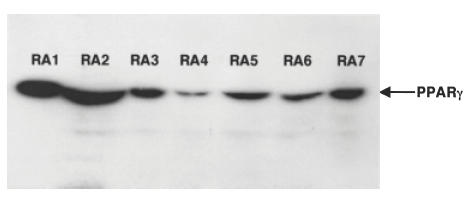
PPARγ protein expression in cultured FLS as determined by Western blotting. Five μg of proteins extracted from cultured FLS obtained from seven RA patients were used for the analysis of PPARγ expression as described in Materials and methods. Note the expression of PPARγ in each FLS sample.
Fig. 2.
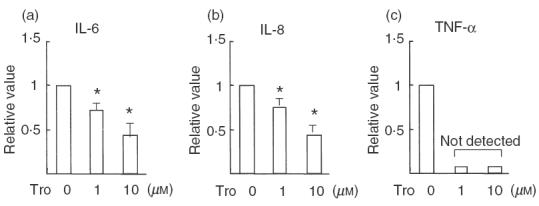
Inhibition of cytokine production from FLS by troglitazone. FLS (samples from six RA patients) were cultured with or without troglitazone (1 or 10 μm) for 72 h, and the protein concentrations of (a) IL-6, (b) IL-8 and (c) TNF-α in the cultured supernatants were examined by ELISA, respectively. Data are mean ± s.d. concentrations relative to the corresponding values in unstimulated FLS from six RA samples. Note that the production of IL-6 and IL-8 from FLS was dose-dependently suppressed by troglitazone, and that the TNF-α concentrations in the FLS culture media decreased to undetectable levels in the presence of troglitazone. * P <0·01 ( Student's t-test).
Fig. 3.
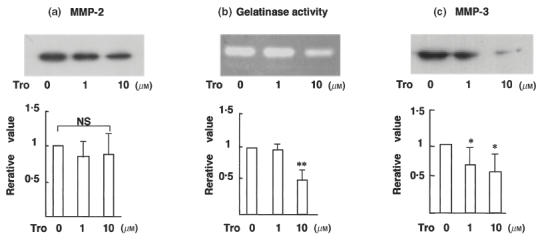
Troglitazone inhibits MMP-3 production and gelatinase activity, but not that of MMP-2, in FLS culture media. Western blot analysis of (a) MMP-2 (n = 7), (b) MMP-3 (n = 7) and (c) gelatin zymography (n = 5) for the analysis of functional gelatinase activity. The upper panel shows representative films, and the lower panel shows the mean ± s.d. values relative to the corresponding values in unstimulated FLS. FLS were incubated for 24 h for the analysis of MMP-2 production, 72 h for MMP-3 production and 24 h for the assay of gelatinase activity. Note that the production of MMP-3 and the functional gelatinase activity present in FLS culture media were both inhibited by troglitazone. Troglitazone did not suppress MMP-2 production by FLS. * P <0·05; ** P <0·01 ( Student's t-test); n.s.: no significant differences among groups.
Troglitazone inhibits NF- κB nuclear activity in FLS
NF-κB is an important nuclear factor and induces the expression of a variety of genes in response to cytokine stimulation [25]. Unstimulated FLS did not exhibit any nuclear NF-κB activity at baseline conditions, as determined by EMSA (Fig. 4a). Stimulation of FLS with TNF-α or IL-1β augmented nuclear NF-κB DNA-binding activity, and PPARγ stimulation markedly suppressed NF-κB activity in response to TNF-α or IL-1β (Fig. 4a). Addition of cold oligonucleotide almost completely inhibited NF-κB binding activity of TNF-α- and IL-1β-treated FLS (Fig. 4b). These results indicate the specificity of NF-κB nuclear activity as determined in the present study. The nuclear NF-κB activity of MT-2 cells was demonstrated as a positive control, and was also clearly inhibited by adding cold oligonucleotide (Fig. 4c).
Fig. 4.
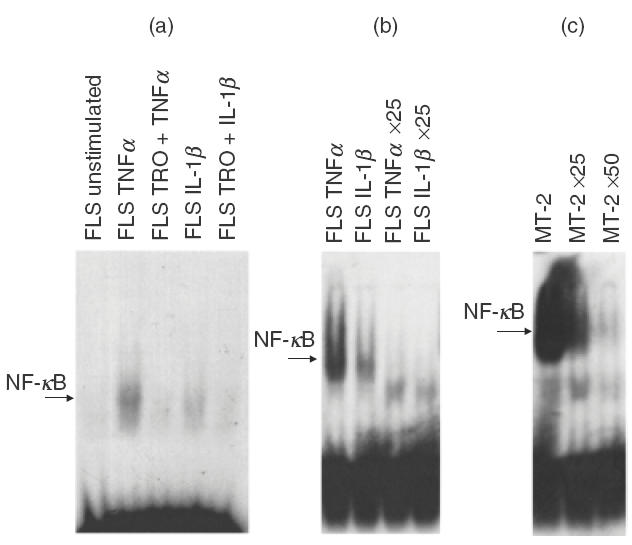
Troglitazone inhibits NF-κB nuclear activity in FLS. FLS were cultured with or without 10 μm troglitazone for 24 h, and further incubated in the presence or absence of TNF-α (200 IU/ml) or IL-1β (20 IU/ml) for 30 min. After incubation, nuclear NF-κB DNA binding activity in FLS was investigated by EMSA. (a) Representative experiment using FLS isolated from RA patients. Note the lack of basal NF-κB nuclear activity in FLS; however, NF-κB activity was induced in FLS cells treated with TNF-α and IL-1β. The latter response was abrogated by pretreatment of troglitazone (10 μm). TRO: pretreated with 10 μm of troglitazone for 24 h. Results shown are representative data of six experiments. (b) Inhibition of NF-κB nuclear activity in TNF-α- and IL-1β-treated FLS by adding excess cold oligonucleotide. ×25 : Addition of 25 times the high molar concentration of cold oligonucleotide. Results shown are representative data of three experiments. (c) Positive control for NF-κB nuclear activity determined in MT-2 cells. Note the detection of NF-κB nuclear activity in MT-2 cells, which was clearly inhibited by adding excess cold oligonucleotide (×25 : addition of 25 times the high molar concentration of cold oligonucleotide; ×50 : addition of 50 times the high molar concentration of cold oligonucleotide).
Differentiation of FLS into adipocyte-like cells by PPARγ stimulation
We then examined whether FLS could differentiate into adipocytes following troglitazone treatment in long-term cell cultures. A 1-week culture of FLS with troglitazone (1 or 10 μm) induced dose-dependent significant changes in cell morphology, such as round-shape transformation and the appearance of lipid-like vesicles in the cytoplasm (data not shown). Furthermore, 3 weeks of culture with troglitazone augmented such morphological changes in FLS (Fig. 5a,b,c), and resulted in the marked accumulation of lipids within the cells, as demonstrated by the strong cell staining with Oil Red O (Fig. 5d,e,f). Furthermore, endogenous cytokine production from FLS-differentiated adipocyte-like cells was apparently lower compared with non-transformed FLS (IL-6 production; patient 1 : 133 pg/ml in adipocyte-like cells and 226 pg/ml in non-transformed FLS; patient 2 : 45 pg/ml in adipocyte-like cells and 747 pg/ml in non-transformed FLS).
Fig. 5.
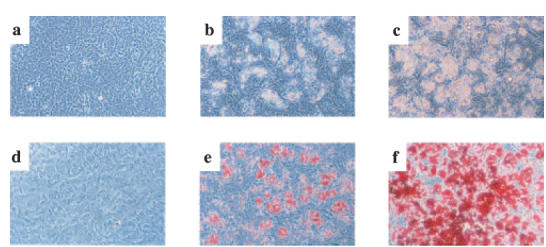
Troglitazone-mediated differentiation of FLS into adipocyte-like cells. FLS were cultured with or without troglitazone for three weeks. a-c: Phase contrast microscopy, demonstrating morphological changes in FLS. (a) unstimulated FLS; (b) FLS cultured with 1 μm of troglitazone. c: FLS cultured with 10 μm of troglitazone. Original magnification, 200×. The morphological changes included transformation to round cells and the appearance of lipid-like vesicles in the cytoplasm. These changes were troglitazone dose-dependent. (d–f) Detection of lipid accumulation in FLS by Oil Red O staining. (d) unstimulated FLS; (e) FLS cultured with 1 μm of troglitazone; (f) FLS cultured with 10 μm of troglitazone. Original magnification, ×200. Note that lipid accumulation in FLS was troglitazone dose-dependent. Results shown are representative data of five experiments.
DNA fragmentation (the presence of hypodiploid DNA+ cells) as well as disruption of mitochondrial function (disruption of mitochondrial transmembrane potential), both indicators of cell apoptosis, were not observed in FLS cultured under the above conditions in the presence of troglitazone at 1 μm or 10 μm (data not shown). It should be noted that 1 and 10 μm troglitazone are lower concentrations than that used to induce apoptosis of synovial cells (40 μm troglitazone) in a previous report [18]. However, the addition of higher concentrations of troglitazone (30–50 μm) induced apoptosis of confluent FLS (data not shown).
DISCUSSION
PPARγ is a multi-functional and ligand-dependent transcriptional factor expressed in various cell lineages [12–18]. Recent studies have reported the functional expression of PPARγ in cultured human FLS and that activation of the PPARγ signalling pathway by its ligands can induce apoptosis of the cells [18]. However, other functional properties of PPARγ in rheumatoid synovial cells have not been fully investigated. The concentrations of troglitazone used in the present study were up to 10 μm. At these concentrations, troglitazone did not induce apoptosis of the cultured FLS. However, at these concentrations, troglitazone clearly modulated the functions of FLS, as manifested by the inhibition of IL-6, IL-8, TNF-α and MMP-3 production. These cytokines and MMP-3 are considered to play pivotal roles in the pathological process of RA [1–3,26,27]. Thus, PPARγ stimulation using a relatively low concentration of its ligand may reduce the chronic inflammatory responses in rheumatoid synovial tissue without inducing apoptosis. Our cultures of FLS contained dexamethasone, and therefore troglitazone-mediated inhibition of cytokine and MMP production by FLS could be associated with the biological effects of glucocorticoids.
NF-κB, which is expressed and activated in rheumatoid synovial cells in vivo, is an important transcriptional factor for perpetuating rheumatoid synovitis [4–6]. In our study, unstimulated cultured FLS did not show a detectable nuclear NF-κB activity by EMSA. However, its activity was clearly induced in FLS in response to TNF-α or IL-β, and the induced activity was significantly suppressed by pretreatment with troglitazone. TNF-α and IL-1β are considered key proinflammatory molecules in the cytokine network of RA. Activation of NF-κB triggered by these cytokines in the synovial tissue plays a central role in the progression of chronic inflammation observed in rheumatoid synovial tissues [1–6]. Therefore, PPARγ-mediated NF-κB suppression highlights the therapeutic potential of PPARγ ligands in patients with RA. As mentioned above, the endogenous production of cytokines and MMP-3 by FLS was also inhibited by troglitazone. The troglitazone-mediated suppression in unstimulated FLS could also be mediated by the inhibition of a small amount of physiologically active NF-κB that cannot be determined by EMSA. Furthermore, other nuclear factors, such as AP-1 and STATs [15], could also be involved in troglitazone-mediated inhibition of cytokines and MMP-3 production. In addition, recent studies have indicated that the anti-inflammatory effect of PPARγ ligands on macrophage lineage cells is PPARγ-independent [28]. Therefore, further studies of PPARγ ligand-mediated inhibition of cytokine and MMP-3 production in FLS are necessary to determine the underlying molecular mechanisms.
We succeeded in differentiating FLS into adipocyte-like cells by incubating the cells with PPARγ ligand for relatively long periods of time. In this regard, recent studies have reported the presence of cells expressing mesenchymal lineage cell markers in the rheumatoid synovium, and indicated that cultured rheumatoid FLS also express the same cell marker [19]. Moreover, Pittenger et al. [29] have shown that human adult mesenchymal stem cells in the bone marrow could potentially differentiate into multilineage cell types. In our study, long-term culture of FLS with troglitazone resulted in the phenotypic change of a sizable proportion of FLS into adipocyte-like cells. Although we could not determine that the differentiated adipocyte-like cells were genuine adipocytes, it is conceivable that rheumatoid FLS are derived from mesenchymal stem cells, and could potentially differentiate into adipocytes under proper stimulation.
Kawahito et al. [18] have indicated the possible usefulness of PPARγ ligands in the treatment of RA through the induction of synovial cell apoptosis. The present study identified another mechanism for PPARγ ligand in modulating synovial cell function without inducing apoptosis. PPARγ stimulation of FLS appears to inhibit the proinflammatory activity of NF-κB. Furthermore, long-term cell culture of FLS with PPARγ ligand resulted in differentiation of these cells into adipocyte-like phenotype, and endogenous cytokine production from adipocyte-like cells was also inhibited compared with non-transformed FLS. Taken together, stimulation of PPARγ expressed in rheumatoid synovial cells enhance our understanding of the factors that could potentially modulate synovial cell function and differentiation.
Acknowledgments
The authors thank Dr Itaru Furuichi and Takahiko Aoyagi (Department of Orthopedics, National Ureshino Hospital, Saga, Japan) for providing synovial samples of RA.
REFERENCES
- 1.Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990;33:768–73. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- 2.Arend WP, Dayer JM. Cytokines and cytokine inhibitor or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33:305–15. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 3.Arend WP, Dayer JM. Inhibition of the production and effect of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–60. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 4.Fujisawa K, Asahara H, Okamoto K, et al. Therapeutic effect of the anti-Fas antibody on arthritis in HTLV-1 tax transgenic mice. J Clin Invest. 1996;98:271–8. doi: 10.1172/JCI118789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miagkov AV, Kovalenko DV, Brown CE, et al. NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–64. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomita T, Takeuchi E, Tomita N, et al. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor κB decoy oligonucleotides as a therapy. Arthritis Rheum. 1999;42:2532–42. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann JM, Moore LB, Smith-Oliver TA, et al. An antidiabetic thiazolidinenedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ) J Biol Chem. 1995;270:12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 8.Forman BM, Tontonoz P, Chen J, et al. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 9.Kliewer SA, Lenhard JM, Willson TM, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 10.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ, a lipid-activated transcription factor. Cell. 1994;79:1147–56. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPARγ and C/EBP. Proc Natl Acad Sci USA. 1995;92:9856–60. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang C, Ting AT, Seed B. PPARγ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 13.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator-activated receptor γ negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem. 1999;274:32048–54. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 14.Yang XY, Wang LH, Chen T, et al. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor γ (PPARγ) agonists. J Biol Chem. 2000;275:4541–4. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 15.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 16.Tsuboichi Y, Sano H, Kawahito Y, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor γ agonist thorough induction of apoptosis. Biochem Biophys Res Commun. 2000;270:400–5. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
- 17.Padilla J, Kaur K, Cao HJ, et al. Peroxisome proliferator-activated receptor γ agonist and 15-deoxy-delta (12, 14) (12, 14)–PGJ2 induce apoptosis in normal and malignant B–lineage cells. J Immunol. 2000;165:6941–8. doi: 10.4049/jimmunol.165.12.6941. [DOI] [PubMed] [Google Scholar]
- 18.Kawahito Y, Kohno M, Tsubouchi Y, et al. 15-deoxy-Δ12,14-PGJ2 induces synoviocytes apoptosis and suppresses adjuvant-induced arthritis in rats. J Clin Invest. 2000;106:189–97. doi: 10.1172/JCI9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinova-Mutafchieva L, Taylor P, Funa K, et al. Mesenchymal cells expressing bone morphogenetic protein receptors are present in the rheumatoid arthritis joint. Arthritis Rheum. 2000;43:2046–55. doi: 10.1002/1529-0131(200009)43:9<2046::AID-ANR16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami A, Nakashima T, Sakai H, et al. Regulation of synovial cell apoptosis by proteasome inhibitor. Arthritis Rheum. 1999;42:2440–8. doi: 10.1002/1529-0131(199911)42:11<2440::AID-ANR23>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Desvergne A, Wahli W. Peroxisome proliferator-activated receptors. Nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 23.Hirai Y, Migita K, Honda S, et al. Effects of nitric oxide on matrix metalloproteinase-2 production by rheumatoid synovial cells. Life Sci. 2001;68:913–20. doi: 10.1016/s0024-3205(00)00998-x. [DOI] [PubMed] [Google Scholar]
- 24.Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113–6. [Google Scholar]
- 25.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 26.Okada Y. Matrix-degrading metalloproteinases and their roles in joint destruction. Mod Rheumatol. 2000;10:121–8. doi: 10.3109/s101650070018. [DOI] [PubMed] [Google Scholar]
- 27.Ribbens C, Andre B, Jasper JM, et al. Matrix metalloproteinase-3 serum levels are correlated with disease activity and predict clinical response in rheumatoid arthritis. J Rheumatol. 2000;27:888–93. [PubMed] [Google Scholar]
- 28.Chawla A, Barak Y, Liao D, et al. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]


