Abstract
Antibodies to the degenerate repeats of EB200, a part of the Plasmodium falciparum antigen Pf332, are protective in monkeys. To analyse the prevalence, magnitude and specificity of antibodies to EB200 in malaria-exposed humans, the IgG antibody reactivity with recombinant EB200 protein as well as with crude malaria antigen was determined in Senegalese donors (n = 100; 4–87 years). Antibody reactivity with EB200 was low or absent in children below 15 years but was prevalent and significantly higher in older donors. In comparison, all individuals displayed reactivity with a crude malaria antigen preparation, which also increased with age. The reactivity with the crude malaria antigen was correlated to the reactivity with EB200, suggesting that the low levels of IgG to EB200 found in some adult donors reflected a limited degree of recent exposure to parasites rather than a selective non-responsiveness to Pf332. Comparison of serological and clinical data showed that high levels of antibodies to crude malaria antigen and to EB200 were predictive of fewer future clinical attacks of malaria. A reactivity pattern very similar to that found in Senegalese donors was observed in Liberian adults where 80% of the sera showed reactivity with EB200 and all peptides were recognized by between 60 and 100% of the donors. This strong reactivity with EB200-derived overlapping peptides suggests that the epitopes in EB200, to a large extent, are linear. In the light of previous data on the parasite neutralizing capacity of antibodies to Pf332, the present results emphasize the potential interest of Pf332-derived sequences for inclusion in a subunit vaccine against P. falciparum malaria.
Keywords: antibody specificity, B-cell epitope, Pf332, Plasmodium falciparum
INTRODUCTION
The humoral immune response plays an important role in protection against the asexual erythrocytic stages of Plasmodium falciparum parasites, the phase of malaria infection associated with clinical symptoms [1,2]. Mechanisms whereby antibodies directly interfere with parasites have been demonstrated in vitro and include inhibition of merozoite invasion or schizont rupture [3,4]. Antibodies, particularly of IgG1 and IgG3 subclasses, also act as opsonins, thereby mediating cellular elimination of parasitized red blood cells (PRBC) [5]. Characterization of antibody responses to parasite antigens and evaluation of their biological effects are key features for the evaluation of vaccine candidate antigens.
The P. falciparum asexual blood stage antigen Pf332 is expressed when parasites reach the trophozoite stage and it is translocated to the surface of mature schizonts [6,7]. Neither the function of the antigen, nor its fate after schizogony are known. Pf332 is a large protein of approximately 750 kDa [8], the complete sequence of which was recently available in the P. falciparum Genome Database (http://www.tigr.org/tdb/pfa1/htmls/index.shtml). It comprises predominantly degenerate repeats of about 11 amino acids with pairs of glutamic acids often spaced by mainly hydrophobic amino acids [7,8]. In addition to Pf332, a large number of other malaria proteins contain repetitive regions, frequently with a high content of glutamic acid [9]. These repetitive regions are often highly immunogenic and have been suggested to act as an immunological smokescreen by diverting the antibody response away from other more important epitopes [10,11]. However, antibodies to malaria antigen repeats can also display parasite-neutralizing capacities, both in vivo and in vitro [12,13]. Antibodies specific for Pf332 repeats inhibit parasite growth in vitro by interfering with intraerythrocytic parasite development or schizont rupture [4].
The recombinant Pf332-derived fragment EB200 is recognized by P. falciparum-reactive antibodies from monkeys infected with asexual blood stage parasites [13]. Such antibodies opsonize PRBC, a mechanism whereby Pf332-reactive antibodies may mediate cellular elimination of the parasite [14]. Immunization using EB200 also enhanced the level of opsonizing IgG in P. falciparum-exposed monkeys [13] although EB200 may also induce protection in monkeys in the absence of opsonizing antibodies [15].
Characterization of the antibody responses to EB200 following natural infection is of interest for a better understanding of the development of immunity to malaria as well as the immunogenicity of Pf332 during natural exposure. In the present study, human IgG antibody responses to the Pf332-derived fragment EB200 were examined with respect to prevalence, magnitude and specificity. Sera collected from Senegalese individuals, 4–87 years old, and from Liberian adults were analysed for reactivity with recombinant EB200. The Senegalese donors were monitored clinically for a period of 3 years prior to and 1 year after sampling. The fine specificity of EB200-reactive antibodies was analysed using a panel of peptides overlapping EB200. The results indicate that IgG antibody reactivity to EB200 is induced after repetitive exposure to P. falciparum parasites and is associated with total increased IgG reactivity with malarial antigens and with increased immunity to malaria. Analysis of the specificty of EB200-reactive antibodies demonstrated that antibodies induced by natural malaria infection are highly reactive with linear epitopes within the Pf332 molecule, suggesting that EB200 could be of interest for induction of protective immune responses if included in a subunit vaccine.
MATERIALS AND METHODS
Recombinant proteins
Two recombinant proteins containing EB200, a 135 amino acid region of the P. falciparum antigen Pf332 [7], were produced in Escherichia coli. EB200 was linked to either Schistosoma japonicum glutathione-S-transferase (GST) [16] (GST-EB200) or to ZZ [17], two IgG-binding domains of staphylococcal protein A (ZZ-EB200). Production and purification of these recombinant proteins as well as GST alone have been described elsewhere [7,16,18].
Peptides
Seventeen peptides, together overlapping EB200 were synthesized, P-1 to - 17 (Table 1). The peptides were 16 amino acids long with eight amino acid overlaps. Fourteen peptides were synthesized by Boc chemistry by Neosystems (Strasbourg, France) whereas the remaining three peptides (P-1, - 13 and - 14) were synthesized by Fmoc chemistry, as described previously [19]. Peptide 6 × 4, corresponding to six tetramer (EENV) repeats of Pf155/RESA, was obtained from Bachem (Bubendorf, Switzerland). All peptides were C-terminally amidated with a free amino terminus. Linear peptides displayed one major peak in reverse phase HPLC [20]. Peptides P-1 to -4, -8, -13 and -14 were analysed further by plasma desorption mass spectrometry, which verified that the peptides had the expected size.
Table 1.
Synthetic peptides overlapping the EB200 sequence
| Peptide* | Amino acid sequence |
|---|---|
| P-1 | ILVEGSVTEEVVGEEK |
| P-2 | EEVVGEEKLVSEEIVT |
| P-3 | LVSEEIVTEEGSVAQE |
| P-4 | EEGSVAQEIVEEDAPA |
| P-5 | IVEEDAPATEEIDEIE |
| P-6 | TEEIDEIESVTEEVVE |
| P-7 | SVTEEVVEEEGPVDEE |
| P-8 | EEGPVDEEIVQEEGTV |
| P-9 | IVQEEGTVTEEIIQGE |
| P-10 | TEEIIQGESKVEEVVE |
| P-11 | SKVEEVVEEQGSENEE |
| P-12 | EQGSENEEIFVEEVSA |
| P-13 | IFVEEVSASQEIVQNE |
| P-14 | SQEIVQNESGTEEILE |
| P-15 | SGTEEILEKVSASQEI |
| P-16 | KVSASQEIVQDGSVTE |
| P-17 | VQDGSVTEQIIEELFP |
Peptides P-1 to -17 span the entire sequence of the Pf332 fragment EB200 with eight amino acid overlaps [7]. Each peptide is 16 amino acids which overlaps the following peptide with eight amino acids.
Crude malaria antigen preparation
Cultures of the P. falciparum parasite strain F32 [21] were used to prepare a crude malaria antigen extract as described previously [22].
Immunization of rabbits
Two New Zealand white rabbits were immunized intramuscularly in the hind legs with 100 μg each of ZZ-EB200 emulsified in Freund's complete adjuvant (FCA, Difco, Bacto, Detroit, MI, USA). Booster injections were given three weeks later using the same amount of antigen in Freund's incomplete adjuvant (FIA). Venous blood to obtain antiserum to ZZ-EB200 was drawn from the ear.
Human serum samples
A panel of 100 sera was collected from donors aged 4–87 years living in the P. falciparum-endemic area of Dielmo, Senegal. All samples were collected in March 1994 and each individual was monitored for clinical attacks of malaria for a period of 36 months prior to the sampling and up to 12 months afterwards. The donors had spent 90·5 ± 17% of the 3 previous years in Dielmo (95% confidence interval = 87·0–94·0%). Clinical attacks of malaria were defined as febrile episodes with temperature = 38·5°C coinciding with parasitaemia levels over an age-dependent pyrogenic threshold defined for this village [23]. The sex of the individuals was distributed evenly in all age groups; 4–9, 10–14, 15–19 and >20 years of age, and for each individual the G6PD level and Hb phenotype was determined. A panel of 83 sera were collected at the LAMCO Hospital, Yekepa, Liberia, from healthy adult (>15 years) blood donors living in a P. falciparum malaria holoendemic and perennial area (kindly provided by Dr A. Björkman). Sera from nine Swedish donors not exposed to malaria served as controls. All samples were collected with informed consent and the study was approved of by the Ministry of National Health in Senegal and the National Ethic Committee in Sweden.
ELISA
High-binding EIA/RIA plates (Costar, Cambridge, MA, USA) were adsorbed for 12 h at 4°C with antigens in phosphate-buffered saline (PBS) at concentrations of 2 μg/ml for recombinant proteins, 10 μg/ml for crude malaria antigen extract and 10 μg/ml for peptides conjugated to bovine serum albumin (BSA) [20]. Duplicate wells were blocked in PBS with 1% BSA at 20°C for 2 h followed by incubation of sera in fivefold dilutions ranging from 1 : 200 to 1 : 25000 in PBS with 0·1% BSA and 0·05% Tween for 1 h at 37°C. To detect specific IgG, goat antirabbit IgG (Mabtech, Stockholm, Sweden) or rabbit antihuman IgG (Sigma Chemical Co., St Louis, MO, USA) conjugated to alkaline phosphatase were incubated as above followed by addition of p-nitrophenylphosphate (Sigma). Absorbance at 405 nm was measured using a Vmax microplate reader (Molecular Devices, Menlo Park, CA, USA). Between incubations, plates were washed in PBS containing 0·05% Tween. Human IgG reactivity in ELISA is displayed either as absorbance values or as a percentage of the reactivity of a human antimalaria serum standard (1 : 1000) reacting with the Pf155/RESA-derived peptide (EENV)6 at a fixed absorbance value (2·0). In the cases where cut-off levels for positivity were determined, those were calculated as the mean reactivity + 3 s.d. of nine Swedish control sera.
Immunofluorescence assay (IF)
Indirect IF was performed on monolayers of non-fixed air-dried P. falciparum (strain F32) cultures as described previously [24].
Affinity purification of human antibodies on Pf332 peptides
Human antibodies were affinity purified on four of the Pf332-derived peptides: P-2, -6, -12 and -17. CNBr-activated Sepharose 4B (1·5 ml) (Pharmacia, Uppsala, Sweden) was charged with 0·2 mg of peptide according to instructions of the manufacturer. Two ml of serum diluted with two ml PBS were applied to the column and incubated on a roller drum overnight at 4°C. After extensive washing, antibodies were eluted with 3m KSCN and dialysed against PBS with 0·1m glycine. Affinity purified antibodies were analysed for reactivity with parasite antigen in IFA or with recombinant EB200 and synthetic peptides in ELISA.
Statistical analysis
Logarithmic transformation was applied to our data and used for statistical analysis in order to reduce the levels of skewness [25]. We used either LogIgG and LogG6PD or Log (1 + malaria attacks) to allow for zero values. For univariate analysis of continuous data, unpaired, two-tailed Student's t-test was used and correlations were tested by linear regression analysis. For multivariate analysis, the association between the pattern of antibody response and the number of malaria attacks observed over a 1-year period was tested using a stepwise logistic regression model available in the JMP® software (SAS Institute Inc., Cary, NC, USA). Age, sex, G6PD (dichotomized as <6·5 or ≥6·5 units), EB200 IgG and parasite extract IgG values were considered as covariates and the logistic model included the haemoglobin phenotype, which was categorized into AA or non-AA. Non-significant covariates were eliminated through the likelihood ratio criterion. The test of the goodness-of-fit of the model was not significant, indicating that the model fitted the data well, which were then tested in a non-linear fit model using a Poisson distribution.
RESULTS
Antigenicity of recombinant EB200 and EB200-derived peptides
Rabbit antisera to ZZ-EB200 were used to analyse the antigenicity of GST-EB200 and a panel of peptides spanning EB200. The sera stained the cytoplasm of non-fixed late stage PRBC in IF at dilutions up to 1 : 15000 (data not shown) in a pattern obtained previously with other antibodies to Pf332 [6,26]. No IF staining was seen using GDA-fixed PRBC, indicating that the sera were not cross-reactive with Pf155/RESA repeats [26,27]. In ELISA, the antisera reacted well with GST-EB200 but not GST (Fig. 1). All EB200-derived peptides except for P-10 were also well recognized by at least one of the antisera, provided the peptides were conjugated to BSA (Fig. 1). The lack of reactivity with non-conjugated peptides was probably due to their inability to adhere to plastic [28].
Fig. 1.
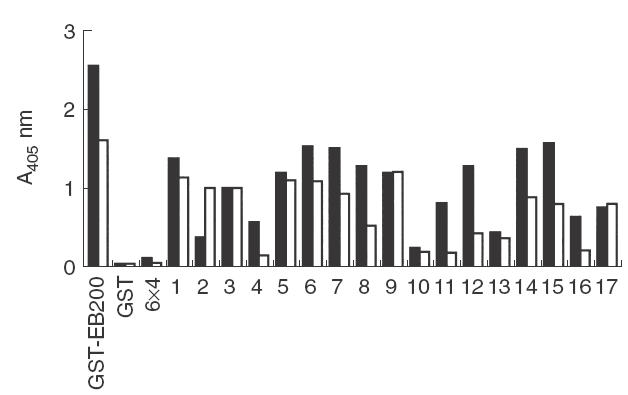
Specificity of rabbit antisera to EB200. Sera from two rabbits immunized with ZZ-EB200 were analysed for reactivity with GST-EB200 and a panel of peptides spanning EB200 (P-1 to -17) in ELISA. GST and the RESA repeat-derived peptide 6 × 4 (EENV)6, were included for comparison. The graph displays reactivity of sera diluted 1 : 500 with one serum indicated by black bars and the other with white bars.
Human serum IgG reactivity with crude malaria antigen and EB200
To investigate the natural antibody response to asexual blood stage malaria antigens and in particular to Pf332, sera from donors residing in the malaria-endemic village Dielmo in Senegal were assessed by ELISA for their reactivity with crude malaria antigen extract or recombinant EB200 (Fig. 2). Results presented and used for statistical analysis were obtained with sera diluted 1 : 1000. The reactivity at this dilution reflected the proportional serum reactivities analysed at dilutions ranging from 1 : 200 to 1 : 25000. For univariate statistical analysis, the donors were divided into age groups of 4–9, 10–14, 15–19 and >20 years.
Fig. 2.
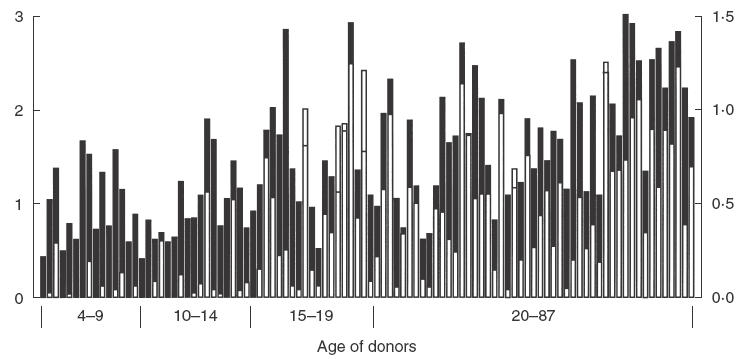
Reactivity of sera from Senegalese donors with crude malaria antigen and EB200. Sera from 100 donors were analysed at a dilution of 1 : 1000 for reactivity with crude malaria antigen extract as well as with EB200 (GST-EB200 reactivity minus GST reactivity) in ELISA. The individual donors (4–87 years) are displayed according to age and the different age groups are indicated below the bars. White bars display reactivity with EB200 with absorbance values indicated on the right y-axis. Reactivity with crude malaria antigens is shown as black bars with the absorbance values displayed on the left x-axis. The level of background reactivity of Swedish control sera was 0·150 ± 0·031 (crude malaria antigen), 0·094 ± 0·024 (GST-EB200) and 0·088 ± 0·024 (GST). In cases where the white bars mask the black bars, the height of the black bar is indicated by a horizontal line. □, Recombinant EB200; ▪, crude malaria antigen.
Antibody reactivity to crude malaria antigen extract was found in children in the age of 4–9 years and increased with age (R = 0·585; R2 = 0·342; P ≤ 0·0001; n = 100) (Fig. 2). Sera from all individuals displayed IgG reactivity well above the background reactivity of Swedish control sera. When the levels of IgG reactivity were compared between age groups, no significant difference was seen between the age groups 4–9 and 10–14 years, while there was a significant increase in IgG reactivity in the age group 15–19 years (Table 2). No further increase was observed in individuals above 20 years.
Table 2.
IgG antibody reactivity with crude malaria antigen and EB200 in Senegalese individuals grouped by age and data on their experience of clinical attacks of malaria
| IgG reactivity in ELISA (mean ± s.d.)* | |||||
|---|---|---|---|---|---|
| Age group (n) (years) | Crude antigen | EB200‡ | Individuals with clinical attack† | Number of attacks over 1 year | Mean number of attack per person |
| 4–9 (17) | 0·47 ± 0·21 | 0·10 ± 0·16 | 52·9% | 23 | 1·35 (0–5) |
| 10–14 (17) | 0·51 ± 0·19 (P = 0·45) | 0·24 ± 0·35 (P = 0·22) | 29·4% | 15 | 0·88 (0–8) |
| 15–19 (14) | 0·73 ± 0·27 (P = 0·013) | 0·91 ± 0·75 (P = 0·001) | 7·1% | 1 | 0·07 (0–1) |
| 20–87 (49) | 0·92 ± 0·33 (P = 0·054) | 1·11 ± 0·67 (P = 0·27) | 6·1% | 3 | 0·06 (0–1) |
| Total (97) | 0·74 ± 0·28 | 0·73 ± 0·54 | 18·5% | 42 | 0·43 |
| Controls (9) | 0·15 ± 0·03 | 0·01 ± 0·01 | |||
Sera were assessed for antibody reactivity at dilutions of 1 : 1000. Logarithmic absorbance values were used for unpaired Student's t-test. The P-values refer to the statistical difference between the indicated group and the age group above.
Percentage of individuals in the group that suffered from one or more clinical attacks of malaria as defined by febrile illness (temperature = 38·5°C) coinciding with parasitaemia level over an age-dependent threshold level previously defined in this village [23]. The 97 individuals shown in the table were actively monitored for a period of 1 year starting immediately after the blood sampling for antibody analysis was taken. They were present in the village during 94 ± 15% of the year of follow-up.
Reactivity with EB200 was estimated by subtracting the reactivity with GST from the reactivity with GST-EB200.
Further, the antibody reactivity with EB200 correlated well with age (R = 0·554; R2 = 0·307; P ≤ 0·0001; n = 100) but, in contrast to what was seen with the crude malaria antigen, most sera from the age groups 4–9 and 10–14 years exhibited low or no reactivity with EB200 (Fig. 2). There was a slight increase in reactivity, although not statistically significant, in the children from the age group 10–14 years. Similar to what was seen with the reactivity with crude malaria antigen, there was a sharp increase in the group 15–19 years compared to children of 10–14 years (P = 0·0027). There was also a strong correlation between IgG reactivity with crude malaria antigen extract and EB200 (R = 0·711; R2 = 0·506; P ≤ 0·0001; n = 100) (Fig. 2).
The EB200 reactivity was estimated by subtracting the reactivity with S. japonicum GST from the reactivity with GST-EB200. Antibodies cross-reacting with GST did thus not interfere with the analysis of EB200 reactivity. The reactivity with GST was generally low, irrespective of donor age (mean A405 <0·11 for all age groups), and comparable to that of Swedish control sera (mean A405 ± s.d. = 0·088 ± 0·024).
Comparison of serological and clinical data
All Senegalese individuals were monitored actively and continuously for clinical attacks of malaria during a period of 1 year starting immediately after the blood sampling used for antibody analysis. The observations carried out on 97 of 100 inhabitants of Dielmo who were present for 94% of the year (i.e. who were residing for a mean of 343 ± 56 days, 95% CI = 332–355) are shown (Table 2). In children 4–9 years of age, nine of 17 experienced one or more attacks. The number of individuals suffering from clinical attacks was lower in the age groups of 10–14 years (five of 17) and 15–19 years (one of 14). Three of 49 individuals above 20 years of age had a malaria attack during the year of follow-up. The mean number of attacks was also the highest in children of the age groups 4–9; this number declined in individuals 10–14 years and was lowest after 14 years of age. The levels of antibody reactivity with crude malaria antigen extracts, as well as with EB200, were higher in the 79 individuals who did not experience any clinical attack over the year of study compared to the 18 who had clinical attacks (mean O.D. value ± 1 s.d. = 0·799 ± 0·327 versus 0·483 ± 0·263 for reactivity with P. falciparum and 0·877 ± 0·734 versus 0·195 ± 0·251 for reactivity with EB200). Age was different between these two groups of individuals (28 ± 18 years for inhabitants without malaria attack versus 13 ± 9 years for patients with malaria attack). Antibody responses against the parasite extract or EB200 were then tested in an automated stepwise regression program including age, sex, G6PD, Hb phenotype, the actual time spent in the village during the 3 previous years and the number of malaria attacks observed during the year following the blood sampling. Among the all one-variable tested, and after backward stepwise elimination of the non-significant terms, the best predictors of a decreased number of malaria attacks were the antibody responses against the parasite extract (F ratio = 16·61; P <0·0001) or that against EB200 (F ratio 13·34; P = 0·0004) when the age variable was retained in the program. A Poisson model was established with iteratively reweighted least squares to fit to the significant variables which included age and antibody responses. Even when age was included in the fitted model, an effect of the antibodies was found for anti- P. falciparum extract (χ2 = 7·61; P = 0·0058) and for anti-EB200 (χ2 = 5·70; P = 0·017). Seven women pregnant during part of the study period were excluded from the analysis but inclusion of these individuals did not modify the results of the analysis.
Specificity of serum IgG for EB200-derived peptides
The fine specificity of IgG reactive with EB200 was studied using a panel of sera from 83 adult Liberians (Fig. 3a). Sera were analysed for reactivity with EB200 as well as with 17 EB200-derived overlapping peptides (P-1 to -17). Almost 80% of the sera showed reactivity with EB200 and all peptides were recognized by between 60 and 100% of the donors. Some of the most frequently recognized peptides, especially P-6 and -8, showed high levels of reactivity with a high proportion of the donors. In contrast, a major proportion of positive responders to P-1, -11 and -13 to -16 reacted weakly, albeit still above the cut-off level. When comparing the peptide specificity of EB200-reactive sera from Senegalese donors, a very similar reactivity pattern was found (data not shown).
Fig. 3.
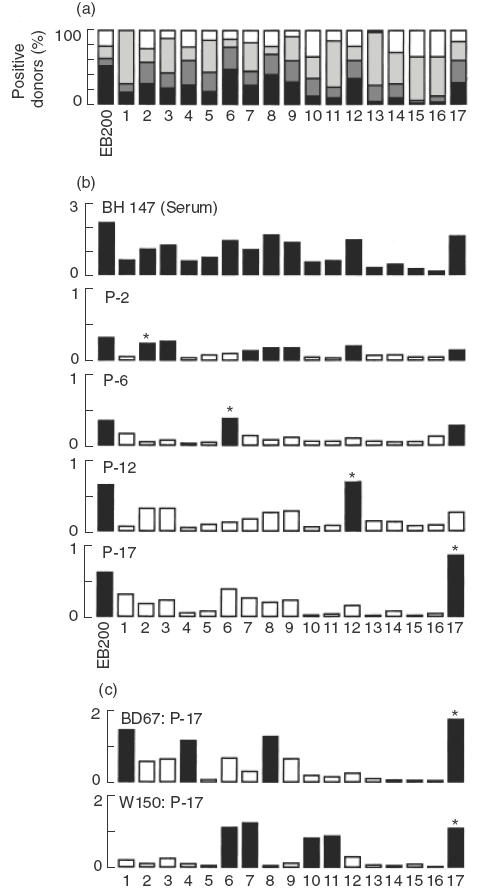
Fine specificity of human IgG antibodies reactive with EB200. (a) Sera from 83 Liberian adults were analysed at a dilution of 1 : 1000 for antibody reactivity with EB200 and a panel of peptides together spanning the EB200 sequence in ELISA. The reactivity was defined as percent reactivity of a human immune serum reactive with peptide 6 × 4 and was graded as below cut off, >cut off 15%, >15%–25% and >25%. Cut-off for each ELISA antigen was defined as the mean reactivity of Swedish control sera +3 s.d. (b) Antibodies affinity purified on peptide P-2, -6, -12 or -17 were tested for reactivity with EB200 as well as with P-1 to -17 in ELISA. Reactivity with EB200 and peptides displayed by serum from donor BH147 diluted 1 : 1000 and affinity purified IgG from the same donor diluted 1 : 300. (c) Peptide specificity of antibodies affinity purified on P-17 from donor BD67 and W150, respectively. (b and c) The y axes present the ELISA absorbance values at 405 nm. Reactivities of >50% of the reactivity with the peptide used for purification are indicated with black bars. Reactivities of less than 50% are depicted with white bars. Asterisks indicate the peptide used for purification. □, ≤Cut-off;  , <cut-off 15%;
, <cut-off 15%;  , >15–25%; ▪, >25%.
, >15–25%; ▪, >25%.
The sequence homologies between the EB200-derived peptides make it reasonable to expect a certain degree of cross-reactivity of antibodies to epitopes within these peptides. In order to analyse the level of cross-reactivity, peptides P-2, -6, -12 and -17 were used to affinity purify antibodies from four human sera displaying high reactivity with these peptides. The eluted antibodies stained the cytoplasm of nonfixed PRBC in IF with the same pattern as the rabbit antisera to EB200 (data not shown) and recognized EB200 in ELISA (Fig. 3a,b).
The affinity purified antibodies were further analysed for reactivity with P-1 to P-17. As shown with antibodies from one donor, purified antibodies recognized the peptide used for purification but displayed different reactivity patterns with the other peptides (reactivity defined as ≥50% of the absorbance observed with the peptide used for purification) (Fig. 3b). Thus, antibodies purified on P-2 reacted equally well with P-2 and the overlapping peptide P-3 as well as several other peptides. Antibodies purified on the other three peptides reacted predominantly with the peptide used for purification, although antibodies to P-6 also recognized P-17. Antibodies purified from other sera also reacted well with the homologous peptide but displayed variable reactivity patterns with the other peptides, as exemplified with two additional antibody fractions purified on P-17 (Fig. 3c). These reactivity patterns indicate that antibody populations from different donors, although recognizing the same peptide, may differ in their fine specificity.
DISCUSSION
Antibody responses to EB200, a part of the P. falciparum asexual blood stage antigen Pf332, in humans continuously exposed to malaria were investigated. The interest in Pf332 derives partly from the observation that the Pf332-reactive human monoclonal antibody 33G2 inhibits parasite growth and cytoadherence in vitro [27,29]. Antibody 33G2 is, however, highly cross-reactive with other repetitive malaria antigens and thus the involvement of Pf332 itself in cytoadherence is uncertain [26]. However, the direct parasite-inhibitory action has been confirmed with various polyclonal antibodies specific for Pf332 [4,26]. In addition, others have reported that EB200-reactive IgG antibodies act as opsonins [13,14].
In sera from the Senegalese children analysed herein, antibodies reactive with both a crude malaria antigen preparation and recombinant EB200 were detected, although many children displayed no or low reactivity with EB200. Antibody levels to both the crude P. falciparum antigen and EB200 increased significantly by age and most donors above 15 years had antibodies to EB200. Thus, although some children between 4 and 14 years had antibodies to EB200, it appears for many children that a long period of continuous malaria exposure is required before high antibody levels to Pf332 are detected, as measured by recombinant EB200 ELISA. However, it is possible that the children may occasionally experience high, but short-lived, peaks of antibodies, which rapidly decline after resolution of the infection. IgG subclasses were not analysed in this study, but a previous study in the same village demonstrated that IgG3 is the predominant anti-EB200 subclass [30]. The IgG subclass pattern of the anti-EB200 antibodies in this area is, thus, compatible with the late appearance of these antibodies after repeated exposure to P. falciparum [31].
In some studies, consistently low levels of antibodies to certain malaria antigens have been observed at an individual level, despite continuous malaria exposure [32–34]. The mechanisms underlying such low responsiveness to particular antigens are unclear and have been suggested to be dependent on HLA haplotypes [32] or other host genetic factors [35] but could also be due to other stochastic events [33,34]. Although immune responses to EB200 have been shown to be genetically restricted in mice [18], no indications suggest that genetic restriction limits the responsiveness to EB200 in humans. Sera from adults that displayed low reactivity with EB200 had low antibody levels to the crude malaria antigens as well. Thus, poor reactivity with EB200 in adults is likely to reflect a low degree of recent exposure to malaria rather than a marked low responsiveness to Pf332. Moreover, when T-cell responses to EB200 were analysed in donors from Dielmo, no correlation between low or high responsiveness with particular HLA molecules was found [36].
A previous study in Dielmo, Senegal showed that approximately 90% of children between 4–9 and 10–14 years of age carried P. falciparum at least once during June–September (1992) while the number of parasitaemic individuals in older age groups was lower, 77% in 15–19 years and below 60% in older individuals (estimated between June and September 1990) [37]. The significant increase in antibody levels to the crude malaria antigen as well as to EB200 observed in the present study between the age groups 10–14 and 15–19 years thus coincides with decreased parasite prevalence in these groups. The correlation between high antibody levels to malaria antigens as well as to EB200 and fewer clinical attacks seen in the present study suggested that both the levels of antibodies against the P. falciparum extract and against EB200 were predictors of protection.
Many of the EB200-derived peptides were well recognized in ELISA by sera from Liberian and Senegalese adults analysed herein. The reactivity pattern was similar to that seen in a previous study, analysing a limited number of adult sera from Senegal [36]. The finding that certain peptides were more frequently recognized than others suggests that some of the degenerate repeats of Pf332 form immunogenically and/or antigenically more attractive B-cell epitopes. The recognition of these peptides and the fact that antibodies affinity purified on EB200-derived peptides recognize native Pf332 indicate that epitopes within the Pf332 repeats to a large extent are of a continuous nature.
Adult sera from Liberia and Senegal recognized some of the EB200-derived peptides with a prevalence higher than for recombinant EB200. However, many of these peptides were recognized rather weakly and it is uncertain whether or not an EB200 peptide ELISA would provide a better tool for detection of Pf332 reactivity than a recombinant EB200 ELISA. Nevertheless, using a Pf332-derived peptide as an ELISA antigen has previously been shown to detect a high prevalence of Pf332-reactive antibodies in Tanzanian children as early as the age of 1–2 years [38].
There is limited information regarding the variability of the Pf332 gene and gene product. There appears to be a certain degree of variation in the Pf332 gene due to its subtelomeric position in chromosome 11 [39]. Breaking and healing of genes occurring in subtelomeric regions are known to influence the gene variation [39]. However, so far it appears as if the degenerate repeats of Pf332 are expressed in all parasites isolates analysed [40,41] and that the expression is stable when parasites are passaged in monkeys [42].
The role of repetitive gene sequences in malaria proteins is not known. One function of repeats may be involvement in molecular mimicry of the RBC cytoskeleton protein spectrin [43]. However, one prevailing theory has been that repeats act as an immunological smokescreeen by eliciting low affinity antibody responses and antibodies with a high degree of cross-reactivity without protective capacity [10]. It has also been suggested that these repeats induce T-cell independent B-cell responses [11]. Although the cross-reactive nature of antibodies to repeats is indisputable, which has been confirmed in this study, other findings speak against the nonprotective nature of repeats; antibodies to repeats have been shown to have antiparasite effects both in vivo and in vivo and have also been shown to elicit T-cell dependent immune responses [4,13,26,36].
Taken together, the EB200 region of Pf332 fulfills a number of criteria of importance for a vaccine candidate antigen. The present study suggests that the EB200 region of Pf332 is highly immunogenic in humans and that increased IgG anti-EB200 responses are associated with a decreased number of malaria attacks. Overall, the antibody response directed against EB200 appears as a valuable biological marker of protection and, as suggested recently in a study from the same village [30], the antigen might be the target of an immunological mechanism of protection against P. falciparum. In this case, inclusion of EB200 in a subunit vaccine could thus lead to an earlier and more rapid onset of potentially protective IgG antibody responses in children which are highly susceptible to P. falciparum malaria infection.
Acknowledgments
This work was supported by grants from UNDP/World Bank/WHO Special Programme for Research in Training in Tropical Diseases, the Swedish Medical Research Council and the Swedish Agency for Research Cooperation with Developing Countries (SIDA/SAREC). The authors thank Mrs Ingegärd Andersson for excellent technical assistance and Dr Denise Mattei for providing the plasmid encoding GST-EB200. The fieldwork in Senegal was supported by a grant from the Ministère de la Coopération (Paris) and we are grateful to the inhabitants of Dielmo for their active participation and continuing collaboration in this long-term project. We also thank all field doctors, nurses, technicians and field workers which were involved in health care, data collection and laboratory tests.
REFERENCES
- 1.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–41. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen S, McGregor IA, Carrington S. Gamma-globulin and aquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 3.Blackman MJ, Heidrich H-G, Doniachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–82. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahlborg N, Iqbal J, Björk L, Ståhl S, Perlmann P, Berzins K. Plasmodium falciparum: differential parasite growth inhibition mediated by antibodies to the antigens Pf332 and Pf155/RESA. Exp Parasitol. 1996;82:155–63. doi: 10.1006/expr.1996.0020. 10.1006/expr.1996.0020. [DOI] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria. Evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–81. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinterberg K, Scherf A, Gysin J, et al. Plasmodium falciparum. The Pf332 antigen is secreted from the parasite by a Brefeldin A dependent pathway and is translocated to the erythrocyte membrane via the Maurer's clefts. Exp Parasitol. 1994;79:279–91. doi: 10.1006/expr.1994.1091. 10.1006/expr.1994.1091. [DOI] [PubMed] [Google Scholar]
- 7.Mattei D, Scherf A. The Pf332 gene codes for a megadalton protein of Plasmodium falciparum asexual blood stages. Mem Inst Oswaldo Cruz. 1992;87:163–8. doi: 10.1590/s0074-02761992000700026. [DOI] [PubMed] [Google Scholar]
- 8.Wiesner J, Mattei D, Scherf A, Lanzer M. Biology of giant proteins of Plasmodium: resolution on polyacrylamide-agarose composite gels. Parasitol Today. 1998;14:38–40. doi: 10.1016/s0169-4758(97)01155-1. [DOI] [PubMed] [Google Scholar]
- 9.Berzins K, Anders RF. The malaria antigens. In: Wahlgren M, Perlmann P, editors. Malaria molecular and clinical aspects. Chur, Switzerland: Harwood Academic Publishers; 1999. pp. 181–216. [Google Scholar]
- 10.Anders RF. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986;8:529–39. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 11.Schofield L, Uadia P. Lack of Ir gene control in the immune response to malaria. I. A thymus-independent antibody response to the repetitive surface protein of sporozoites. J Immunol. 1990;144:2781–8. [PubMed] [Google Scholar]
- 12.Berzins K, Perlmann H, Wåhlin B, et al. Passive immunization of Aotus monkeys with human antibodies to the Plasmodium falciparum antigen Pf155/RESA. Infect Immun. 1991;59:1500–6. doi: 10.1128/iai.59.4.1500-1506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perraut R, Mercereau-Puijalon O, Mattei D, et al. Induction of opsonizing antibodies after injection of recombinant Plasmodium falciparum vaccine candidate antigens in preimmune Saimiri sciureus monkeys. Infect Immun. 1995;63:554–62. doi: 10.1128/iai.63.2.554-562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gysin J, Gavoille S, Mattei D, et al. In vitro phagocytosis inhibition assay for the screening of potential candidate antigens for sub-unit vaccines against the asexual blood stage of Plasmodium falciparum. J Immunol Meth. 1993;159:209–19. doi: 10.1016/0022-1759(93)90159-5. [DOI] [PubMed] [Google Scholar]
- 15.Perraut R, Mercereau-Puijalon O, Mattei D, et al. Immunogenicity and efficacy trials with Plasmodium falciparum recombinant antigens identified as targets of opsonizing antibodies in the naive squirrel monkey Saimiri sciureus. Am J Trop Med Hyg. 1997;56:343–50. doi: 10.4269/ajtmh.1997.56.343. [DOI] [PubMed] [Google Scholar]
- 16.Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-tranferase. Gene. 1988;6:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson B, Moks T, Jansson B, et al. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1:107–13. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 18.Ahlborg N, Sterky F, Haddad D, et al. Predominance of H-2d and H-2k-restricted T-cell epitopes in the highly repetitive Plasmodium falciparum antigen Pf332. Mol Immunol. 1997;34:379–89. doi: 10.1016/s0161-5890(97)00046-1. [DOI] [PubMed] [Google Scholar]
- 19.Sällberg M, Rudén U, Magnius LO, Norrby E, Wahren B. Rapid ‘tea-bag’ peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol Lett. 1991;30:59–68. doi: 10.1016/0165-2478(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 20.Berzins K, Perlmann H, Wåhlin B, et al. Antibodies to repeated amino acid sequences in Pf155, a merozoite associated antigen of Plasmodium falciparum. Mem Inst Oswaldo Cruz. 1986;81(Suppl. 2):77–81. [Google Scholar]
- 21.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Troye-Blomberg M, Perlmann H, Patarroyo ME, Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983;53:345–53. [PMC free article] [PubMed] [Google Scholar]
- 23.Rogier C, Commenges D, Trape JF. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg. 1996;54:613–9. doi: 10.4269/ajtmh.1996.54.613. [DOI] [PubMed] [Google Scholar]
- 24.Perlmann H, Berzins K, Wahlgren M, et al. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early stages of Plasmodium falciparum. J Exp Med. 1984;159:1686–704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuinness D, Bennett S, Riley E. Statistical analysis of highly skewed immune response data. J Immunol Meth. 1997;201:99–114. doi: 10.1016/s0022-1759(96)00216-5. [DOI] [PubMed] [Google Scholar]
- 26.Ahlborg N, Iqbal J, Hansson M, et al. Immunogens containing sequences from antigen Pf332 induce Plasmodium falciparum-reactive antibodies which inhibit parasite growth but not cytoadherence. Parasite Immunol. 1995;17:341–52. doi: 10.1111/j.1365-3024.1995.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 27.Udomsangpetch R, Carlsson J, Wåhlin B, et al. Reactivity of the human monoclonal antibody 33G2 with repeated sequences of three distinct Plasmodium falciparum antigens. J Immunol. 1989;142:3620–6. [PubMed] [Google Scholar]
- 28.Geerlings HJ, Weijer WJ, Bloemhoff W, Welling GW, Welling-Wester S. The influence of pH and ionic strength on the coating of peptides of herpes simplex virus type 1 in an enzyme-linked immunosorbent assay. J Immunol Meth. 1988;106:239–44. doi: 10.1016/0022-1759(88)90203-7. [DOI] [PubMed] [Google Scholar]
- 29.Udomsangpetch R, Aikawa M, Berzins K, Wahlgren M, Perlmann P. Cytoadherence of knobless Plasmodium falciparum infected erythrocytes and its inhibition by a human monoclonal antibody. Nature. 1989;338:763–5. doi: 10.1038/338763a0. [DOI] [PubMed] [Google Scholar]
- 30.Perraut R, Mercereau-Puijalon O, Diouf B, et al. Seasonal fluctuation of antibody levels to Plasmodium falciparum parasitized red blood cell-associated antigens in two Senegalese villages with different transmission conditions. Am J Trop Med Hyg. 2000;62:746–51. doi: 10.4269/ajtmh.2000.62.746. [DOI] [PubMed] [Google Scholar]
- 31.Wahlgren M, Berzins K, Perlmann P, Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti- P. falciparum antibodies in different sera. Clin Exp Immunol. 1983;54:135–42. [PMC free article] [PubMed] [Google Scholar]
- 32.Quakyi IA, Otoo LN, Pombo D, et al. Differential non-responsiveness in humans of candidate Plasmodium falciparum vaccine antigens. Am J Trop Med Hyg. 1989;41:125–34. [PubMed] [Google Scholar]
- 33.Riley EM, Bennett S, Jepson A, et al. Human antibody responses to Pfs230, a sexual stage-specific surface antigen of Plasmodium falciparum: non-responsiveness is a stable phenotype but does not appear to be genetically regulated. Parasite Immunol. 1994;16:55–62. doi: 10.1111/j.1365-3024.1994.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 34.Taylor RR, Egan A, McGuinness D, et al. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but is reminiscent of clonal imprinting. Int Immunol. 1996;8:905–15. doi: 10.1093/intimm/8.6.905. [DOI] [PubMed] [Google Scholar]
- 35.Modiano D, Chiucchiuini A, Petrarca V, et al. Humoral response to Plasmodium falciparum Pf155/ring-infected erythrocyte antigen and Pf332 in three sympatric ethnic groups of Burkina Faso. Am J Trop Med Hyg. 1998;58:220–4. doi: 10.4269/ajtmh.1998.58.220. [DOI] [PubMed] [Google Scholar]
- 36.Kulane A, Siddique AB, Sarthou J-L, et al. Human immune responses to the highly repetitive Plasmodium falciparum antigen Pf332. Am J Trop Med Hyg. 1999;61:141–8. doi: 10.4269/ajtmh.1999.61.141. [DOI] [PubMed] [Google Scholar]
- 37.Trape J-F, Rogier C, Konate L, et al. The Dielmo project. a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–37. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 38.Warsame M, Wernsdorfer WH, Perlmann H, et al. A malariometric survey in a rural community in the Muheza District, Tanzania. age profiles in the development of humoral immune responses. Acta Trop. 1997;68:239–53. doi: 10.1016/s0001-706x(97)00100-9. [DOI] [PubMed] [Google Scholar]
- 39.Mattei D, Scherf A. Subtelomeric chromosome instability in Plasmodium falciparum: short telomere-like sequence motifs found frequently at healed chromosome breakpoints. Mutat Res. 1994;324:115–20. doi: 10.1016/0165-7992(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 40.Mercereau-Puijalon O, Jacquemot C, Sarthou JL. A study of the genomic diversity of Plasmodium falciparum in Senegal 1. Typing by Southern blot analysis. Acta Trop. 1991;49:281–92. doi: 10.1016/0001-706x(91)90079-y. [DOI] [PubMed] [Google Scholar]
- 41.Haddad D, Snounou G, Mattei D, et al. Limited genetic diversity of Plasmodium falciparum in field isolates from Honduras. Am J Trop Med Hyg. 1999;60:30–4. doi: 10.4269/ajtmh.1999.60.30. [DOI] [PubMed] [Google Scholar]
- 42.Fandeur T, Vazeux G, Mercereau-Puijalon O. The virulent Saimiri-adapted Palo Alto strain of Plasmodium falciparum does not express the ring-infected erythrocyte surface antigen. Mol Biochem Parasitol. 1993;60:241–8. doi: 10.1016/0166-6851(93)90135-k. [DOI] [PubMed] [Google Scholar]
- 43.Werner EBE, Taylor WR, Holder AA. A Plasmodium chabaudi protein contains a repetitive region with a predicted spectrin-like structure. Mol Biochem Parasitol. 1998;94:185–96. doi: 10.1016/s0166-6851(98)00067-x. [DOI] [PubMed] [Google Scholar]


