Abstract
The new immunosuppressive agent mycophenolate mofetil (MMF) has been shown recently to exert a protective effects in certain animal models of autoimmunity, including diabetes in diabetes-prone bio-breeding (BB) rats. In the present study, the immunomodulatory potential of MMF was investigated in autoimmune diabetes induced by multiple low doses of streptozotocin (MLD-STZ) in genetically susceptible DA rats 20 mg STZ/kg body weight (b.w.) for 5 days] and CBA/H mice (40 mg STZ/kg b.w. for 5 days). In both species, short time treatment of animals with MMF (25 mg/kg) during the early development of the disease, as well as continuous MMF treatment, prevented the appearance of hyperglycaemia and inflammatory infiltrates in the pancreatic tissue. Moreover, clinical manifestations of diabetes were suppressed by application of the drug after the onset of clinical symptoms. Treatment with guanosine (1 mg/kg) in parallel with MMF completely reversed MMF activity in vivo, indicating that inhibition of inosine monophosphate dehydrogenase (IMPDH) was responsible for the observed suppressive effects. MMF-mediated protection from diabetes correlated with reduced ex vivo spontaneous spleen mononuclear cell (MNC) proliferation and defective adhesive cell interactions. MMF-treated animals also had lower local production of IFN-γ, as well as IL-12 and nitric oxide (NO) production by peripheral tissues (spleen and peritoneal cells), compared to that in control diabetic groups, while IL-10 level was elevated. Together, these data demonstrate that MMF interferes with autoimmune process in streptozotocin-induced diabetes at multiple levels, including lymphocyte proliferation and adhesion, as well as pro/anti-inflammatory cytokine balance.
Keywords: adhesion molecules, autoimmune diabetes, cytokines, mycophenolate mofetil, streptozotocin
INTRODUCTION
In insulin-dependent diabetes mellitus (IDDM), the β-cell loss and subsequent hyperglycaemia are the outcome of an autoimmune process leading to inflammatory reaction with accumulation of macrophages and T cells in the islets [1–3]. Although results obtained with pancreas or islet transplantation are promising, the interference with deleterious autoimmune response by using immunosuppressive or immunomodulating agents still remains a conceivable strategy for IDDM treatment. However, conventional calcineurine-inhibiting immunosuppressants CsA and FK506 are not appropriate for such a task, because their actions include decreased insulin secretion, increased insulin resistance or direct toxic effect on the β-cells [4,5]. Accordingly, both drugs can cause post-transplantation diabetes mellitus [6], while CsA was also found to exacerbate the disease symptoms in an animal model of IDDM [7].
Mycophenolate mofetil (MMF) is a novel xenobiotic immunosuppressant that has proved effective in the therapy of transplant rejection, as well as in various animal models of autoimmunity (for review, see [8]). Through its active metabolite mycophenolic acid (MPA), MMF inhibits the activity of inosine monophosphate dehydrogenase (IMPDH), a rate-limiting enzyme in the synthesis of guanosine nucleotides [9]. MPA exerts more potent antiprolifferative effects on lymphocytes than on other cell types, as it is fairly selective for the isoform II of IMPDH, which is expressed mainly in activated T and B cells [10]. Moreover, through depletion of intracellular GTP levels and subsequent suppression of glycosylation and the expression of some adhesion molecules, MPA interferes with the recruitment of lymphocytes and monocytes into sites of inflammation [11,12]. Hao et al. [13] reported that MMF prevented spontaneous development of autoimmune diabetes in genetically predisposed bio-breeding (BB) rats, but the protection ceased with the termination of the therapy. Furthermore, the mechanisms responsible for the beneficial action of MMF in this diabetes model were not investigated.
In the present study we have explored the effect of MMF in the autoimmune diabetes induced in rats and mice by multiple low doses of streptozotocin (MLD-STZ), which offers a number of experimental advantages. The islet lesions of MLD-STZ diabetes, initiated mainly by infiltrating T cells and macrophages, resemble closely those of human disease [14]. Moreover, the onset of disease is controlled and the animals involved do not have immune abnormalities that complicate studies in models of spontaneous autoimmune diabetes [15]. Here we present evidence that MMF provides a long-lasting protection in rodent MLD-STZ-induced diabetes, interfering with autoimmune diabetogenic process at several levels, including lymphocyte proliferation and adhesiveness, as well as the production of proinflammatory mediators.
MATERIALS AND METHODS
Reagents
Streptozotocin (STZ, S-0130), mycophenolic acid (MPA), sulphanilamide and naphthylethylenediamine dichidrochloride were purchased from Sigma (St Louis, MO, USA). Mycophenolate mofetil (MMF, CellCept®) was obtained from Roche (Basel, Switzerland). RPMI-1640 supplemented with 1mm HEPES buffer, 5% fetal calf serum (FCS), 1% sodium pyruvate, 2 mm l-glutamine (all from Flow Laboratories, Irvine, UK), penicillin/streptomycin (ICN, Yugoslavia) and 5 × 10–5m 2-mercaptoethanol (Sigma) was used as a culture medium. Recombinant rat IL-1β was from Genzyme (Boston, MA, USA). Mouse MoAbs specific for rat CD4 (W3/25, Serotec, Oxford, UK), MHC class II (OX6, HBT, Leiden, the Netherlands), IFN-γ (DB-1, HBT) and monocytes/macrophages (ED-1, kindly provided by Dr C. D. Dijkstra, Medical Faculty, Free University, Amsterdam, the Netherlands), as well as rat MoAbs specific for mouse CD4 (GK1·5) and CD8 (YTS 169) (both from Serotec) were used for immunohistochemical staining.
Animals and treatments
Male Dark Agouti (DA) rats and CBA/H mice were obtained from our own facility (Institute for Biological Research ‘Sinisa Stankovic’, Belgrade, Yugoslavia) and used when 10–16 weeks old. Diabetes was induced into two rodent species with multiple subtoxic doses of STZ 20 mg/kg body weight (b.w.)/day in rats, and 40 mg/kg b.w./day in mice], given i.p. for 5 consecutive days. In order to evaluate the effect of MMF on the disease development in rats, the drug was administrated i.m. at a dose of 25 mg/kg/day by three treatment protocols: continuously throughout the experiment (7 weeks), or as a 10-day treatment started either jointly with MLD-STZ doses (the first injection of MMF given at day 0), or after MLD-STZ (starting at day 5 in relation to the last STZ dose). Mice were treated with the same dose of MMF, with or without guanosine (1 mg/kg b.w./day), for the 2 weeks starting jointly with MLD-STZ. Control animals received saline in the same way as MMF. Plasma glucose was determined by a glucose-oxidase method using a glucometer (Glucotronic C; Macherey-Nagel, Duren, Germany) once a week for up to 7 weeks. Clinical diabetes was defined by hyperglycaemia in non-fasted animals (blood glucose >11mmol/l). In order to collect cells and tissues for further analysis, a groups of rats and mice were sacrificed on day 10 or 15, respectively, when the treatment with MMF was completed.
Immunohistochemical analysis of pancreas
At sacrifice, pancreata were taken out and fixed overnight in 4% paraformaldehyde in PBS and subsequently transferred to 30% sucrose at 4°C. Fixed pancreata were embedded in PolyFreeze tissue freezing medium (Polyscience, Warrington, PA, USA) and sections were cut in a cryomicrotome. The air-dried specimens (10–12 μm thick) on gelatinized slides were fixed for 10–15min in acetone at −20°C. After blocking of endogenous peroxidase with 0·3% H2O2 in methanol, sections were incubated for 1h with primary antibodies (W3/25, ED-1, DB-1, OX6, GK1·5, YTS 169). Staining of rat tissue was performed using mouse ExtrAvidin peroxidase staining kit (Sigma), according to the manufacturer's instruction. For mouse tissue, peroxidase-conjugated rabbit anti-rat IgG (DAKO, Hamburg, Germany) was used as a secondary antibody. All incubations were performed at room temperature (RT) and between each step slides were washed with PBS. Finally, sections were incubated for 2–5min with diaminobenzamidine (DAB, Dako) as a substrate diluted in 0·02m PBS pH 7·2 and then counterstained with Mayer's haematoxylin. Slides were mounted with Canada balsam and analysed under the light microscope. Immunohistochemical examination was undertaken in a blind fashion by two independent observers. A minimum of 100 islets per animal were analysed for each marker. Islets were graded as follows according to mononuclear cell infiltration: grade 0, normal islets totally free of any mononuclear cells stained for a given marker; grade 1, two to five positively stained mononuclear cells within the islet; grade 2, more than five positively stained mononuclear cells within the islet. The histology score gives the mean percentages of infiltration grade in each category of the islets analysed.
Ex vivo lymphoproliferative response
Spleen mononuclear cells (MNC) were obtained as previously described [16]. The cells (5 × 105/well in 200 μl) were cultured in triplicate in flat-bottom 96-well plates with 1 μCi [3H]-thymidine (specific activity 5 Ci/mmol; ICN, Costa Mesa, CA, USA) at 37°C in 5% CO2 for 24 h, harvested on an automatic harvester and [3H]-thymidine incorporation was measured in a liquid scintilation counter. In order to determine ConA-induced proliferative response, cells were cultured with or without ConA (1μg/ml) for 48 h, then pulsed with [3H]-thymidine for the last 18h and counted by liquid scintilation.
Quantitative cell aggregation assay
The analysis of spontaneous cell aggregation was performed as described previously [17]. Briefly, spleen MNC (1 × 105/well in 100 μl) were placed in flat-bottom 96-well plates and incubated at 37°C alone or in the presence of MPA (10 μm). After 2h of incubation, free cells were counted in a haemocytometer. The percentage of cells in aggregates was calculated as follows: 100 × 1 − (number of free cells in the test/number of free cells in control], where control represented the total number of free cells (1 × 106/ml).
Colorimetric assay for splenocyte adherence to fibroblasts and to plastic
To measure the adhesion of spleen MNC to fibroblasts or plastic, we used a modification of crystal violet assay for adherent cells staining [18]. Pancreatic fibroblasts were obtained as previously described [19], incubated overnight in 96-well flat-bottom plates (6 × 103/well) and stimulated with IL-1β (10ng/ml) for additional 24h. Afterwards, fibroblast cultures were washed and spleen MNC (2·5 × 105 in 100 μl) were added. Alternatively, MNC were added to empty wells to test their adherence to plastic. Following 2h of incubation at 37°C, non-adherent cells were removed by gentle washing with warm Hanks's medium supplemented with 2% FCS. Adherent cells were then fixed with methanol and stained with 1% crystal violet solution for 10 min. Plates were washed thoroughly with PBS, 100 μl 33% acetic acid was added to each well and the absorbance of dissolved dye, corresponding to the number of adherent cells, was measured in a microplate reader at 570nm.
Cytokine and nitric oxide measurement
Spleen MNC, pancreatic islets and peritoneal macrophages used for ex vivo detection of cytokines and nitric oxide (NO) have been prepared as previously described [16]. Cells were incubated in culture medium for 48h at 37°C in 5% CO2 and supernatants were collected for cytokine and nitrite determination. Alternatively, nitrites were measured in PEC supernatants after 24h of incubation. Nitrite accumulation, an indicator of NO production, was measured using Griess reagent [20]. Briefly, 50 μl of culture supernatants were mixed with an equal volume of Griess reagent (mixture at 1:1 of 0·1% naphthyletilenediamine dihydrochloride and 1% sulphanilamide in 5% H3PO4), and incubated at RT for 10 min. The absorbance at 570 nm was measured in a microplate reader. Nitrite concentration was calculated from a NaNO2 standard curve. Rat IFN-γ was determined by enzyme-linked immunosorbent assay (ELISA) detection kit (Biosource International, Camarillo, CA, USA) according to manufacturer's instructions. Mouse IL-12p40, IFN-γ and IL-10 were measured by ELISA with paired antibodies (all from Pharmingen, San Diego, CA, USA). The assay sensitivity limit was <13 pg/ml for rat IFN-γ, and <20 pg/ml for all other cytokines.
Statistical analysis
The statistical significance of differences between examined groups was evaluated using the analysis of variance (anova), followed by Student–Newman–Keuls test for multiple comparisons. A P-value less than 0·05 was considered significant.
RESULTS
MMF reduces clinical and histological parameters of MLD-STZ-induced diabetes
Preliminary experiments have shown that MMF administration for 2 weeks did not significantly alter blood glucose level, nor glucose tolerance in healthy animals, indicating that the drug itself was not toxic to β-cells (data not shown). In order to evaluate the ability of MMF to interfere with the diabetogenic process induced by external agents, streptozotocin, we used both murine and rat model of autoimmune diabetes. Although the dose regimens of β-cell toxin STZ for mice and rats were not identical in both rodent species, as reported previously [21,22], repeated injections with five subtoxic daily doses of STZ induced delayed and chronic hyperglycaemia 10–15 days after completion of the treatment (Fig. 1). Continuous application of MMF during the 7 weeks significantly reduced blood glucose level in DA rats (Fig. 1a). Moreover, animals exposed to either early or late 10-day MMF treatment kept normoglycaemic status even after the treatment was terminated (Fig. 1a). Similarly, blood glucose level was significantly lower in CBA/H mice receiving MMF for 2 weeks concomitantly with MLD-STZ, compared to control diabetic animals.
Fig. 1.
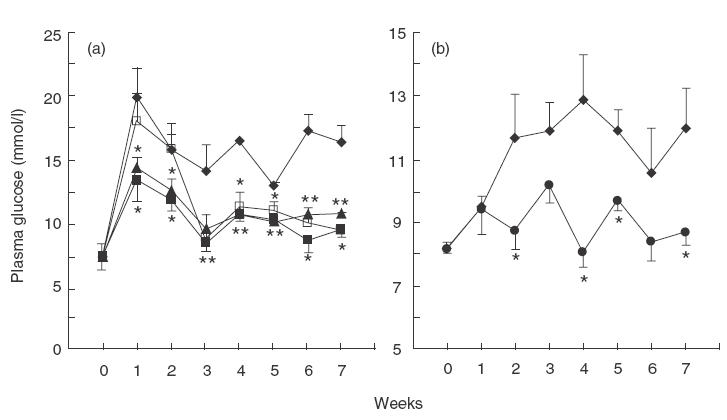
Effects of MMF treatment of rats and mice on MLD-STZ-induced hyperglycaemia. Plasma glucose levels in control DA rats (a), or CBA/H mice (b) receiving MLD-STZ only, or treated with MLD-STZ in conjunction with MMF (25 mg/kg/day) for different time regimes, as indicated. Results from a representative experiment are presented as mean + s.e.m. for five to six animals/group. * P <0·05 refers to treatment with MLD-STZ. (a) ◊, Control; ▴, MMF 0–9 days; ▪, MMF 0-49 days; □, MMF 10–19 days. (b) ◊, Control; •, MMF 0–14 days.
The interference of MMF with a pathological process leading to islet disfunction has further been studied at the level of target tissue. Rats and mice were killed on days 10 and 15 in relation to the first STZ injection, respectively, the time at which histological changes were maximal [1,3], and immunostaining of pancreata was performed. At this time, analysis of control MLD-STZ-induced diabetic mice revealed heavy mononuclear infiltrates in some of the islets (Fig. 2b), while in rats small infiltrates were seen rarely and infiltrating cells were scattered throughout the islets (Fig. 2a). Immunohistochemical analysis confirmed our earlier findings [3,21] that in rats the majority of mononuclear cells participating in insulitis process were blood-borne ED-1+ and MHC class II+ macrophages, while CD4+ and IFN-γ+ cells were also detected (Fig. 2a). In comparison to diabetic rats, pancreatic islets obtained from animals treated with MMF during the induction of diabetes were mainly without infiltrated cells, and only few spatially distributed cells positive for these markers could be found in a small number of the islets (Fig. 2a). Similarly, in MMF treated mice there were much larger numbers of normal islets without inflammatory cells, or islets with only mild infiltrates of CD4+ and CD8+ cells (Fig. 2b) in comparison to diabetic control mice. Apoptotic cells with characteristic condensation of nuclear chromatin or fragmented into small apoptotic bodies were noticed in the pancreatic sections of both diabetic rats (Fig. 2a) and mice (Fig. 2b), but were almost completely absent in MMF-treated animals. Although we were not able to confirm the origin of apoptotic cells, they were most probably β-cells because they did not stain for the markers characteristic for the infiltrated lymphocytes or macrophages. In general, although MMF prophylaxis could not suppress insulitis development and β-cell damage completely, it drastically reduced its severity, which was sufficient for the maintenance of normoglycaemic status (Fig. 1).
Fig. 2.
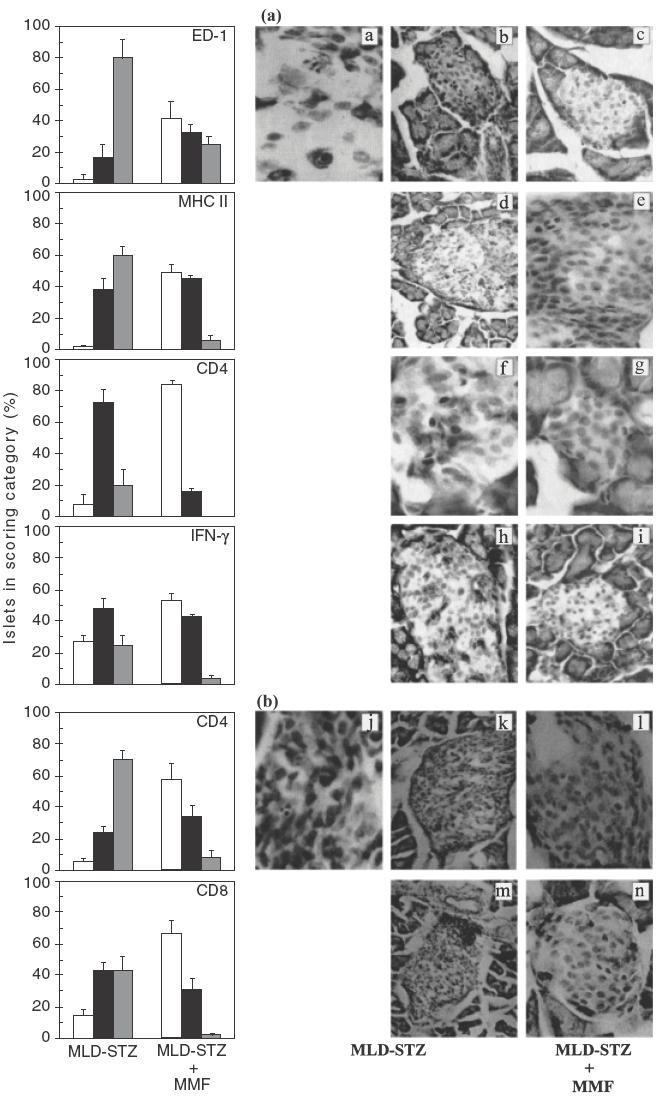
Effects of MMF treatment of rats and mice on inflammatory infiltration of the islets. Immunohistological analysis of islet-infiltrating cells in DA rats (a) or CBA/H mice (b) receiving MLD-STZ only versus animals treated with MLD-STZ plus MMF from day 0 to day 9 (a), or from days 0–14 (b). One day after termination of MMF treatments the sections of pancreata from five animals per group were stained with monoclonal antibodies specific for rat (a) ED-1 (a, b, c), MHC II (d, e), CD4 (f,g) and IFN-γ (h, i), or murine (b) CD4 (j, k, l) and CD8 (m, n) and were graded as described in Materials and methods. Bars indicated the mean of islets + s.e.m. in each category of mononuclear cell infiltration scored. Photos are paired and represent the staining pattern of control MLD-STZ-treated animals (left and middle panel), or MLD-STZ plus MMF-treated animals (right panel). Note phagocytosis of one cell undergoing apoptosis by the adjacent non-endocrine cell (a), and apoptotic cell surrounded by the infiltrated lymphocytes (j). □, Gr0; ▪, Gr1;  , Gr2.
, Gr2.
MMF down-regulates the proliferation and adhesive properties of spleen MNC
We next assessed the impact of MMF treatment on spontaneous, as well as mitogen-stimulated lymphoproliferative response in MLD-STZ-treated animals. As shown in Fig. 3 spontaneous proliferation of mononuclear spleen cells obtained from diabetic rats and mice was elevated significantly in comparison with healthy animals, probably reflecting the activation of autoreactive lymphocytes [16]. The MMF treatment almost completely reverted the proliferative activity of splenocytes to the control level. Because it was previously shown that MMF treatment could cause leukopenia in transplant recipients [23], there was a possibility that similar MMF effect might be operative in our experiments. However, while diabetic rats were leukopenic, the leucocyte number in MMF-treated rats was similar to that of healthy animals (Fig. 3c). Moreover, MMF treatment did not affect ConA-induced proliferative response of spleen MNC (Fig. 3d). To examine the influence of the drug on adhesive interactions, we performed ex vivo cell aggregation assay in the presence or absence of MMF active metabolite MPA. While spontaneous aggregation of splenocytes from diabetic rats was increased in comparison with cells from healthy animals, the treatment of rats with MMF strongly inhibited adhesive interactions of splenocytes (Fig. 4a). The addition of MPA down-regulated the increased cell clustering in splenocyte cultures of MLD-STZ-treated rats, but did not affect low aggregation of spleen MNC obtained from healthy or MMF-treated rats (Fig. 4a). MMF treatment of diabetic mice also inhibited ex vivo spontaneous cell aggregation (not shown), and caused a significant reduction of splenocyte adhesion to both plastic (Fig. 4c) and fibroblasts (Fig. 4b) as detected by colorimetric assay.
Fig. 3.
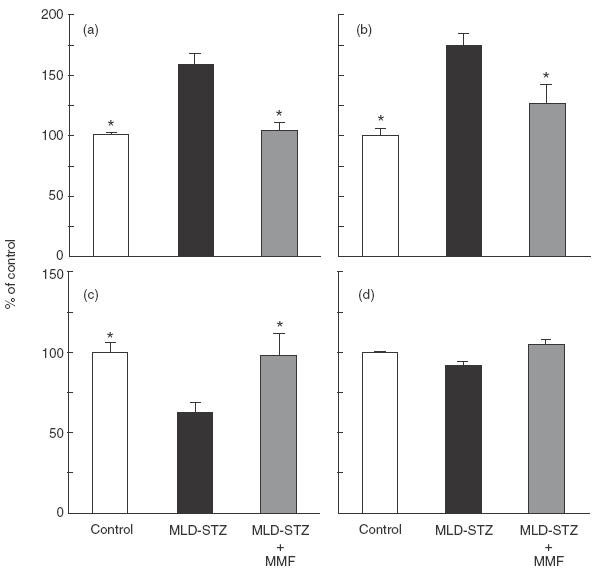
Effect of MMF on spleen mononuclear cell proliferation and peripheral blood leucocytes number. Incorporation of [3H]-thymidine by rat (a) and mouse (b) splenic MNC, as well as ConA-induced proliferation (d) of rat spleen MNC were determined as indicated in Materials and methods. Results from a representative experiment, presented as percentage of control (controls were 1749 cpm for (a), 8977 cpm for (b) and 254527 cpm for (d)) are given as mean ± s.e.m. for three to five animals per group. Peripheral blood leucocytes number (c) was counted after the completion of continuous MMF treatment in rats. Results, presented as percentage of control (value for control healthy animals was 14·59 × 109/l), are given as mean ± s.e.m. for five to six rats per group. * P <0·05 refers to MLD-SZ treatment.
Fig. 4.
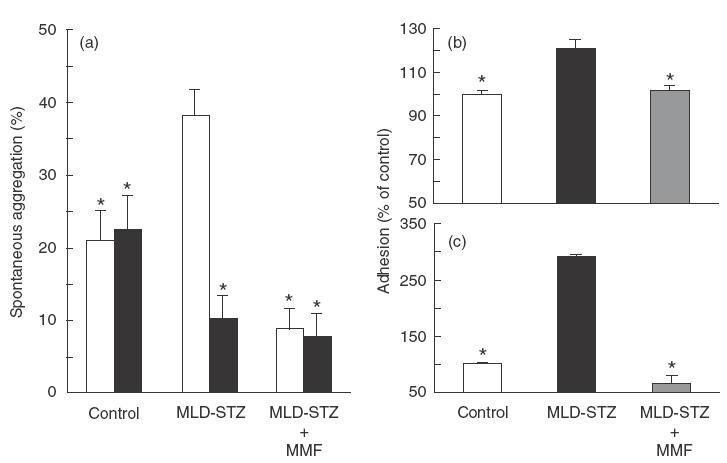
Effect of MMF on adhesive properties of spleen MNC. Spontaneous cell aggregation (a) was determined in spleen MNC obtained from control, MLD-STZ and MLD-STZ + MMF-treated rats on day 10. Cells were additionally cultured in the presence or absence of MPA (10 μm). Results represented mean + s.e.m. of individual analyses for three rats from each group. Colorimetric assays for adhesion of murine cells to IL-1-stimulated fibroblasts (b) and to plastic (c) were performed with splenocytes derived from the same groups of animals on day 15. Results from a representative experiment, presented as a percentage of control value of O.D. 570 (1·338 for (b); 0·144 for (c)), are means + s.e.m. for three mice per group. * P <0·05 refers to MLD-SZ treatment. □, Medium; ▪ MPA.
MMF suppresses IL-12 and NO production ex vivo
Interleukin-12 is a cardinal cytokine leading to induction of IFN-γ-producing Th1 cells which may participate in β cell destruction, partly through stimulation of NO release (for review, see [24]). We therefore assessed the influence of MMF treatment on the ex vivo production of proinflammatory mediators IL-12, IFN-γ and NO, and the data are summarized in Fig. 5. In the cell cultures from healthy animals, the levels of these mediators were below the limit of detection (data not shown). The accumulation of IL-12 and NO could not be detected in pancreatic islet cultures of diabetic animals (not shown). However, the production of IL-12 was observed readily in PEC and spleen MNC cultures obtained from MLD-STZ-treated mice (Fig. 5b). Following 14 days' treatment of mice with MMF, a significant reduction of IL-12 production was observed in both peritoneal macrophages and spleen cells. Similarly, NO production by PEC and spleen MNC cultures was up-regulated in diabetic mice and rats, but it was lower in animals that received MMF (Fig. 5a). While cultured pancreatic islets from diabetic mice did not release measurable amounts of IFN-γ, we observed low but detectable IFN-γ accumulation in supernatants of islet cultures from MLD-STZ-treated rats (Fig. 5a). Concordant with immunohistochemical findings (Fig. 2), IFN-γ production by pancreatic islets was significantly lower in rats which received MMF. On the other hand, IFN-γ synthesis by splenic MNC from streptozotocin-injected rats or mice was not affected by MMF administration (Fig. 5a). Interestingly, while anti-inflammatory cytokine IL-10 was not detectable in the islets (not shown), a clear increase of IL-10 synthesis was observed in splenocyte and PEC cultures from MMF-treated mice (Fig. 5b). Therefore, the protective effect of MMF in streptozotocin diabetes is associated with reduced ability of macrophages and spleen cells to produce IL-12 and NO, as well as with enhanced IL-10 release.
Fig. 5.
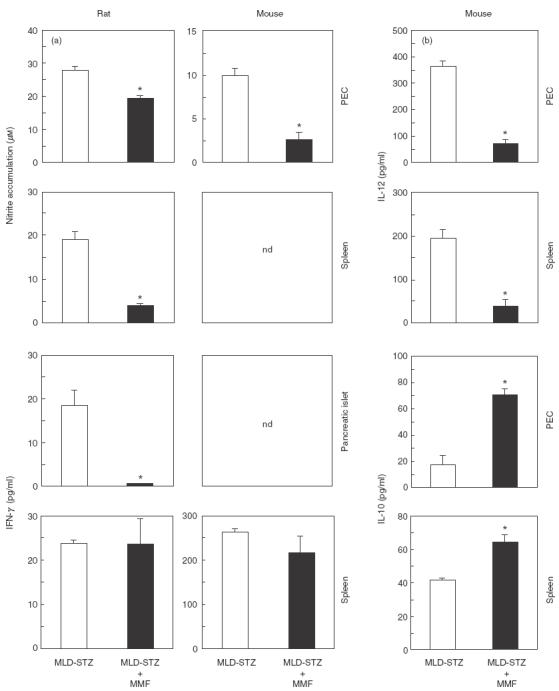
Effect of MMF on nitric oxide and cytokine production. Nitrite accumulation and production of IFN-γ (a), as well as IL-12 and IL-10 (b) were measured in the 24- or 48-h culture supernatants of cells derived from MLD-STZ and MLD-STZ + MMF-treated animals, as indicated in Materials and methods. Results from a representative experiment are presented as means of triplicate observations + s.e.m. for the pool of three to five animals pre group.* P <0·05 refers to MLD-SZ treatment; nd: not detectable.
Guanosine counteracts MMF-mediated protection in MLD-STZ diabetes
The inhibition of IMPDH with subsequent reduction of guanosine nucleotides has been considered a primary mechanism responsible for MMF immunosuppressive action. To assess whether this MMF action was also involved in down-regulation of streptozotocin-induced autoimmune diabetes in our study, we tested the effect of simultaneous guanosine administration on MMF mediated protection. The application of guanosine in the mouse MLD-STZ diabetes neutralized completely the protection afforded by MMF, reverting the plasma glucose concentration to the levels observed in animals which did not receive therapy. Thus, at day 28, the time when MLD-STZ-induced persistant hyperglycaemia was established (Fig. 1b), results of a representative experiment with five animals per group showed that glucose concentrations in mice concomitantly treated with MMF and guanosine and in control diabetic mice were similar (13·1 ± 0·5 mmol/l and 12·9 ± 1·4 mmol/l, respectively), in contrast to MMF-treated (8·1 ± 0·5 mmol/l), or normal healthy mice (8·2 ± 0·2 mmol/l), P <0·05. It should be noted that guanosine did not have any effect on blood glucose levels in healthy or MLD-STZ-treated animals in the absence of MMF (not shown). These data strongly suggest that the beneficial effect of MMF in our experiments was mediated exclusively through interference with de novo synthesis of guanosine nucleotides.
DISCUSSION
In the present study the immunosuppressive agent MMF exerted a strong long-lasting protection in a streptozotocin model of autoimmune diabetes in rodents. Destructive autoimmune response leading to loss of pancreatic β-cell function was impaired by the drug at multiple levels – the lymphocyte proliferation and adhesion, followed by their infiltration into pancreas, as well as the production of important proinflammatory mediators IL-12 and NO, were all inhibited by MMF treatment. While the beneficial effect of MMF in spontaneously diabetic BB rats has been previously reported [13], this is the first study providing insights into the mechanisms behind antidiabetogenic effect of this immunosuppressant.
The well-documented antiproliferative action of MMF affects mainly activated T and B cells due to drug selectivity for IMPDH isoform II, which is expressed in activated lymphocytes. Accordingly, the spontaneous, presumably self-antigen driven splenocyte proliferation in our study was suppressed by MMF treatment. On the other hand, the proliferative response to ConA was not impaired in MMF-treated animals, indicating that naive T lymphocytes retained their capacity for mitogen-induced activation following the treatment applied. Moreover, the development of leukopenia in MLD-STZ-injected rats [25] was prevented completely by MMF treatment in our experiments. A similar MMF-mediated protection from leukopenia associated with autoimmunity was observed previously in a murine model of systemic lupus erythematosus [26]. In addition to impaired spontaneous proliferation, the mononuclear cells from MMF-treated animals exhibited reduced ability for intercellular adhesion or adhesion to plastic, which is the first report on such MMF action in an autoimmune disease. This is consistent with the findings that MPA-mediated GTP depletion and subsequent impairment of N-linked glycosilation decreases the in vitro binding activity of T cell and monocyte adhesion molecules [11,27]. Interestingly, the increased adhesion of mononuclear cells from diabetic animals was down-regulated by MMF active component MPA in vitro, indicating that the drug presence during leucocyte activation was not required for its anti-adhesive action. This ability of MPA to interfere with the adhesion of already activated lymphocytes and macrophages might be an important mechanism for the antidiabetogenic effect of late MMF administration, presumably preventing the recruitment of activated immune cells into the site of inflammation. A similar MMF-mediated reduction of inflammatory infiltration of the target tissue was recently observed in rat model of EAE, which resulted in the recovery from already established disease [28]. In addition, recently described reduction by MPA of selectin expression on endothelial cells [12] might contribute further to drug-mediated protection of pancreatic islets from inflammatory infiltration and autoimmune destruction.
The immunohistochemical examination confirmed that MPA-mediated interference with the proliferation of autoreactive lymphocytes and leucocyte adhesiveness correlated with reduced severity of pancreatic inflammation. The protective effect of MMF in rats was associated with reduced number of ED-1+ and CD4+ cells in the islets, which is consistent with the findings that ED-1+ macrophages are the first cells to enter the endocrine pancreas in rat MLD-STZ diabetes [21], while subsequent infiltration of CD4+ cells is necessary for the development of disease [3,29]. The observation that MMF-injected animals had lower number of MHC class II+ cells in their islets is important in light of the data describing protection from MLD-STZ insulitis by administration of anti-class II antibodies [30]. Because MHC class II expression during streptozotocin-induced insulitis seems to be restricted to monocytes/macrophages infiltrating the pancreas and presumably presenting β-cell antigens [31], the MHC class II+ cells in our study are likely to correspond to ED-1+ macrophages. While CD8+ T cells apparently do not contribute to the induction of MLD-STZ diabetes in rats [32], both CD4+ and CD8+ T lymphocytes are required for disease development in mouse [29,33,34], and their numbers were decreased markedly in the islets of MLD-STZ-treated mice after MMF treatment. Recent findings indicate that the death of β-cells, as a final consequence of the autoimmune attack in diabetes, can take a form of apoptosis [35]. Indeed, we have observed numerous cells with the morphological appearance of apoptotic ones in the islets of diabetic, but not in MMF-treated animals, thus suggesting further another level of MMF mediated protection.
In addition to impaired lymphocyte proliferation and leucocyte adhesion, an important feature of MMF anti-diabetogenic effect is the reduced production of macrophage proinflammatory mediators IL-12 and NO. Interleukin-12 is a heterodimeric cytokine required for the development of destructive Th1 phenotype of auto-reactive T cells [36], with its inducible p40 chain observed readily during progression of auto-immune diabetes in spontaneously diabetic NOD mice and BB rats, as well as in MLD-STZ-treated mice ( [37–39] and the present study). Somewhat surprisingly, down-regulation of IL-12p40 production in peritoneal and spleen cells of MMF-treated mice was associated with unaltered splenocyte production of the signature Th1 cytokine IFN-γ. However, the amount of IFN-γ produced during the priming of T cells does not necessarily correlate with their polarization towards Th1 phenotype. The striking example is the action of IL-18, which in the absence of IL-12 markedly enhances IFN-γ secretion in the first contact of T cells with antigen, but leads eventually to the development on IFN-γ non-producing Th2 cells [40]. Therefore, the reduction of IL-12 production in MMF-treated mice could still interfere with the induction of Th1 response and might partially account for the observed decrease of IFN-γ synthesis within the microenvironment of the pancreas, even in the absence of IFN-γ down-regulation during priming in the spleen. The destructive actions of Th1 cell-derived IFN-γ include the induction of macrophage free radical NO, which is directly toxic to β-cells [41]. Regardless of unchanged splenic IFN-γ production, the NO production by spleen and peritoneal macrophages was abolished almost completely by MMF treatment in our study. While, due to the low sensitivity of the method employed for NO detection, we could not observe any NO release in the cultured islets, it seems conceivable to assume that impaired NO synthesis by peripheral macrophages might reflect a similar disability of the macrophages infiltrating the target tissue. Interestingly, although MPA suppressed NO production by endothelial cells in vitro [42], it did not affect either IL-12 or NO production in IFN-γ + LPS-stimulated macrophages or splenocytes (our unpublished data). Thus, it appears that the drug acted through IFN-γ-independent interference with cytokine network regulating macrophage activation, rather than direct modulation of macrophage activity. An intriguing possibility for such MMF effect is the in vivo up-regulation of macrophage-deactivating cytokine IL-10, observed previously in endotoxaemic mice [43], as well as in the present study.
Finally, our data indicate that MMF exerted the protection in MLD-STZ diabetes through its best defined intracellular action – the inhibition of guanosine nucleotide synthesis, reported previously to cause the inhibition of lymphocyte proliferation and adhesion in vitro (for review, see [8]). Recent evidence suggests that guanosine depletion might affect GTP binding protein-dependent signal transduction machinery such as MAP kinase pathway [44], which could be a mechanism for MMF interference with cytokine and NO synthesis. Having in mind a favourable ratio between the beneficial and the side-effects of MMF, our study indicate that its potential use for treatment of human IDDM is worthy of further investigation.
Acknowledgments
This work was supported partly by grants from the Ministry of Science and Technology, Republic of Serbia, Yugoslavia. MMF was kindly provided by Roche Representative Office, Belgrade.
REFERENCES
- 1.Kolb-Bachofen V, Epstein S, Kiesel U, et al. Low dose streptozotocin induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes. 1988;37:21–7. doi: 10.2337/diab.37.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Yuan J, Goddard A, et al. Interferon expression in the pancreas of patients with type I diabetes. Diabetes. 1995;44:658–64. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- 3.Lukic ML, Stosic-Grujicic S, Shahin A. Effector mechanisms in low dose streptozotocin induced diabetes. Dev Immunol. 1998;6:119–28. doi: 10.1155/1998/92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drachenberg CB, Klassen DK, Weir MR, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Lohmann T, List C, Lamesch P, et al. Diabetes mellitus and islet cell specific autoimmunity as adverse effects of immunsuppressive therapy by FK506/tacrolimus. Exp Clin Endocrinol Diabetes. 2000;108:347–52. doi: 10.1055/s-2000-8127. [DOI] [PubMed] [Google Scholar]
- 6.Jindal RM, Sidner RA, Milgrom ML. Post-transplant diabetes mellitus. The role of immunosuppression. Drug Saf. 1997;16:242–57. doi: 10.2165/00002018-199716040-00002. [DOI] [PubMed] [Google Scholar]
- 7.Sai P, Maugendre D, Loreal O, et al. Effects of cyclosporin on autoimmune diabetes induced in mice by streptozotocin. Diabetes Metabolisme. 1988;14:455–62. [PubMed] [Google Scholar]
- 8.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. 10.1016/S0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 9.Brazelton TR, Morris RE. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin Immunol. 1996;8:710–20. doi: 10.1016/s0952-7915(96)80090-2. [DOI] [PubMed] [Google Scholar]
- 10.Hager PW, Collart FR, Huberman E, et al. Recombinant human inosine monophosphate dehydrogenase type I and II proteins. Purification and characterization of inhibitor binding. Biochem Pharmacol. 1995;49:1323–9. doi: 10.1016/0006-2952(95)00026-v. [DOI] [PubMed] [Google Scholar]
- 11.Laurent AF, Dumont S, Poindron P, et al. Mycophenolic acid suppresses protein N-linked glycosylation in human monocytes and their adhesion to endothelial cells and to some substrates. Exp Hematol. 1996;24:59–67. [PubMed] [Google Scholar]
- 12.Blaheta RA, Leckel K, Wittig B, et al. Mycophenolate mofetil impairs transendothelial migration of allogenic CD4 and CD8 T-cells. Transplant Proc. 1999;31:1250–2. doi: 10.1016/s0041-1345(98)01984-8. [DOI] [PubMed] [Google Scholar]
- 13.Hao L, Chan S-M, Lafferty KJ. Mycophenolate mofetil can prevent the development of diabetes in BB rats. Ann NY Acad Sci. 1993;696:328–32. doi: 10.1111/j.1749-6632.1993.tb17168.x. [DOI] [PubMed] [Google Scholar]
- 14.Like AA, Rosini AA. Streptozotocin-induced pancreatic insulitis. a new model of diabetes mellitus. Science. 1976;193:415–7. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 15.Herold KC, Vezys V, Sun Q, et al. Regulation of cytokine production during development of autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1996;156:3521–7. [PubMed] [Google Scholar]
- 16.Stosic-Grujicic S, Maksimovic D, Badovinac V, et al. Antidiabetogenic effect of pentoxifylline is associated with systemic and target tissue modulation of cytokines and nitric oxide production. J Autoimmun. 2001;16:47–58. doi: 10.1006/jaut.2000.0456. 10.1006/jaut.2000.0456. [DOI] [PubMed] [Google Scholar]
- 17.Stosic-Grujicic S, Dimitrijevic M, Bartlett R. Leflunomide protect mice from multiple low dose of streptozotocin (MLD-SZ)-induced insulitis and diabetes. Clin Exp Immunol. 1999;117:44–50. doi: 10.1046/j.1365-2249.1999.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draskovic-Pavlovic B, Van Der Laan LJW, Pejnovic N, et al. Differential effects of anti-rat CD11b monoclonal antibodies on granulocyte adhesiveness. Immunology. 1999;96:83–9. doi: 10.1046/j.1365-2567.1999.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pechold K, Patterson NB, Craighead N, et al. Inflammatory cytokines IFN-γ plus TNF-α induce regulated expression of CD80 (B7–1) but not CD86 (B7–2) on murine fibroblasts. J Immunol. 1997;158:4921–9. [PubMed] [Google Scholar]
- 20.Hibbs JB, Taintor Z, Vavrin E, et al. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1989;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 21.Lukic ML, Al-Sharif R, Mostarica M, et al. Lymphatic tissues and in vivo immune responses. New York: Marcel Dekker; 1991. Immunological basis of the strain differences in susceptibility to low-dose streptozotocin-induced diabetes in rats; pp. 643–7. [Google Scholar]
- 22.Lukic ML, Stosic-Grujicic S, Ostojic N, et al. Inhibition of nitric oxide generation affects the induction of diabetes by streptozotocin in mice. Biochem Biophys Res Commun. 1991;178:913–20. doi: 10.1016/0006-291x(91)90978-g. [DOI] [PubMed] [Google Scholar]
- 23.Squifflet JP, Backman L, Claesson K, et al. Dose optimization of mycophenolate mofetil when administered with a low dose of tacrolimus in cadaveric renal transplant recipients. Transplantation. 2001;72:63–9. doi: 10.1097/00007890-200107150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Santamaria P. Effector lymphocytes in autoimmunity. Curr Opin Immunol. 2001;13:663–9. doi: 10.1016/s0952-7915(01)00276-x. [DOI] [PubMed] [Google Scholar]
- 25.Oon BB, Muggleston D, Warley A. A diet enriched in essential fatty acids protects against the loss of lymphocytes which occurs in rats suffering from streptozotocin-induced diabetes. Exp Physiol. 1992;77:185–90. doi: 10.1113/expphysiol.1992.sp003572. [DOI] [PubMed] [Google Scholar]
- 26.Corna D, Morigi M, Facchinetti D, et al. Mycophenolate mofetil limits renal damage and prolong life in murine lupus autoimmune disease. Kidney Int. 1997;51:1583–9. doi: 10.1038/ki.1997.217. [DOI] [PubMed] [Google Scholar]
- 27.Allison AC, Kowalski WJ, Muller CJ, et al. Mycophenolic acid and brequinar, inhibitors of purine and pyrimidine synthesis, block the glycosylation of adhesion molecules. Transplant Proc. 1993;25:67–70. [PubMed] [Google Scholar]
- 28.Tran GT, Carter N, Hodgkinson SJ. Mycophenolate mofetil treatment accelerates recovery from experimental allergic encephalomyelitis. Int Immunopharmacol. 2001;1:1709–23. doi: 10.1016/s1567-5769(01)00081-9. [DOI] [PubMed] [Google Scholar]
- 29.Elliott JI, Dewchand H, Altmann DM. Streptozotocin-induced diabetes in mice lacking αβ T cells. Clin Exp Immunol. 1997;109:116–20. doi: 10.1046/j.1365-2249.1997.4241319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiesel U, Kolb H. Suppressive effect of antibodies to immune response gene product on the development of low-dose streptozotocin-induced diabetes. Diabetes. 1983;32:869–71. doi: 10.2337/diab.32.9.869. [DOI] [PubMed] [Google Scholar]
- 31.Farr AG, Mannschreck JW, Anderson SK. Expression of class II MHC antigens in murine pancreas after streptozotocin-induced insulitis. Diabetes. 1988;37:1373–9. doi: 10.2337/diab.37.10.1373. [DOI] [PubMed] [Google Scholar]
- 32.Stosic-Grujicic S, Mijatovic S, Ejdus L, et al. Enhancement of Th2-type activity downregulated diabetes induction. Transplant Proc. 1996;28:3259. [PubMed] [Google Scholar]
- 33.Herold KC, Montag AG, Fitch FW. Treatment with anti-T-lymphocyte antibodies prevents induction of insulitis in mice given multiple doses of streptozotocin. Diabetes. 1987;36:796–801. doi: 10.2337/diab.36.7.796. [DOI] [PubMed] [Google Scholar]
- 34.Herold KC, Bluestone JA, Montag AG, et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41:385–91. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien BA, Harmon BV, Cameron DP, et al. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J Pathol. 1996;178:176–81. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/ interleukin-12-receptor system. Role in normal and pathologic immune responses. Annu Rev Immun. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 37.Rothe H, Burkart V, Faust A, et al. Interleukin-12 gene expression is associated with rapid development of diabetes mellitus in non-obese diabetic mice. Diabetologia. 1996;39:119–22. doi: 10.1007/BF00400422. [DOI] [PubMed] [Google Scholar]
- 38.Zipris D, Greiner DL, Malkani S, et al. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J Immunol. 1996;156:1315–21. [PubMed] [Google Scholar]
- 39.Li X, Kaminski NE, Fischer LJ. Examination of the immunosuppressive effect of delta9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes. Int Immunopharmacol. 2001;1:699–712. doi: 10.1016/s1567-5769(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Trajkovic V, Hunter D, et al. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30:3147–56. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their role in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–49. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 42.Senda M, DeLustro B, Eugui E, et al. Mycophenolic acid, an inhibitor of IMP dehydrogenase that is also an immunosuppressive agent, suppresses the cytokine- induced nitric oxide production in mouse and rat vascular endothelial cells. Transplantation. 1995;60:1143–8. doi: 10.1097/00007890-199511270-00015. [DOI] [PubMed] [Google Scholar]
- 43.Durez P, Appelboom T, Pira C, et al. Antiinflammatory properties of mycophenolate mofetil in murine endotoxemia: inhibition of TNF-α and upregulation of IL-10 release. Int J Immunopharmac. 1999;21:581–7. doi: 10.1016/s0192-0561(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 44.Vallee S, Fouchier F, Braguer D, et al. Ribavirin-induced resistance to heat shock, inhibition of the Ras-Raf-1 pathway and arrest in G(1) Eur J Pharmacol. 2000;404:49–62. doi: 10.1016/s0014-2999(00)00596-3. [DOI] [PubMed] [Google Scholar]


