Abstract
Severe combined immunodeficiency (SCID) is a heterogeneous group of disorders characterized by defect of T- and B-cell immunity. In many cases of autosomal recessive SCID, thus far described, the molecular alteration involves genes encoding for molecules that participate in the signal transduction. We report on a patient affected by a combined immunodeficiency, characterized by severe T-cell functional impairment, in spite of a close to normal number of circulating mature type T and B cells. NK cells were absent. Associated with the immunodeficiency, this patient also showed short stature characterized by very low growth velocity, delayed bone age and absence of increase of the plasma levels of Insulin growth factor-I (IGF-I) after growth hormone (GH) in vivo stimulation indicating peripheral hyporesponsiveness to GH. Evaluation of the protein tyrosine phosphorylation events occurring following either T-cell receptor (TCR) or GH receptor (GHR) triggering revealed striking abnormalities. No molecular alteration of GHR gene was found, thus suggesting the presence of postreceptorial blockage. Mutational screening and expression analysis failed to reveal any molecular alteration of JAK2 and STAT 5 A/B genes thus ruling out the involvement of these genes in the pathogenesis of this form of SCID. Mutational analysis of IL2Rγ chain gene revealed the presence of a L183S missense mutation, thus indicating an atypical and a more complex clinical presentation of this X-linked form of SCID. At our knowledge, this is the first report on the GH hyporesponsiveness in this disease
Keywords: Severe combined immunodeficiency, janus kinase 2, signal transducer and activators of transcription 5, idiopathic short stature
INTRODUCTION
Severe combined immunodeficiency (SCID) comprises a group of genotipically and phenotipically heterogeneous hereditary diseases characterized by profound deficiency of both T- and B- cell immunity [1]. SCID phenotypes have been tentatively classified according to the presence or absence of B cells in the peripheral blood in T−B+ or T−B− forms of SCID [2]. Of note, all the molecular alterations causing a severe combined immunodeficiency phenotype thus far described involve genes encoding for molecules that participate in the signal transduction process [3]. This is the case of the γ chain, a common element of several receptors, and the Janus kinase 3 (JAK3) molecule that associates with γ element, whose alteration results in a T−B+ SCID and absence of natural killer (NK) cells [4,5]. Mutations of the Zap-70 kinase that associates with the ζ element of the T-cell receptor (TCR)/ CD3 complex [6,7], or decreased expression of the p56lck tyrosine kinase due to an alteration of the splicing pattern result in SCIDs characterized by a selective decrease of CD8+ or CD4+ T cells [8], respectively. Both of these forms are associated with normal NK cell number. Even though further molecular mechanisms have been recently elucidated, which involve molecules as Rag-1, Rag-2, CD-45, IL-7Rα, CD3ɛ, CD3γ, in about 30% of the cases of SCID the underlying genetic defect is still unidentified [2,3,9]
Here, we report on a SCID patient whose immunodeficiency is predominantly a T-cell activation defect, and is part of a multisystemic disorder being associated with peripheral GH insensitivity and abnormal haematopoiesis. The pathogenic mechanism underlying this complex phenotype involves signal transduction through both TCR and GHR. These receptors, as many other receptors, transduce signals through a cascade of biochemical events involving phosphorylation/dephosphorylation processes [10]. Even though a few signalling molecules are tissue specific, most of the signalling pathway are not restricted to a certain cell type. We first focused our attention on the JAK2/STAT5 molecules involved in signalling through several cytokine receptors even included GHR [11,12]. JAK2 is activated following receptor stimulation with several factors, as erythropoietin, GH, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin- (IL-)3, IL-5, IL-6, IL-12 [13,14]. It is also required for a proper interferon (INF)-γ response [15,16]. In turn, activated JAK2 phosphorylates the STAT5 thus resulting in the activation of transcription of target genes. In particular, experimental evidence and knock out mice indicate that alterations of STAT5A and 5B result in an impaired response to a number of cytokines, including GM-CSF, IL-2, IL-3, IL-5, GH, and erythropoietin (Epo) [17,18]. However, molecular studies led us to rule out alteration of GHR, JAK2 and STAT5A/B encoding genes. Remarkably, sequence analysis of the IL-2Rγ chain gene revealed a L183S missense mutation, thus indicating that also this form of SCID may exhibit a more colmplex and atypical clinical presentation
SUBJECT AND METHODS
Case report
The patient DG was born at 40 weeks of gestation to unrelated healthy parents. His birth weight was 2850g and height was 48 cm. No morphological anomalies were appreciable. At the age of 4 months, the patient presented with severe respiratory distress due to an interstitial pneumopathy. A Pneumocystis carinii infection was diagnosed and, due to the persistence of the infectious problem, an extensive immulogical evaluation was carried out leading to a diagnosis of SCID as below described in detail. Humoral immunity studies revealed very low IgG (195 mg/dl) and IgA (9 mg/dl) serum levels at 12 months of age. IgM were I44 mg/dl. Allohemagglutinins were undetectable. A severe hyporegenerative anaemia requiring blood transfusion represented a further clinical problem until the age of 10 months. Moreover, since the age of 1 years a short stature not explained on the basis of endocrinological, nutritional or infectious causes was present as below described. Short limb dwarfism was ruled out by the measurement of body proportions which were normal. Prophylaxis against bacterial, fungal and viral infections was successfully established while the patient, at the time of the study, was pending a bone marrow transplantation
IGF-I generation test
To assess the ability to respond to exogenous GH, DG patient underwent an IGF-I generation test through daily subcutaneous injections of recombinant GH (0·1 IU/kg) for 4 consecutive days. Blood samples were taken before the first injection and on day 5 for the measurements of IGF-I levels. IGF-I was measured by using a two-site immunoradiometric assay (IRMA) kit (Diagnostic Systems Laboratories, Webster, Texas). Values were expressed as Standard International Units (μg/l)
Phenotypic analysis and proliferative assays
The expression of surface-membrane antigens on peripheral blood mononuclear cells (PBMC) was examined by flow cytometry (Becton Dickinson, San Jose, CA, USA) using the following monoclonal antibodies: anti-CD3 (Leu-4), anti-CD4 (Leu-3a), anti-CD8 (Leu-2a), anti-CD19 (Leu-12), anti-CD56 (Leu-19). The activation marker HLA-DR, CD25 (IL-2 receptor α chain), and CD71 (transferrin receptor), were evaluated on resting or phytohemagglutinin- (PHA)-stimulated PBMC in two-colour immunofluorescence using anti-CD3 and the corresponding monoclonal antibody from the same source (BD). The proliferative response was evaluated by 3H]thymidine (Amersham International, Buckinghamshire, UK) incorporation in PBMC cultured at a concentration of 2 × 105 cells per well for 72h with PHA (8 μg/ml), concanavalin A (ConA; 8 μg/ml) (Difco Laboratories, Detroit, MI, USA), 20 ng/ml phorbol-myristate acetate (PMA) and ionomicin (0·5 mm). CD3 cross-linking (CD3 X-L) was performed by precoating tissue colture plates with anti-CD3 monoclonal antibody at optimal or suboptimal concentrations (10 and 1 ng/ml) (Ortho Diagnostics, Raritan, NJ, USA)
GH and TCR induced protein tyrosine phosphorylation
Transducing properties were studied through the analysis of protein tyrosine phosphorylation in PBMC after stimulation with GH, PMA or CD3 X-L. GH stimulation (500 ng/ml) was performed through 5–15 min stimulation. The pattern of tyrosine phosphorylated (Ptyr) proteins was analysed on whole cell lysates. After stimulation, 3 × 106 cells were lysed in a buffer containing 20 mm Tris, pH 8, 10% glycerol, 137 mm NaCl, 1% Nonidet P-40, 10 mmol EDTA, 1 mm phenyl methane sulphonyl fluoride (PMSF), 1 mm sodium orthovanadatum (Na3Vo4), 5 μg/ml leupeptin and 5 μg/ml aprotinin. Proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then blocked with 3% bovine serum albumin. Immunoblotting was performed by a 1–4 h incubation with anti-Ptyr. Densitometric analysis of the signals was performed
GH receptor, STAT5A and B, JAK2 and γ chain mutational analysis
Genomic DNA and RNA were isolated from peripheral blood leucocytes [19]. Automated sequence analysis of exons 2–10 of GHR (X06562·1) was performed in DG patient. The coding portion of exon 10 was amplified in three overlapping fragments [20]. STAT5A and STAT5B mutational analysis was performed using primers designed on published sequence for a total of 18 primer sets for each gene. Each exon and corresponding donor and acceptor splice site was individually amplified by PCR. PCR conditions were as follows 35 cycles of 95°C for 30 s, 52°C for 45 s, 72°C for 1 min JAK2 cDNA was obtained by reverse transcription (RT) using 1 μg purified RNA and random examers in a buffer supplied by the manufacturer (Boehringer Roche, Mannheim, Germany). Twelve sets of primers were synthesized to amplify and sequence human JAK2 cDNA (AF005216·1) both coding and noncoding strands
STAT5A and B cDNAs (NM012448·1 and NM003152·1, respectively) were amplified using RT-PCR performed with primers designed to amplify both cDNAs (position 1636 STAT5B/ 2130 STAT5A CCGTGCCTGACAAAGTGCTG and position 2284 STAT5B/2763 STAT5A TTCACAAACTCAGGGAC CAC). One μl of32P]-dCTP was included in the reaction. Amplified products were subsequently Hinf1 digested and separated by electrophoresis on 5% polyacrilamide gel. Densitometric analysis of the signals was performed
IL-2Rγ chain mutational analysis was performed using primers designed on published sequence (L19546) for a total of 8 primer sets [21]. Each exon and corresponding donor and acceptor splice site was individually amplified by PCR. Amplified products were automatically sequenced. ABI Prism dye terminator cycle sequencing kit and an ABI 377 Automated DNA Sequencer were used
Sequence of primers used in molecular analysis of STAT 5 A/B and γ chain are available upon request (ursini@iigbna.iigb. na.cnr.it)
RESULTS
Cell proliferation and activation markers
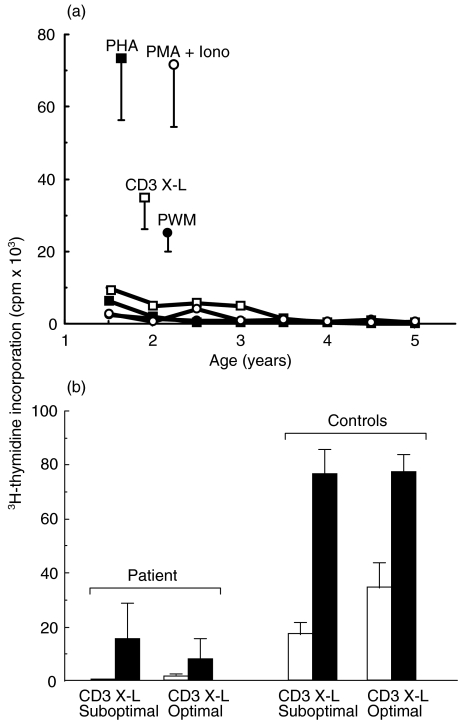
The proliferative response was consistently reduced during the 5 years follow-up as depicted in Fig. 1a. The addition of either suboptimal or optimal rIL-2 to the cultures of patient's PBMC stimulated through CD3 triggering, as shown in Fig. 1b, was unable to restore the proliferation. The functional impairment was associated with the presence of some circulating mature CD3+ T cells, ranging during the follow up between 23 and 85%. Table 1 shows the representative percentage values at 2 and 5 years of age indicating a spontaneous increase of CD3+ cells bearing TCR α/β chains. CD4+ cells were persistently reduced during the follow-up ranging from 13 and 16%, while CD8+ cells ranged between 10 and 48%. TCR γ/δ+ cells increased over the time. However, most of these cells were CD4 and CD8 double negative. CD19+ B cells were present and inversely correlated to the number of T cells, ranging between 70 and 4%. NK cells were always absent
Fig. 1.
Proliferative responses in the SCID patient during the 5 years follow-up. Proliferative response evaluated through3H]-thymidine incorporation following stimulation of PBMC by PHA, PMA + Iono, PWM, and CD3 cross-linking (a). Proliferation was analysed at regular time during the 5 years follow-up. The results are expressed as cpm. Bars indicate mean value + SD of control values. (b) Proliferative response following optimal or suboptimal stimulation through CD3 cross-linking in the presence (▪) or absence (□) of exogenous rIL-2 used at a concentration of 100 U/ml.
Table 1.
Patient's immunophenotype
| Age | ||||
|---|---|---|---|---|
| 2 years old | 5 years old | |||
| Patient* | Control† | Patient* | Control† | |
| Total lymphocyte count | 915 | 3600 | 919 | 3600 |
| Surface markers | ||||
| CD3+ | 23.8 (218) | 64 (22232–2484) | 85.3 (784) | 64 (2232–2484) |
| CD3+TCR αβ | 14.1 (129) | 66 (1980–2592) | 49.4 (459) | 66 (1980–2592) |
| CD4+ | 13.4 (123) | 37 (1080–1440) | 16.0 (147) | 37 (1080–1440) |
| CD8+ | 10.3 (94) | 29 (900–1152) | 48.5 (451) | 29 (900–1152) |
| CD4−CD8−TCR γδ | 1.0 (9.2) | abs | 17.3 (158) | abs |
| CD19+ | 70.9 (652) | 24 (756–1008) | 4.3 (39) | 24 (756–1008) |
| CD3+DR+ | 12.8 (117) | 9 (216–576) | 65.9 (604) | 9 (216–576) |
| CD56+ | 0.6 (5) | 11 (288–540) | 0.1 (0.9) | 11 (288–540) |
Values given as
% (cells/mm3)
%(25 th–75thcentile).
The evaluation of the activation markers HLA-DR, α chain of IL-2R (CD25) and transferrin receptor (CD71) on patient's T cells confirmed on a per cell basis that there was a cell activation defect, as illustrated in Table 2. PHA stimulation for 48h of the patient's PBMC failed to induce up-regulation of the membrane expression of all 3 activation markers tested, differently from controls, whose cells exhibited a 3–20 fold increase of the surface expression
Growth rate, peripheral sensitivity to GH and GHR analysis
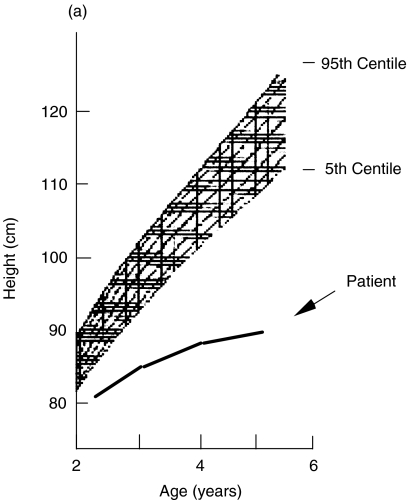
The patient DG showed a clearly abnormal growth curve, being under the 5th centile for height, as shown in the Fig. 2a. Bone age was always markedly delayed as compared to the chronological age. The more common causes of short stature, including malnutrition, chronic diseases, and endocrinological disorders were ruled out by standard diagnostic procedures. Due to the efficacy of the anti-infectious prophylaxis, short stature could not be explained by chronic infections. In 2 out of 3 pharmacological stimulation tests, GH increase was normal, thus leading to a descriptive diagnosis of ISS
Fig. 2.
(a) Growth curve over the 5 years follow-up of the patient DG compared to sex-matched percentile. (b) IGF-I response following in vivo generation test. GH, at a dosage of (□) 0·10 and (○) 0·16 U/kg/day, was administered for 4-days in DG. Values of IGF-I are expressed as μg/l.
Insulin-like growth factor-I (IGF-I) is a hepatically derived circulating mediator of growth hormone, which is regulated by GH, and mediates many of the GH biological functions, being also involved in the maintenance of lymphoid mass and functions [11]. Therefore, we sought to investigate the peripheral sensitivity to GH by examining the increase of IGF-I plasma levels following in vivo provocation test. As reported in Fig. 2b, peripheral hyporesponsiveness to GH was demonstrated in vivo on the basis of the low baseline IGF-I-values and the absence of plasma IGF-I increase during GH treatment performed at 2 different concentrations. An IGF-I increase below 15 μg/l during the generation test was considered a diagnostic criteria for GH insensitivity [22]
To rule out a genetic alteration of the GHR gene in the patient, a GHR sequence analysis of the exons 2–10 was performed. All 11 amplified products have sequence identical to the wild type, thus ruling out a GHR alteration. The patient was heterozygote for the already described G168 and I526L polymorphisms [23]
Defective pattern of protein tyrosine phosphorylation after TCR and GHR engagement
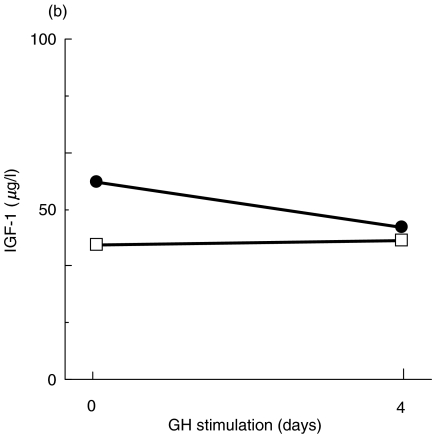
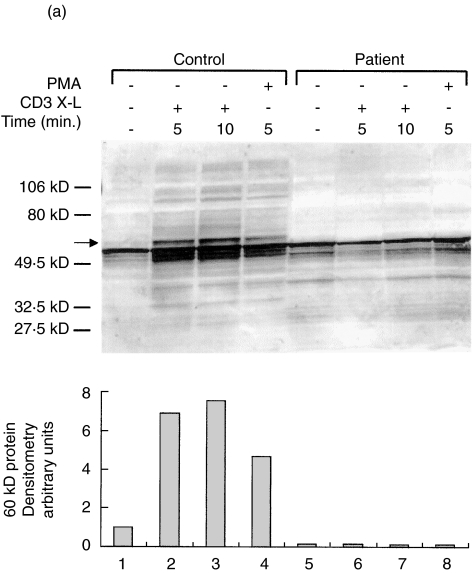
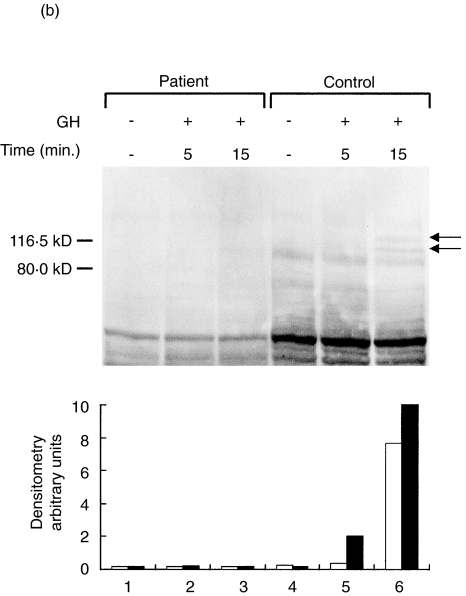
We next investigated the overall signal transduction properties following TCR and GHR ligation by analysing the number and the timing of the proteins phosphorylated on tyrosine residues. Figure 3a,b illustrate immunoblots with anti-Ptyr of whole cell lysates from patient's or control's PBMC following stimulation through CD3 X-L, PMA or GH. In contrast to what observed in control cells, both stimuli failed to induce substantial increase of tyrosil phosphorylated proteins in patient cells. In particular, CD3 X-L failed to increase phosphorylation of proteins of 42–44, 56–60, 70 and 85 kD usually phosphorylated upon this activation [24]. Similarly, after the GH stimulation there was no increase in the signal regarding the 2 proteins of 105 and 119 kD generally phosphorylated upon GH stimulation of PBMC, presumably corresponding to JAK2 and STAT5 molecules
Fig. 3.
Protein tyrosine phosphorylation induced by CD3 cross-linking or GH stimulation. Representative experiments showing the pattern of tyrosil phosphorylated proteins after stimulation of PBMC from DG and a control. (a) TCR triggering for 5 (lanes 2 and 6) or 10 min (lanes 3 and 7) or PMA stimulation (lanes 4 and 8) in the control's PBMC (lanes 1–4) and in the patient (lanes 5–8). Molecular markers are indicated. The lower panel illustrates the densitometric analysis of the 60 kD protein, which represents the major phosphorylated protein after TCR triggering. (b) GH stimulation for 5 or 15 min of the patient's PBMC (lanes 1–3) and of the control (lanes 4–6). The lower panel illustrated the densitometric analysis of the (▪) 105 and (□) 119 kD proteins. In the patient, an abnormal pattern of protein tyrosine phosphorylation was observed following both stimulations. Western blots were performed using whole cell lysates and an antiphosphotyrosine antibody.
Molecular analysis of JAK2, STAT 5 A/B and IL-2Rγ chain molecules
By taking advantage of knock out mice knowledge, we focused our attention on JAK2/STAT5 signalling pathway involved in several cytokine receptors including GHR [15,16]. Since the DG PBMC contain JAK2 mRNA (data not shown), we used cDNA produced by reverse RT-PCR and JAK2 specific oligodeoxynucleotide primers to scan the entire gene. All 12 amplified products have sequence identical to the wild type. DNA fragments appeared quantitatively and qualitatively indistinguishable from those observed in several unrelated controls
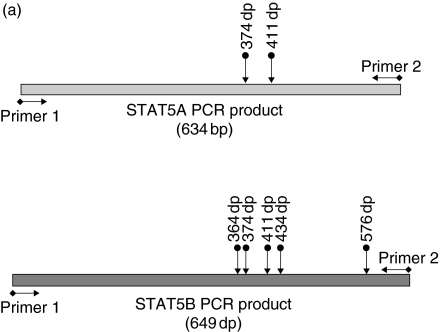
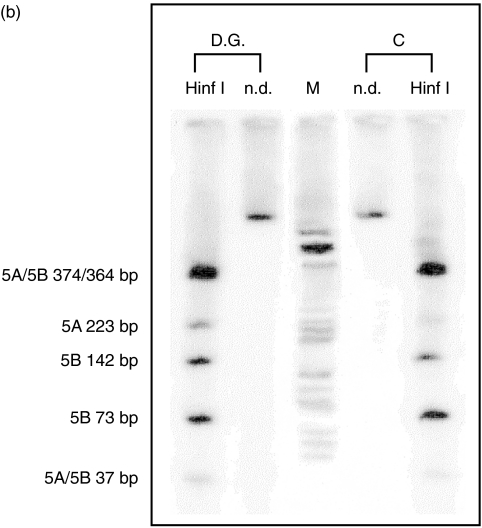
Recently, the human genomic sequence of STAT5A and STAT5B was solved by some of us thus allowing a complete mutational analysis of each individual gene (unpublished observation). However, we found that all 18 amplified products corresponding to coding exons of each STAT gene have sequence identical to the wild type (data not shown). In addition, we analysed the expression and the relative abundance of both STAT5A and STAT5B mRNAs in the patient PBMC by RT-PCR performed with primers able to amplify both STAT 5 A/B cDNAs. Amplified products were subsequently Hinf1 digested and separated by electrophoresis to distinguish fragments of each gene; therefore, this analysis also reflects the STAT 5 A/5B specific mRNA ratio (Fig. 4a). Reference pattern was obtained from age-matched control. We observed that the two mRNA-derived amplified products were qualitatively and quantitatively indistinguishable from those obtained from control PBMC mRNA (Fig. 4b)
Fig. 4.
Evaluation of RNA expression of STAT5A and B in the patient and a control. (a) Schematic representation of amplified products from STAT5A and B cDNA. Arrows indicate HinfI restriction sites. Primers were designed on cDNA conserved regions sequence of both STAT5A and B. (b) HinfI restriction pattern of patient and control STAT5A and B amplified products. Fragments were separated by 5% polyacrilamide gel electrophoresis. (C, control; D.G., proband; n.d., not digested; M, marker). Size of the fragments are indicated.
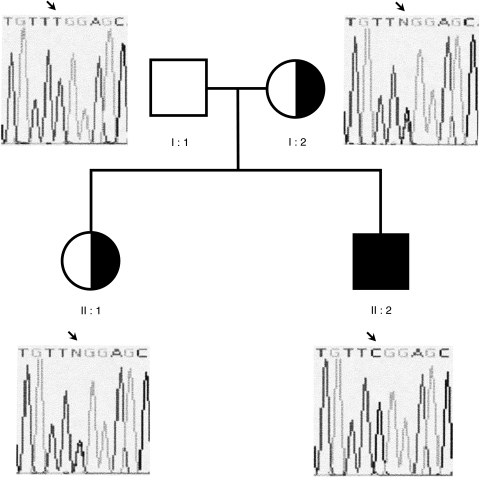
Ultimately, a mutational analysis of the 8 encoding exons of the γ chain gene was perfomed and revealed the presence of a single base pair change T548 > C (numbered from the ATG) resulting in a change in coding from leucine to serine at residue 183 in the region coding for the extracellular domain of the protein (Fig. 5). We also found that the mother and the sister of the proband are heterozigous for this mutation. Amplification of γ chain specific cDNA appeared quantitatively and qualitatively indistinguishable from those observed in several unrelated controls
Fig. 5.
Pedigree of the famly and sequence analysis of the IL-2Rγ chain in family members. The proband is indicated by the arrow. The sequence analysis revealed the presence of T548C (causing the L183S missense mutation) at the hemizygous state in the proband and at the heterozigous state in the mother and sister.
DISCUSSION
We reported here on an atypical form of SCID in a patient presenting with severe hyporegenerative anaemia, and severe functional impairment of apparently mature T cells, as shown by the absence of proliferative response to a variety of mitogens and up-regulation of activation markers. The number of B cells was normal, but they were non functional, whereas NK cells were undetectable. The patient was also affected by idiopathic short stature with a reduced serum levels of IGF-1 and peripheral insensitivity to GH. This last defect has frequently been associated with GHR mutation [20,23,25]. However, in DG we were unable to find any mutation in GHR gene. Of note, an abnormal pattern of protein tyrosine phosphorylation was found in DG PBMC either after GH stimulation or following TCR/CD3 perturbation
Mouse mutations have become an important genetic tool for the identification of new clinical phenotypes that may help unravel complex human disorders and identify new pathogenic gene alterations [26]. The STAT5A/B mutant mice, but not the individual mutants, have a profound T-cell functional defect. In addition to prolactin, STAT5 proteins are activated by GH, Epo, GM-CSF and IL-2 [27,28]. GHR belongs to cytokine/ haematopoietin receptor super family and shares with other members of this family several signal transducers even included JAK2, which, in turn, phosphorylates STAT5 [11,12]. STAT5 heterodimers are phosphorylated in response to TCR stimulation, thus indicating their involvement in T-cell proliferation [29]. Even though the JAK2 knockout in mice is embrionically lethal, this gene plays an essential non redundant role in the function of a number of cytokine receptors including Epo, GM-CSF, IL-5 and IL-3, and presumably of GHR as well [15,16]. Therefore, we first focused our attention on the JAK2/STAT5 signalling pathway. However, our molecular studies led us to rule out a direct involvement of these molecules in the pathogenesis of the disease
The absence of NK cells is also present in SCID related to mutation of γ chain or Jak3 genes. In these forms of SCID, generally there are no T cells. These forms are generally fatal within the first years of life, but a few atypical cases are characterized by a milder phenotype [30–32]. In our patient, we documented the presence of a L183S hemyzigous mutation of the γ chain. This alteration modifies the extracellular domain of the protein, as also described for other several mutations associated with a typical phenotype of this form of SCID [21,32]
In conclusion, our finding indicates that the immunodeficiency here described is an X-linked form of SCID due to an activation deficiency associated with peripheral insensitivity to GH, resulting in a profound growth failure. We here documented that in vitro triggering of both TCR/CD3 complex and GHR was associated with an abnormal pattern of protein tyrosine phosphorylation events, raising the intriguing question on themechanism by which the γ-chain defect is the common underlying pathogenic mechanism for both endocrinological and immunological problems. Of note, mutational analysis of a few additional candidate genes functionally related to short stature and T-cell activation failed to reveal any further alteration that could explain the atypical and complex phenotype. This observation is in keeping with what reported on other genetic diseases, showing that patients with an identical mutation may have different clinical and immunological features. To address the issue of the role of interfering genes in modifying the clinical expression of a genetic disease new developing technologies (e.g. biochip) are now available. We believe that unusual clinical and laboratoristic observations may be very useful to unravel complex diseases and help find novel gene-function relationship toward targeted therapeutic approaches
Acknowledgments
Supported by Telethon #E0934
REFERENCES
- 1.WHO report. Primary immunodeficiency diseases. Clin Exp Immunol. 1997;109:1–28. [PubMed] [Google Scholar]
- 2.Fischer A, Cavazzana-Calvo M, De Saint Basile G, et al. Naturally occurring primary deficiencies of the immune system. Annu Rev Immunol. 1997;15:93–124. doi: 10.1146/annurev.immunol.15.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Arnaiz-Villena A, Timon M, Gallego CR, Blas MP, Correl A, Villa JMM, Regueiro JR. Human T-cell activation deficiencies. Immunol Today. 1992;13:259–65. doi: 10.1016/0167-5699(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–57. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 5.Macchi P, Villa A, Giliani S, et al. Mutation of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–8. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 6.Chan AC, Kadlecek TA, Elder ME, et al. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 7.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T Cell tyrosine kinase. Science. 1994;264:1596–8. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 8.Goldman FD, Ballas ZK, Schutte BC, Kemp J, Hollenback C, Noraz N, Taylor N. Defective expression of p56lck in an infant with severe combined immunodeficiency. J Clin Invest. 1998;102:421–9. doi: 10.1172/JCI3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villa A, Santagata S, Bozzi F, et al. Partial V (D) J recombination activity leads to Omenn syndrome. Cell. 1998;93:885–96. doi: 10.1016/s0092-8674(00)81448-8. [DOI] [PubMed] [Google Scholar]
- 10.Klausner RD, Samelson LE. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991;64:875–8. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- 11.Leonard WJ, O'Shea JJ. Jaks and Stats: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 12.Argetsinger LS, Carter-Su C. Mechanism of signaling by growth hormone receptor. Physiol Rev. 1996;76:1089–107. doi: 10.1152/physrev.1996.76.4.1089. [DOI] [PubMed] [Google Scholar]
- 13.Winston LA, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270:30837–40. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- 14.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O'Shea JJ, Johnston JA. Interleukin 12 induces tyrosine phosphorylation of Jak2 and Tyk2: differential use of janus family tyrosine kinases by IL-2 and IL-12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 16.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 17.Teglund S, Mckay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and non essential, or redundant, roles in cytokine responses. Cell. 1998;93:841–50. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 18.Socolovsky M, Fallon AEJ, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a −/−5b−/− mice: a direct role for Stat5 in Bcl-Xl induction. Cell. 1999;98:181–91. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Goddard AD, Covello R, Luoh SM, et al. Mutations of the growth hormone receptor in children with idiopathic short stature. N Engl J Med. 1995;333:1093–8. doi: 10.1056/NEJM199510263331701. [DOI] [PubMed] [Google Scholar]
- 21.Clark PA, Lester T, Genet S, Jones AM, Hendriks R, Levinsky RJ, Kinnon C. Screening for mutations causing X-linked severe combined immunodeficiency in the IL-2R gamma chain gene by single-strand conformation polymorphism analysis. Hum Genet. 1995;96:427–32. doi: 10.1007/BF00191801. [DOI] [PubMed] [Google Scholar]
- 22.Woods KA, Dastot F, Preece MA, et al. Phenotype: genotype relationships in growth hormone insensitivity syndrome. J Clin Endocrinol Metab. 1997;82:3529–35. doi: 10.1210/jcem.82.11.4389. [DOI] [PubMed] [Google Scholar]
- 23.Goddard AD, Dowd P, Chernausek S, et al. Partial growth-hormone insensitivity. the role of growth-hormone receptor mutations in idiopathic short stature. J Pediatr. 1997;131:s51–5. doi: 10.1016/s0022-3476(97)70012-x. [DOI] [PubMed] [Google Scholar]
- 24.Del Giudice E, Gaetaniello L, Matrecano E, Cosentini E, Ursini MV, Racioppi L, Pignata C. Brain migration disorder and T-cell activation deficiency associated with abnormal signaling through TCR/CD3 complex and hyperactivity of Fyn tyrosine kinase. Neuropediatrics. 2000;31:265–8. doi: 10.1055/s-2000-9234. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez JE, Perera E, Baumbach L, Cleveland WW. Growth hormone receptor mutations in children with idiopathic short stature. J Clin Endocrinol Metab. 1998;83:4079–83. doi: 10.1210/jcem.83.11.5238. [DOI] [PubMed] [Google Scholar]
- 26.Kokron CM, Bonilla FA, Oettgen HC, Ramesh N, Geha RS, Pandolfi F. Searching for genes involved in the pathogenesis of primary immunodeficiency diseases: lessons from mouse knockouts. J Clin Immunol. 1997;17:109–26. doi: 10.1023/a:1027322314256. [DOI] [PubMed] [Google Scholar]
- 27.Davey HW, Wilkins RJ, Waxman DJ. STAT5 signaling in sexually dimorphic gene expression and growth patterns. Am J Hum Genet. 1999;65:959–65. doi: 10.1086/302599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriggl R, Topham DJ, Teglund S, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–59. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 29.Welte T, Leitenberg D, Dittel BN, et al. STAT5 interaction with the T cell receptor complex and stimulation of T cell proliferation. Science. 1999;283:222–5. doi: 10.1126/science.283.5399.222. [DOI] [PubMed] [Google Scholar]
- 30.Di Santo JP, Rieux-Laucat F, Dautry-Varsat A, Fischer A, de Saint Basile G. Defective human interleukin 2 receptor γ chain in an atypical x chromosone-linked severe combined immunodeficiency with peripheral T cells. Proc Natl Acad Sci USA. 1994;91:9466–70. doi: 10.1073/pnas.91.20.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharfe N, Shahar M, Roifman CM. An interleukin-2 receptor γ chain mutation with normal thymus morphology. J Clin Invest. 1997;100:3036–43. doi: 10.1172/JCI119858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Notarangelo LD, Giliani S, Mazza C, et al. of genes and phenotypes: the immunological and molecular spectrum of combined immune deficiency. Defects of the gamma (c)-JAK3 signaling pathway as a model. Immunol Rev. 2000;178:39–48. doi: 10.1034/j.1600-065x.2000.17812.x. [DOI] [PubMed] [Google Scholar]