Abstract
Denatured syngeneic liver tissue prepared by mechanical procedures was intraperitoneally injected into adult C57BL/6 mice. In parallel with a decrease in the total number of lymphocytes in the liver, spleen, and thymus from days 1–7 after the injection, the proportion of the CD4+NK1·1+CD3int subset of these cells (i.e. natural killer T or NKT cells) increased in the liver. Even the absolute number of these NKT cells increased in the liver on days 14 and 21. In response to the injection of denatured liver tissue, tissue damage was induced in the liver, as shown by elevated levels of serum transaminases and hepatocyte degeneration observed by electron microscopy. Sera obtained on days 7 and 14 contained autoantibodies including anti-DNA antibodies. The proportion of CD1dhighB cells in the liver was found to decrease on days 1–7. In other words, denatured liver tissue stimulated both NKT cells and certain B cells in the liver. These results suggest that liver lymphocytes might contain not only autoreactive T cells (e.g. CD3int or NKT cells) but also some B cells (e.g. B-1 cells) which produce autoantibodies and that the denatured tissue had the potential to stimulate these lymphocytes and to evoke an autoimmune-like state.
Keywords: natural killer T cells, autoantibody production, denatured liver tissue, CD1dhigh B cells, autoreactive T cells
INTRODUCTION
Recent studies in mice have revealed that a newly observed population of T cells which coexpresses an NK marker, NK1·1 (i.e. natural killer T or NKT cells) recognizes some glycolipid antigens as well as peptide antigens in the context of a monomorphic MHC class I-like antigen (i.e. CD1d) [1–5]. These NKT cells use an invariant Vα14 chain for TCRα and Vβ8, Vβ2, and Vβ7 chains for TCRβ[6–8]. A similar population of T cells has been characterized even in humans [9,10]. In murine studies, it has been proposed that NKT cells are immunoregulatory T cells for the suppression of autoimmune diseases. This proposal was based on the fact that autoimmune mice such as MRL-lpr/lpr[11,12] and NOD mice [13–15] showed a decreased level of NKT cells. However, subsequent studies have raised another possibility, namely, that NKT cells mediate autoreactivity against self-cells when they are activated with IL-12 [16] or α–galactosylceramide (α-GalCer) [17].
In light of these findings, we herein examined whether denatured self-antigens induce the expansion of NKT cells and the production of autoantibodies. Since NKT cells are abundant in the liver [18–20], we injected denatured liver tissue into mice. This idea arose from findings that the number of NKT cells in the liver increased after partial hepatectomy [21]. At such time, damaged hepatocytes and regenerated hepatocytes seemed to stimulate the expansion of NKT cells in the liver. As expected, the injection of denatured liver tissue stimulated NKT cells in the liver. Interestingly, liver damage and autoantibody production were accompanied by the expansion of NKT cells. The possible association of NKT cells and certain B cells (e.g. B-1 cells) with the onset of autoimmune diseases is herein discussed.
There has been a question as to the causes of the onset of autoimmune diseases [1]: Does the genetic background which induces abnormal immune functions permit the generation of autoreactive lymphocytes, resulting in the induction of autoimmune diseases? and [2] Does tissue damage (leaked self-antigens) which is evoked by viral infection or other inflammations stimulate the expansion of autoreactive lymphocytes and induce autoimmune diseases? Although we have to consider both possibilities, we propose the latter possibility in this study.
MATERIALS AND METHODS
Mice
Female C57BL/6 (B6) and MRL-lpr/lpr (lpr) mice were used at the age of 8–25 weeks. All mice were fed under specific pathogen-free conditions in the animal facility of Niigata University.
Treatment of mice
Mice were treated with denatured syngeneic liver tissue. Such tissue was prepared by cutting with scissors, after which it was pressed through 200-gauge stainless steel mesh and suspended in PBS (pH 7·2). After being washed twice, this denatured liver tissue was resuspended in 5ml PBS. Such denatured liver tissue was injected i.p. into mice. The same procedure was used to prepare denatured splenic tissue. In some experiments, α-GalCer (2 μg/mouse) (KRN 7000, Kirin Beer Co., Tokyo, Japan) [17] was i.p. injected. All mice were derived from the same colony and had no infectious diseases.
Cell preparation
Hepatic lymphocytes were isolated by a previously described method [22]. Briefly, mice anaesthetized with ether were killed by exsanguination through incised axillary arteries and veins. To obtain lymphocytes, the liver was removed, pressed through 200-gauge steel mesh, and suspended in Eagle's MEM supplemented with 5mm Hepes (Nissui Pharmaceutical Co., Tokyo, Japan) and 2% heat-inactivated newborn calf serum. After being washed with the medium once, the cells were resuspended in 35% Percoll solution containing 100U/ml heparin and centrifuged at 2000r.p.m. for 15min at room temperature.
Splenocytes and thymocytes were obtained by pressing the spleen and thymus through 200-gauge stainless steel mesh. Erythrocytes in the liver and spleen were disrupted by 0·83% NH4Cl-Tris buffer.
Immunofluorescence tests by a cell analyser
The surface phenotype was identified by using moAbs in conjunction with a two-colour immunofluorescence test [23]. The moAbs used here included FITC, PE, or biotin-conjugated reagents of anti-CD3 (145–2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), and anti-NK1·1 (PK 136) moAbs (PharMingen, San Diego, CA). Biotin-conjugated reagents were developed with either PE- or Tri-Color-conjugated streptavidin (Becton Dickinson Co., Mountain View, CA and Caltag Laboratory, San Francisco, CA). To prevent nonspecific binding of moAbs, CD32/16 (2·4G2) (PharMingen) was added before staining with labelled moAbs. The fluorescence-positive cells were analysed by a FACScan (Becton-Dickinson Co.). Dead cells were excluded by forward scatter, side scatter, and propidium iodide gating.
Reverse-transcription-PCR for Vα14Jα281 mRNA
To detect mRNA of the Vα14Jα281 gene, RNA was reversely transcribed (RT) using the oligo (dT) 15 primer, and such cDNA was further amplified by the PCR method as previously described [24]. Briefly, total RNA was prepared from lymphocytes by an acid guanidinium thiocyanate-phenol-chloroform method. cDNA was synthesized using 1μg RNA with a 1st-strand cDNA synthesis kit (Pharmacia Biotech Co., Tokyo). For PCR amplification, 1μl of cDNA was transferred to individual tubes that contained 10 pm of primers for the Vα14Jα281 gene, Taq DNA polymerase (2·5U; Perkin-Elmer Co., New Jersey, USA), d-NTP (200μm) in 10 × PCR buffer (Perkin-Elmer Co.), and MgCl2 (2 mm). Vα14 sense primer and Jα281 antisense primer yielding a 132-bp fragment were used [24]. A 10-μl portion of each amplified product was examined by 2% agarose gel electrophoresis and stained with ethidium bromide.
Light and electron microscopic studies
Liver specimens for light microscopy were fixed in 10% formalin solution, and the paraffin sections were stained with haematoxylin-eosin. For electron microscopy, liver specimens were quickly fixed in 3% gultaraldehyde, followed by postfixation in 1% OsO4. They were embedded in Epon 812 and ultrathin sections were examined with a JEOL 100B electron microscopy (JEOL, Peabody, MA, USA).
Identification of autoantibodies
To detect anti-DNA antibody, the ELISA method was applied [25]. Coated materials of denatured DNA were derived from salmon. The activity of such anti-DNA antibodies was divided into IgM and IgG fractions in sera by using isotype-specific anti-μ chain moAb and anti-γ chain moAb as secondary antibodies (PharMingen). The titre was determined by using a positive control of sera (100 U/ml) from lpr mice after the onset of autoimmune disease (at the age of 25 weeks). We also examined the titre of anti-hepatocyte antibody by the ELISA method. Instead of denatured salmon DNA, denatured B6 hepatic tissue was coated. The excess tissue was washed out by PBS.
Autoantibodies were also detected by using a HEp-2 cell line in conjunction with an immunofluorescence test [26]. Sera obtained from various mice were used after a dilution 1/20. FITC-conjugated anti-mouse Ig (PharMingen) was used as a secondary antibody.
Statistical analysis
Statistical differences were analysed by Student's t-test.
RESULTS
Increase in the number of NK1·1+CD3int cells (NKT cells) in the liver by an injection of denatured liver tissue
A denatured liver suspension was injected (one liver/mouse) and it was examined how the distribution pattern of lymphocytes and their subsets (including NKT cells) was changed (Table 1). In this experiment, two-colour staining for CD3 and NK1·1 was conducted to identify NKT cells (NK1·1+CD3int) and other subsets. We also examined the effect of denatured splenic tissue (one spleen/mouse). Similar to the case of denatured liver tissue, denatured splenic tissue resulted in a decrease in the number of lymphocytes in the liver on days 1 and 7 (data not shown). However, there was a big discrepancy between the effects of these two injections. Namely, denatured liver tissue induced a prominent expansion of its NK1·1+CD3int subset, whereas denatured splenic tissue did not induce such an expansion. In the case of denatured liver tissue, the increase in the proportion of CD3int cells was most striking on day 1 after the injection (up to 45%) and continued thereafter. Two-colour staining for CD4 and NK1·1+ indicated that the majority of NK1·1+CD3int cells were CD4+ (with a few double-negative CD4−8−) (data not shown).
Table 1.
Variation in the number of whole lymphocytes and NK1·1 + TCRint cells (NKT cells) by the administration of denatured liver tissue
| No. of whole lymphocytes | No. of NKT cells | |||||
|---|---|---|---|---|---|---|
| Day after the injection of denatured liver tissue | Liver (×106) | Spleen (×107) | Thymus (×107) | Liver (×105) | Spleen (×106) | Thymus (×105) |
| – | 4·6 ± 0·6 | 7·6 ± 0·9 | 15·8 ± 1·2 | 8·5 ± 1·6 | 2·0 ± 1·8 | 8·4 ± 1·0 |
| day 1 | 2·1 ± 0·8* | 4·1 ± 0·5* | 7·9 ± 1·0* | 6·0 ± 1·5 | 1·2 ± 0·9* | 6·8 ± 0·6 |
| day 7 | 3·2 ± 0·6* | 5·5 ± 0·6* | 8·0 ± 1·0* | 7·4 ± 1·4 | 1·9 ± 0·9 | 3·9 ± 1·2* |
| day14 | 5·8 ± 0·8 | 8·0 ± 1·0 | 11·5 ± 1·1 | 13·0 ± 1·2* | 2·2 ± 0·8 | 4·6 ± 0·9* |
| day21 | 5·0 ± 0·7 | 9·8 ± 1·0 | 9·8 ± 0·9 | 10·1 ± 1·2* | 2·3 ± 0·8 | 9·9 ± 0·8 |
Lymphocytes were obtained from the liver on days1, 7, 14 and 21 after the injection. Two-colour stainings for NK 1·1 and CD3 were conducted to identify NKT cells (NK1·1+ CD3int cells). The absolute number (per one mouse) of NKT cells was estimated by calculation using the number of total lympocytes and the proportion of NKT cells.
The mean and one SD are represented (n = 4 at each point of time).
P < 0·05.
The number of lymphocytes was enumerated in the liver, spleen, and thymus of mice injected with denatured liver tissue (Table 1). The number of lymphocytes decreased in all tested organs on day 1 (P < 0·05). It thereafter began to recover and returned to the normal level on days 14 and 21. However, even on day 21, the number of lymphocytes in the thymus decreased. The effect of ageing might be associated with this phenomenon.
The absolute number of NKT cells in the liver, spleen, and thymus was estimated by calculation. It was found that the number of NKT cells in the liver increased on days 14 and 21. The number of NKT cells in the thymus decreased, whereas that in the spleen remained unchanged.
Since the proportion of NKT cells increased prominently in the liver of mice injected with denatured liver tissue on day 1, we examined whether these NKT cells showed any sign of Vα14Jα281 mRNA by the RT-PCR method. It was found that such a sign was also prominent on day 1 (data not shown).
Increase in serum level of transaminases
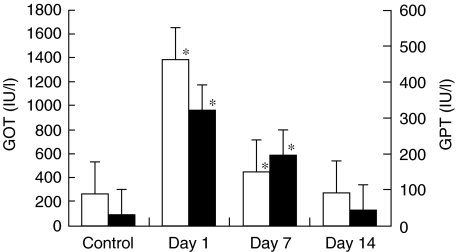
It is known that activated NKT cells can mediate self-reactive cytotoxicity against regenerated hepatocytes [21]. In this regard, we examined whether the serum level of transaminases was elevated after the injection of denatured liver tissue (Fig. 1). On days 1–7, elevated levels of GOT and GPT were detected (P < 0·05), the highest levels being demonstrated on day 1.
Fig. 1.
Elevation of serum levels of transaminases induced by the injection of denatured liver tissue. The levels of transaminases (□ GOT and ▪ GPT) were measured at the indicated points of time (n = 4 at each point). *P < 0·05.
Induction of hepatic damage
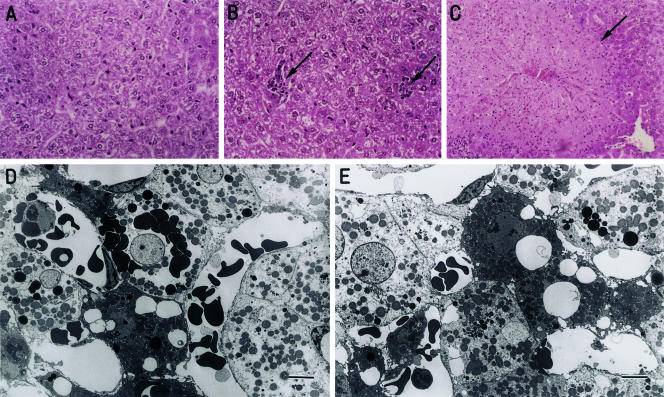
Histology of the liver was compared between control mice and mice injected with denatured liver tissue (day 1) (Fig. 2). In this experiment, we also examined mice injected with α-GalCer (day 1). In comparison with the liver of control mice (Fig. 2A), several clusters of lymphoid cells were seen in the liver of mice injected with denatured liver tissue (Fig. 2B). However, tissue necrosis was not seen in these mice. In contrast, massive necrosis was seen in the liver of mice injected with α-GalCer (Fig. 2C). In the case of the liver of mice injected with denatured liver tissue, some hepatocytes showed scantiness of the cytoplasmic contents as observed by light microscopy. We therefore examined such liver by electron microscopy (Fig. 2D,E). Some hepatocytes were found to have lost their cytoplasmic contents and instead carried erythrocytes into the cytoplasm. Some other hepatocytes showed large vacuoles (lipid droplets) in the cytoplasm.
Fig. 2.
Histology of the liver (A to C, × 400, light microscopy; D and E, Bar = 5μm electron microscopy). A. Normal mice, B. Mice injected with denatured liver tissue (on day 1), C. Mice injected with α-GalCer (2μg/mouse) (on day 3), D, E. Mice injected with denatured liver tissue (on day 1).
Autoantibody production by injection with denatured liver tissue
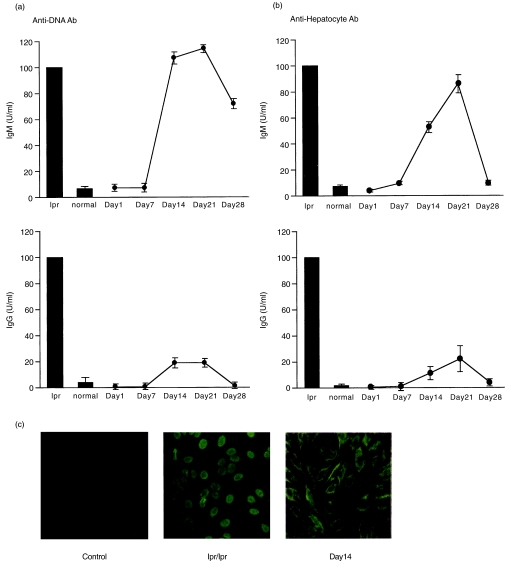
Since hepatic damage was induced by the injection of denatured liver tissue, we examined whether autoantibodies were produced in these mice. Serum was obtained from control and treated mice (on days 1–28). Autoantibodies against DNA were measured by the ELISA method (Fig. 3a). Sera of lpr mice after the onset of disease (at the age of 25 weeks) were used as a positive control. The titre of lpr sera was adjusted as 100U/ml. In this situation, the titres of IgM anti-DNA antibody and of IgG anti-DNA antibody were negligible in normal mice (< 2U/ml). In the case of mice injected with denatured liver tissue, the IgM titre increased on days 14 and 21 and then declined. Although the IgG titre was very low, a similar elevation pattern was observed in these mice. In the case of lpr mice, both IgM and IgG types of anti-DNA antibodies were detected. In contrast, only the IgM type was elevated in mice injected with denatured liver tissue. A similar experiment was conducted and the titre of anti-hepatocyte antibody was determined (Fig. 3b). The results showed the same tendency as those of anti-DNA antibody.
Fig. 3.
Autoantibody production induced by the injected of denatured liver tissue. (a) Anti-DNA antibody, (b) Anti-hepatocyte antibody, (c) Identification of autoantibodies by using a HEp-2 cell line. Sera were obtained on days 1–28 after injection of denatured liver tissue. Serum of lpr mice after the onset of disease (25 weeks of age) was used as a positive control and the titre was adjusted to 100 U/ml. The mean and one SD were produced from three experiments. All sera were used in a 1/20 dilution.
We examined the qualitative difference of autoantibodies between lpr mice and mice injected with denatured liver tissue (Fig. 3c). A HEp-2 cell line was used to detect the autoantibodies in conjunction with a immunofluorescence test. Sera of lpr mice were found to react with only the nucleus but not the cytoplasm of HEp-2 cells. In contrast, sera of mice injected with denatured liver tissue reacted with both the cytoplasm and nucleus, showing a high intensity of the former.
Simultaneous stimulation of hepatic B cells in mice injected with denatured liver tissue
Since autoantibody production was seen in mice injected with denatured liver tissue, it was examined whether some stimuli had also entered B cells in the liver or spleen (Table 2). Two-colour staining for B220 and CD1d and that for IgD and IgM were conducted. B220+B cells carried a higher level of CD1d antigens (mean intensity = 480) on the surface than did the remaining lymphocyte subsets (mean intensity ≤100) (including NK, NKT, and conventional T cells) in the liver. These CD1dhigh B220+B cells expressed surface IgD and IgM (a few cells being IgD+IgM−). After the administration of denatured liver tissue, the proportion of CD1dhigh B220+B cells decreased on days 1–7 (P < 0·05), especially in the liver, but returned to the normal level on day 14. These proportional changes of B cells were not seen in the spleen (data not shown).
Table 2.
Variation in the proportion of B cells in the liver of mice injected with d enatured liver tissue
| % Fluorescence-positive cells | ||
|---|---|---|
| Day after the injection of denatured liver tissue | CD1dhigh B220+ cells | IgM+ IgD+ cells |
| – | 31·6 ± 8·5 | 28·1 ± 7·7 |
| day 1 | 17·9 ± 5·0* | 19·8 ± 4·5* |
| day 7 | 23·8 ± 6·1* | 25·9 ± 4·8* |
| day14 | 32·5 ± 8·0 | 25·1 ± 7·2 |
P < 0·05, n = 4.
Decrease in the expansion of NKT cells and in liver injury in NKT-deficient mice injected with denatured liver tissue
In a final portion of experiments, we used NKT-deficient mice to examine how the expression of CD1d molecule or other MHC antigens was related to the expansion of NKT cells. In addition to B6 mice, CD1d (–/–) and β2m (–/–) mice were used as NKT-deficient mice (Table 3). TAP-1 (–/–) mice were also used as non-NKT-deficient mice. Two-colour staining for CD3 and NK1·1 revealed that B6 and TAP-1 (–/–) mice showed a normal level of NKT cells in the liver and a normal expansion of NKT cells when injected with denatured liver tissue (day 1). In sharp contrast, CD1d (–/–) and β2m (–/–) mice showed a low level of NKT cells in the liver but a moderate expansion of NKT cells resulting from the injection of denatured liver tissue. However, the major population of NKT cells were not CD4+, in CD1d (–/–) and in β2m (–/–) mice. Tissue damage on day 1 was seen in NKT-deficient mice, but the magnitude of damage decreased.
Table 3.
Decrease in the expansion of NKT cells in the liver of NKT-deficient mice induced by the injection of denatured liver tissue
| % Fluorescence-positive cells | |||
|---|---|---|---|
| Mice | Injection of denatured liver tissue | NK1·1+ CD3int | NK1·1+ CD4+ |
| B6 | – | 18·5 ± 4·3 | 16·2 ± 3·6 |
| day 1 | 44·8 ± 11·0* | 34·1 ± 9·8* | |
| TAP-1 (–*–) | – | 11·4 ± 1·2 | 8·7 ± 0·9 |
| day 1 | 25·3 ± 4·6 | 16·7 ± 8·5 | |
| CD1d (–/–) | – | 2·8 ± 0·2 | 0·3 ± 0·1 |
| day 1 | 12·8 ± 4·4* | 1·7 ± 0·2 | |
| β2m (−/−) | – | 2·2 ± 0·1 | 0·6 ± 0·1 |
| day 1 | 11·9 ± 3·3* | 0·7 ± 0·1 | |
P < 0·05, n = 4.
DISCUSSION
We demonstrated that activation of NKT cells and autoantibody production were induced in mice injected with denatured liver tissue. At such time, acute thymic atrophy and a decrease in the number of whole lymphocytes (and conventional T cells) in the periphery occurred. In other words, inverted modulation was seen between primordial T cells (e.g. NKT cells) and conventional T cells in immune organs. In conjunction with evidence for direct effects of NKT cells on hepatic damage when these T cells were activated with IL-12 [16] or α-GalCer [17], NKT cells and certain B cells which produce autoantibodies might be intimately associated with the onset of autoimmune diseases or autoimmune-like diseases.
It is known that B220+B cells express a higher level of CD1d antigens than other lymphocyte subsets [27,28]. We also revealed that the stimulus resulting from the injection of denatured tissue rather decreased these B cells of the liver. There was a certain stimulation of these B cells by denatured liver tissue. By stimulation, lymphocytes sometimes proliferate and increase in number, but they sometimes undergo apoptosis and decrease in number. It is conceivable that CD1dhigh B220+B cells was the latter case. It is known that the stimulation of α-GalCer induces the apoptosis of NKT cells in the liver, similar to the present case [17].
In autoimmune diseases, specific self-antigens have been researched [29–31]. Such self-antigens include single- and double-stranded DNA in systemic lupus erythematosus, type II collagen in rheumatoid arthritis, myelin basic protein in autoimmune encephalomyelitis, etc. However, several anti-self-antigen antibodies or self-reactive T cells are simultaneously induced in many autoimmune diseases. To determine T and B cells associated with autoimmune hepatitis, we applied denatured liver tissue. As a result, we induced autoimmune-like hepatic damage. The autoantibodies induced by denatured liver tissue manifested activities against multiple self-antigens. Namely, not only denatured DNA and hepatocytes (IgM-type antibody) but also the cytoplasms of a HEp-2 cell line were reactive with sera of these mice. In contrast, sera of lpr mice were reactive with denatured DNA and whole hepatocytes (both IgM and IgG type antibodies) but not at all with the cytoplasms of a HEp-2 cell line.
The most prominent population which expanded in the damaged liver was estimated to be NK1·1+CD3int (i.e. NKT) cells. Thus far, attention has been focused on the immunoregulatory function of NKT cells. For example, a decreased level of NKT cells has been reported to be associated with the onset of renal failure in autoimmune MRL-lpr/lpr mice [11,12] and in autoimmune diabetic NOD mice [13–15]. However, we should consider the possibility that NKT cells might be directly associated with the onset of autoimmune diseases. As reported in recent studies [32,33], there is a possibility that NK cells, in conjunction with NKT cells are also associated with effector functions for the present phenomenon. Indeed, the proportion of NK cells, as well, increased by the injection of denatured liver tissue.
In contrast to conventional T cells, NKT cells mediate self-reactive cytotoxicity against syngeneic thymocytes [34,35] or regenerating hepatocytes [21]. It is speculated that NKT cells act as immunoregulatory cells if they mediate their cytotoxicity against autoimmune effector cells. However, if they mediate their cytotoxicity against hepatocytes or other self-cells, they would be the effector cells for autoimmune diseases. Since NKT cells have the ability to produce a large amount of IL-4 [36–39], activated NKT cells might induce Th2 switching resulting in the acceleration of autoantibody production.
Liver injury induced by the injection of denatured liver tissue was estimated by not only the elevation of serum transaminases but also by the morphology. In contrast to the liver injury induced by α-GalCer, massive necrosis was not seen in the above case. The mechanisms underlying denatured liver tissue (e.g. a mild NKT cell function and autoantibodies) and α-GalCer (e.g. a strong NKT effector function) might be different. Many lymphoid cell clusters were seen in the liver of mice in this study. As we have shown previously [40], this picture resembles that in mice injected with an excessive dose of oestrogen.
We detected an autoantibody against a HEp-2 cell line. It is conceivable that liver lymphocytes might comprise some B cells (e.g. B-1 cells) which produce autoantibodies [41,42]. They carry a higher level of CD1d antigens than do other lymphocytes. In this study, we did not identify such B cells by specific markers. Thus, the majority of B cells in the liver lacked CD5 antigens (data not shown) and had a phenotype similar to that of splenic B cells. The only difference of B cells between the liver and spleen was that some hepatic B cells expressed a higher level of IL-7R than did splenic B cells (data not shown). It was interesting that a specific depletion of B220+B cells (IgM+IgD+ and some IgM+IgD−) by stimuli with denatured liver tissue was seen in the liver. As already proposed [27,28], these CD1dhigh B220+B cells might have the potential to present some self-antigens to NKT cells via their CD1d.
We cannot exclude the possibility that some molecules other than CD1d are associated with the self-antigen presentation to NKT cells. This notion originates from the data from the experiments using CD1d (–/–) and β2m (–/–) mice in this study. Since these mice lacked usual CD4+NKT cells and showed the expansion of other phenotype NKT cells (e.g. CD8+ or CD4−8−) induced by the injection of denatured liver tissue, it is speculated that the complex of self-antigens and non-CD1d might interact with these unusual NKT cells. At such time, the magnitude of tissue damage would decrease in these mice.
Although the experimental protocols were different, high frequency of B-1 cells (IgA-committed) and NKT cells in the lacrimal glands of mice was reported by Saitoh-Inagawa et al.[43]. Both the liver and lacrimal glands are the organs where NKT cells are most abundant. These organs often become the target sites for autoimmune diseases.
We noted that thymic atrophy accompanied the present phenomenon. Namely, suppression of the major pathway of T cell differentiation (for TCRhigh cells) in the thymus and activation of an alternative intrathymic pathway (for NKT cells) or extrathymic pathways of T cell differentiation might be the immune states seen in autoimmune diseases. In an earlier study, Sega and Shevach [44] reported that IL-12 unmasks latent autoimmune disease in resistant mice. IL-12 is known to activate NKT cells. Taken together, we have to consider the possibility that NKT cells function not only as immunoregulatory cells but also as autoimmune-effector cells.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan. We wish to thank Mrs Yuko Kaneko for preparation of the manuscript.
REFERENCES
- 1.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A. Positive selection of mouse NK1+ T cells by CD1- expressing cortical thymocytes. J Exp Med. 1995;182:2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR- mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Tangri S, Brossay L, Burdin N, Lee DJ, Corr M, Kronenberg M. Presentation of peptide antigens by mouse CD1 requires endosomal localization and protein antigen processing. Proc Natl Acad Sci USA. 1998;95:14314–9. doi: 10.1073/pnas.95.24.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d- independent NK T cells. J Immunol. 1999;162:6410–9. [PubMed] [Google Scholar]
- 6.Makino Y, Yamagata N, Sasho T, Adachi Y, Kanno R, Koseki H, Kanno M, Taniguchi M. Extrathymic development of Vα14-positive T cells. J Exp Med. 1993;177:1399–408. doi: 10.1084/jem.177.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 8.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Positive selection of invariant Vα14+ T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone marrow-derived cells. Proc Natl Acad Sci USA. 1995;92:1200–4. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins C, Norris S, McEntee G, Traynor O, Bruno L, von Boehmer H, Hegarty J, O'Farrelly C. RAG1, RAG2 and pTα expression by adult human hepatic T cells. Eur J Immunol. 1996;26:1193–8. doi: 10.1002/eji.1830261243. [DOI] [PubMed] [Google Scholar]
- 10.Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. Natural T cells in the human liver. Cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include T cell receptor Vα24-JαQ and γδ T cell receptor bearing cells. Human Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1- positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–64. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mieza MA, Itoh T, Cui JQ, et al. Selective reduction of Vα14+ NKT cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–40. [PubMed] [Google Scholar]
- 13.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach JF, Monteiro RC. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. α/β-T cell receptor (TCR) +CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD) /Lt mice by the influence of interleukin (IL) -4 and/or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. A defect in interleukin 12-induced activation and interferon γ secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999;190:963–72. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita T, Ando K, Kimura K, Ohnishi H, Imawari M, Muto Y, Moriwaki H. IL-12 induces specific cytotoxicity against regenerating hepatocytes in vivo. Int Immunol. 1999;11:657–65. doi: 10.1093/intimm/11.5.657. [DOI] [PubMed] [Google Scholar]
- 17.Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate TCR cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+T cells in various immune organs. NK1.1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 20.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–15. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 22.Tsukahara A, Seki S, Iiai T, et al. Mouse liver T cells: Their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–9. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura H, Kawamura T, Kokai Y, et al. Expansion of extrathymic T cells as well as granulocytes in the liver and other organs of G-CSF transgenic mice: Why they lost the ability of hybrid resistance. J Immunol. 1999;162:5957–64. [PubMed] [Google Scholar]
- 24.Narita J, Kawamura T, Miyaji C, Watanabe H, Honda S, Koya T, Arakawa M, Abo T. Abundance of NKT cells in the salivary glands but absence thereof in the liver and thymus of aly/aly mice with Sjögren syndrome. Cell Immunol. 1999;192:149–58. doi: 10.1006/cimm.1998.1450. [DOI] [PubMed] [Google Scholar]
- 25.Shiraki M, Fujiwara M, Tomura S. Long term administration of cyclophosphamide in MRL/1 mice. I. The effects on the development of immunological abnormalities and lupus nephritis. Clin Exp Immunol. 1984;55:333–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–7. [PubMed] [Google Scholar]
- 28.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710–7. [PubMed] [Google Scholar]
- 29.Matsumoto Y, Hanawa H, Tsuchida M, Abo T. In situ inactivation of infiltrating T cells in the central nervous system with autoimmune encephalomyelitis. The role of astrocytes. Immunology. 1993;79:381–90. [PMC free article] [PubMed] [Google Scholar]
- 30.Hanawa H, Tsuchida M, Matsumoto Y, et al. Characterization of T cells infiltrating the heart in rats with experimental autoimmune myocarditis. Their similarity to extrathymic T cells in mice and the site of proliferation. J Immunol. 1993;150:5682–95. [PubMed] [Google Scholar]
- 31.Arai K, Yamamura S, Hanyu T, Takahashi HE, Umezu H, Watanabe H, Abo T. Extrathymic differentiation of resident T cells in the joints of mice with collagen-induced arthritis. J Immunol. 1996;157:5170–7. [PubMed] [Google Scholar]
- 32.Nakagawa R, Nagafune I, Tazunoki Y, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α-Galactosylceramide in mice. J Immunol. 2001;166:6578–84. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 33.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–30. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamura T, Kawachi Y, Moroda T, et al. Cytotoxic activity against tumour cells mediated by intermediate TCR cells in the liver and spleen. Immunology. 1996;89:68–75. doi: 10.1046/j.1365-2567.1996.d01-719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moroda T, Iiai T, Suzuki S, et al. Autologous killing by a population of intermediate TCR cells and its NK1.1+ and NK1.1− subsets, using Fas ligand/Fas molecules. Immunology. 1997;91:219–26. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto T, Paul WE. CD4+, NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emoto M, Emoto Y, Kaufmann SHE. IL-4 producing CD4+ TCRαβint liver lymphocytes. influence of thymus, β2-microglobulin and NK1.1 expression. Int Immunol. 1995;7:1729–39. doi: 10.1093/intimm/7.11.1729. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–7. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 39.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 40.Narita J, Miyaji C, Watanabe H, et al. Differentiation of forbidden T cell clones and granulocytes in the parenchymal space of the liver in mice treated with estrogen. Cell Immunol. 1998;185:1–13. doi: 10.1006/cimm.1998.1245. [DOI] [PubMed] [Google Scholar]
- 41.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–86. [PubMed] [Google Scholar]
- 42.Ochi H, Takeshita H, Suda T, Nisitani S, Honjo T, Watanabe T. Regulation of B-1 cell activation and its autoantibody production by Lyn kinase-regulated signallings. Immunology. 1999;98:595–603. doi: 10.1046/j.1365-2567.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoh-Inagawa W, Hiroi T, Yanagita M, Iijima H, Uchio E, Ohno S, Aoki K, Kiyono H. Unique characteristics of lacrimal glands as a part of mucosal immune network: high frequency of IgA-committed B-1 cells and NK1.1+αβT cells. Invest Ophthalmol Vis Sci. 2000;41:138–44. [PubMed] [Google Scholar]
- 44.Segal BM, Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996;184:771–5. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]