Abstract
Neutrophils, short-lived leucocytes that die by apoptosis, play an important role in the first stage of defense against bacterial infections. It has been reported that phagocytosis of intact bacteria or Candida albicans can accelerate neutrophil apoptosis. However, the mechanism of phagocytosis-mediated neutrophil apoptosis is not well characterized. In this study, we evaluated whether ingestion of heat-killed Staphylococcus aureus (S. aureus) enhances neutrophil apoptosis and whether this type of apoptosis is mediated by oxidative stress by using antioxidants and polymorphonuclear leucocytes (PMNs) from patients with chronic granulomatous disease (CGD). Co-culture of PMNs with varying doses of S. aureus resulted in accelerated PMN death in a dose- and time-dependent manner. Increased PMN apoptosis was observed by both Annexin V and PI staining. Similar results were observed in PMNs of CGD patients. Dimethyl sulphoxide (DMSO, an OH• scavenger) did not significantly inhibit either S. aureus-ingested PMN apoptosis or spontaneous PMN apoptosis. On the other hand glutathione (GSH, an H2O2 scavenger) significantly inhibited both types of apoptosis.
Our findings suggest that oxygen-independent pathways may mainly operate in the process of phagocytosis-induced apoptosis.
Keywords: neutrophil, apoptosis, phagocytosis, chronic granulomatous disease, hydrogen peroxide
INTRODUCTION
Polymorphonuclear leucocytes (PMNs) die rapidly in circulation by the process of apoptosis [1]. When microorganisms invade a living body, PMNs adhere to blood vessels, transmigrate through the endothelial layer and locomote to the extravascular space to ingest bacteria and kill the ingested bacteria using reactive oxygen species. However, this defence function may often injure even normal tissues. Therefore PMNs need to be disposed of quickly when they have performed their function. It has been demonstrated that PMN apoptosis occurs at sites of inflammation and that PMNs are then phagocytosed by the surrounding cells [1]. It has also been reported that PMN phagocytosis of live E. coli or heat-killed Candida albicans could accelerate neutrophil apoptosis [2,3]. Although the processes of spontaneous and Fas-mediated apoptosis are well known [4], the mechanism of phagocytosis-induced apoptosis has not been well characterized. William et al.[2] reported that ingestion of E. coli induces PMN apoptosis through an oxygen-dependent mechanism.
In the present study, we tried to determine whether the ingestion of S. aureus enhances PMN apoptosis and whether this type of apoptosis is mediated by oxidative stress by using antioxidants and PMNs from patients with chronic granulomatous disease (CGD).
MATERIALS AND METHODS
Reagents
RPMI1640, penicillin G and streptomycin were purchased from Gibco BRL (Santa Clara, CA, USA). Propidium iodide (PI), glutathione (GSH), Histopaque 1077, human AB serum were purchased from Sigma Chemical Co (St. Louis, MO, USA); DMSO and methanol were purchased from Wako Chemicals (Osaka, Japan); dextran T-500 from Amersham Biosciences Co., Piscataway, NJ, USA); PBS from Takara Shuzo Co Ltd (Shiga, Japan); Annexin V from Immunotech, Beckman Coulter Co (Marseille, France); S. aureus (ATCC25923) was kindly supplied by the Shionogi Pharmaceutical Co. (Osaka, Japan).
Isolation and culture of human PMNs
PMNs were isolated from heparinized venous blood of 7 healthy adult donors and 5 patients (average age of patients 6·2 year ± 5·2 SD) with X-linked gp91-phox-defective CGD by a combination of dextran sedimentation and centrifugation through a Ficoll-Hypaque gradient as previously described [5]. Informed consent was obtained from the parents of the patients or directly from the normal controls, and our study was approved by the Human Ethical Committee of the Kansai Medical University. All 5 patients with CGD were diagnosed by simultaneous measurement of phagocytosis and hydrogen peroxide production of neutrophils in whole blood by flow cytometry [6] and Western blot analysis with a gp91-phox-antibody [7], kindly donated by Dr Hiroyuki Nunoi (Department of Paediatrics, Miyazaki Medical University, Miyazaki, Japan). All patients received good medical attention, were free from infections, and were not on interferon-γ. Their leucocyte counts and differentials were normal.
Five hundred microliters of isolated PMNs (1 × 106cells/ml) were suspended in RPMI 1640 medium supplemented with 10% human AB serum, 1% glutamine, and 1% penicillin G and streptomycin with or without heat-killed S. aureus at different ratios ranging from 1:3 to 1:30. The cells were incubated in polypropylene tubes at 37°C in an humidified incubator containing 5% CO2 in air for the indicated periods of time. PMN viability was assessed by trypan blue exclusion.
Quantification of apoptotic cells
Apoptosis of cultured neutrophils was measured, following either staining with propidium iodide (PI, 100 μg/ml) [8] or an Annexin V FITC kit [9,10], by flow cytometry. Briefly, cell pellets obtained by centrifugation were treated with 2 ml of cold 70% ethanol and fixed at −20°C for more than 2h. After washing, the cells were suspended in 0·5ml phosphate-buffered saline. Then 500 μl RNase (1 mg/ml) and 1ml PI (100 μg/ml) were added, and the mixtures were kept at 4°C in the dark until examined. We used an Annexin V FITC kit to detect early apoptotic changes. The cell samples were washed with ice-cold culture medium or PBS after centrifugation for 5 min at 500 × g at 4°C. The cells were finally resuspended in ice-cold, diluted binding buffer at a concentration of 105–106 cells/ml. Five microliters of Annexin V FITC and 5μl of PI solution were added to the cell pellet along with 490μl of binding buffer, and were mixed gently. The tubes were kept on ice and incubated for 10 min in the dark. Fluorescence of individual cells was measured with a flow cytometer (EPICS-XL; Coulter Corp).
Statistical analysis
Data were presented as mean ± SEM with the number of experiments indicated. Data were analysed by Wilcoxon's or Mann–Whitney's signed rank test. Differences were considered significant when P < 0·05.
RESULTS
Co-culture of PMNs with S. aureus resulted in accelerated cell death in a dose- and time-dependent manner (Fig. 1). To elucidate whether cell death is due to apoptosis or necrosis, two stainings – PI staining for hypodiploid DNA and Annexin staining for assessing early apoptotic changes on the cell surface [9,10]– were employed. Increased PMN apoptosis was observed following ingestion of S. aureus by both methods (Fig. 2). This effect was dependent on S. aureus doses (Fig. 2), and was confirmed by microscopy (Fig. 3).
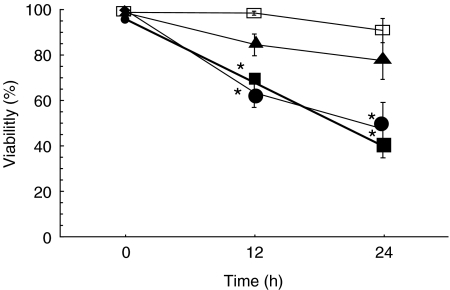
Fig. 1.
Viability (by trypan blue exclusion) of PMNs when incubated with different concentrations of S. aureus. The ratios of PMN:S. aureus of 1:10 and 1:30 resulted in significant increases in PMN apoptosis after incubation for 12 h and 24 h. *P < 0·05 versus control. Control PMN □; PMN + S. aureus (1:30) •, (1:10) ▪, (1:3) ▴.
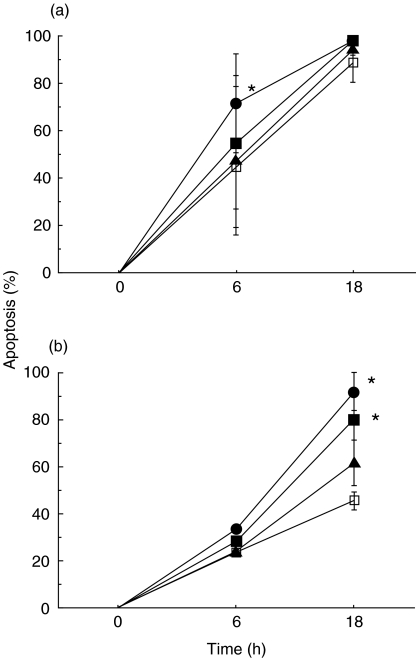
Fig. 2.
Increased PMN apoptosis following ingestion of different concentrations of S. aureus when evaluated by PI (a) and Annexin V (b) staining. Ratio of PMN:S. aureus of 1:10 and 1:30 resulted in significant increases in apoptosis. *P < 0·05 versus control:.Control PMN □; PMN + S. aureus (1:30) •, (1:10) ▪, (1:3) ▴.
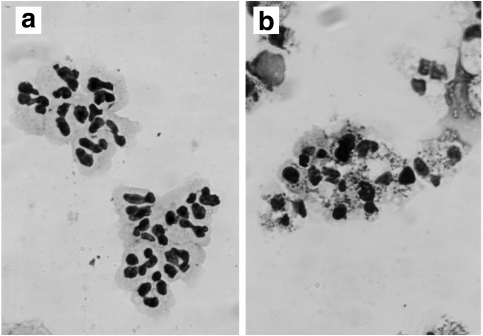
Fig. 3.
Morphological features of apoptotic PMNs cultured with medium only (spontaneous) (a) and medium plus S. aureus (1:30) (b) for 6h. A May–Grünwald-Giemsa stained preparation was photographed at a microscope magnification × 1000. PMNs indicate nuclear condensation (b).
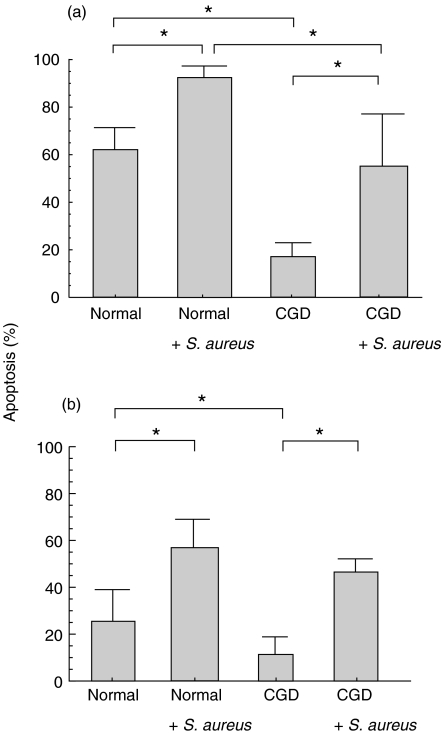
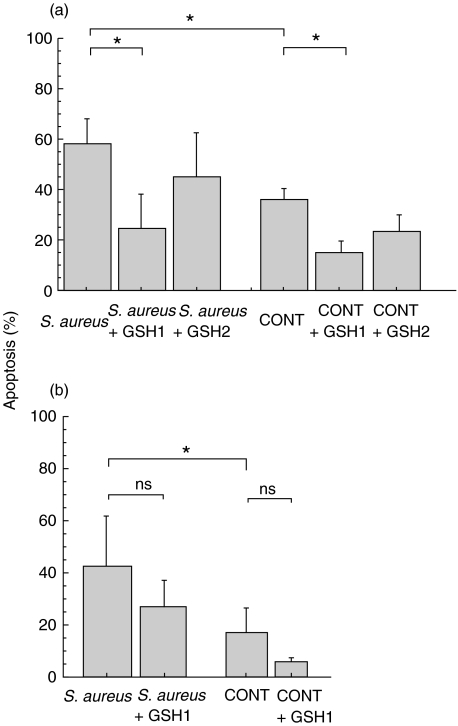
To clarify whether the above findings were mediated by oxygen radicals, we compared S. aureus-induced PMN apoptosis between normal and CGD PMNs. PMN apoptosis in both cell types, cocultured for 18h with a PMN:S. aureus ratio of 1:30, was significantly enhanced compared to the spontaneous rate (Fig. 4). This observation appears to indicate that accelerated PMN apoptosis following ingestion of S. aureus is not only mediated by an oxygen-dependent pathway but also an oxygen-independent pathway and possibly other unknown factors. To confirm this hypothesis, we then evaluated the effect of antioxidants (DMSO and glutathione) on both spontaneous and phagocytosis-related apoptosis. Briefly, DMSO did not inhibit spontaneous or bacteria-ingested apoptosis of either normal or CGD PMNs (data not shown). Glutathione significantly inhibited bacteria-ingested apoptosis in normal PMNs, although it also inhibited spontaneous apoptosis in these cells (Fig. 5a). To exclude a non specific effect of GSH, the effect of GSH on CGD neutrophil apoptosis was evaluated. GSH did not significantly decrease spontaneous or S. aureus-induced apoptosis in such cells (Fig. 5b).
Fig. 4.
PMN apoptosis of both normal and CGD PMNs cocultured with S. aureus for 18 h was significantly enhanced compared to that without S. aureus, respectively. (a) PI, (b) Annexin V.*P < 0·05.
Fig. 5.
Apoptosis of PMNs cocultured with S. aureus (1:30) and glutathione (GSH). (a) In normal PMNs, GSH1 (25mm) significantly inhibited apoptosis of S. aureus-ingested PMNs, although it also inhibited spontaneous PMN apoptosis. (b) In CGD PMNs, however, GSH did not significantly decrease spontaneous or S. aureus-induced apoptosis. *P < 0·05, ns: not significant. GSH 1: glutathione 25mm, GSH 2: glutathione 12·5 mm.
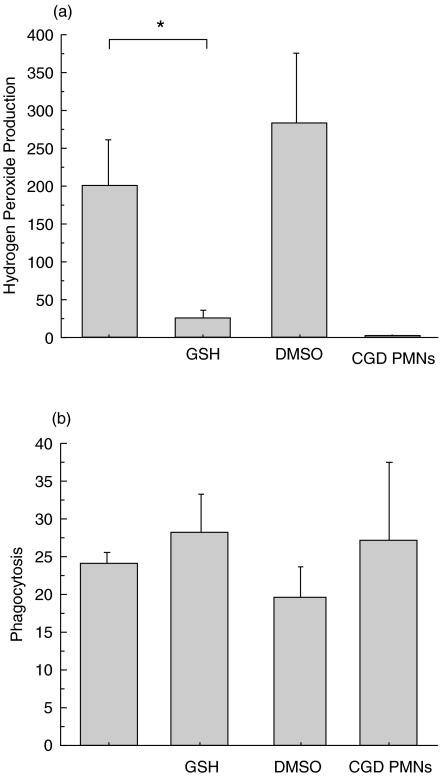
To evaluate the effect of S. aureus-ingestion on intracellular free radical production, the respiratory burst of PMNs was assessed 1h after the addition of S. aureus to the PMN culture according to the method described by Hasui et al.[6]. Although DMSO did not inhibit hydrogen peroxide production following the addition of S. aureus, glutathione remarkably reduced its production (Fig. 6a).
Fig. 6.
Effect of GSH and DMSO on (a) hydrogen peroxide production (DCF) and (b) phagocytosis (PI) of PMNs cocultured with S. aureus (1:30). DMSO neither inhibited hydrogen peroxide production nor phagocytosis. GSH reduced the production but did not alter phagocytosis. Ordinate: mean PI and DCF intensity/cell *P < 0·01 GSH, glutathione; DMSO, dimethyl sulphoxide; DCF, 2′,7′-dichlorofluorescein; PI, propidium iodide.
DISCUSSION
In the present study, we confirmed the previous finding that spontaneous neutrophil apoptosis is due to an oxygen-dependent process [4]. We further extended the finding to indicate that S. aureus-induced neutrophil apoptosis is mainly due to oxygen radical independent pathways, by using CGD PMNs and antioxidants.
The effect of S. aureus on PMN apoptosis was assessed by both PI and Annexin V staining. PI stains DNA and discriminates hypodiploid DNA, and can be used to measure the percentage of apoptotic PMNs [11]. Annexin V has only a very low affinity for phospholipid species such as phosphatidylethanolamine, sphingomyelin and phosphatidyl-choline. The phospholipid-binding property makes Annexin V as a powerful and selective tool for the detection of apoptotic cells, and is particularly suited to detecting early apoptotic changes. Our results from Annexin V staining, however, showed a lower percentage of apoptoic cells than that obtained by PI staining. Cell membrane changes during apoptosis occur through ICE/CED-3, one of the caspase families, once DNA fragmentation has started during apoptosis [12]. It is therefore possible that DNA fragmentation precedes apoptotic changes in the cell membrane. Moreover a previous article has demonstrated that data obtained by of PI staining (70%) showed a much higher percentage of apoptotic cells than that obtained by Annexin V binding (50%) [13].
In this study, we used both PI and Annexin staining to show that the death of PMNs was apoptotic not necrotic. Addition of S. aureus to neutrophil cultures resulted in increased neutrophil apoptosis as compared with the controls (i.e. neutrophils cultured alone), and this effect was dose- and time dependent. This phenomenon implies that phosphatidylserine binding, which binds to Annexin V, is expressed earlier on apoptotic neutrophils following the ingestion of bacteria, thus facilitating phagocytosis of the apoptotic cells by phosphatidylserine receptor bearing macrophages [14]. Similarly, another report indicated that neutrophil apoptosis was accelerated following the ingestion of E. coli[2] when compared with spontaneous apoptosis. Taking these findings together, it could be concluded that neutrophils, following ingestion of bacteria, die easily by apoptosis and that this event is beneficial for a living body to ensure that the surrounding tissues are not damaged by toxic neutrophil products.
CGD is a rare hereditary disorder characterized by a diminished or absent production of oxygen radicals due to a defect in any one of the components of the NADPH oxidase system. Patients with CGD suffer from severe, recurrent bacterial and fungal infections because of the inability of their granulocytes to kill ingested microorganisms. Patients with CGD also show granulomatous formation at infection sites and have widespread infection-unrelated complications subsequent to tissue injury.
The pathogenesis of granuloma formation in CGD patients is unclear. Kasahara et al.[4] have reported that the lack of oxygen radical production by neutrophils of CGD patients may cause neutrophils to fail to apoptose and remain alive at inflammation sites, thus leading to an absence of phagocytosis by tissue macrophages. They also emphasized that live CGD neutrophils were related to granuloma formation. However, they only described spontaneous apoptosis, not phagocytosis-induced apoptosis. In our study, apoptosis of CGD PMNs cocultured with S. aureus was decreased in comparison with that of control PMNs. This observation certainly implies that CGD neutrophils, following phagocytosis of bacteria, remain alive at inflammatory sites and that they could subsequently release several cytokines responsible for the formation of granuloma.
The antioxidants DMSO and glutathione were used in this study to evaluate the possible participation of reactive oxygen species in phagocytosis-induced apoptosis. These two reagents are supposed to be intracellular oxygen scavengers. The antioxidants catalase and superoxide dismutase were not used because of their inability to traverse the cell membrane [15]. The effects of DMSO and glutathione are different. DMSO is an OH• scavenger [16], while glutathione is an H2O2 scavenger [2]. Our results indicate that the signal transduction pathway involved in spontaneous apoptosis may be mediated not by the OH•, but by the H2O2, radical.
Interpretation of the mechanism of spontaneous neutrophil apoptosis is conflicting [4,17–22]. However, it may be mediated by oxygen radicals, particularly H2O2, based on the decreased rate of spontaneous apoptosis in CGD neutrophils and the inhibition of spontaneous apoptosis by glutathione. William et al.[2] reported that E. coli induces neutrophil apoptosis. They also showed that DMSO and GSH significantly reduced E. coli- mediated neutrophil apoptosis but these antioxidants had no effect on spontaneous apoptosis. They concluded that E. coli-induced neutrophil apoptosis is mainly due to an oxygen- dependent signal. Their conclusion is the opposite to our results. The discrepancy may be explained by the fact that they used live E. coli and a different method for detecting apoptosis. Although E. coli-mediated phagocytosis is due mainly to the C3bi receptor, S. aureus-mediated phagocytosis occurs through FcγIIa and FcγIIIb [23]. In addition, they mainly assessed apoptosis by morphology and FcγRIII expression, while we used PI and Annexin V staining. These distinctions allowed us to draw the conclusion that S. aureus-induced apoptosis is not primarily due to H2O2 but to other oxygen-independent signals.
It has also been reported that phagocytosis of Candida albicans stimulates increased apoptosis mediated by the redox state [3]. An oxygen-independent pathway was indicated for immune complex mediated neutrophil apoptosis through the FcγII molecules [24]. There has been no report, however, about the mechanism of S. aureus-induced neutrophil apoptosis. In addition to the antioxidant studies, we also compared S. aureus-induced apoptosis between CGD and normal neutrophils to determine whether and how hydrogen peroxide, which is not produced in CGD leucocytes after phagocytosis of bacteria, might have any effect on apoptosis. The distinctions between normal and CGD leucocytes in our experiments are as follows:
lower spontaneous apoptosis of CGD leucocytes compared with normals as has been reported previously [4];
significantly increased S. aureus-induced apoptosis is observed in CGD neutrophils in comparison to spontaneous apoptosis in both normal and CGD neutrophils; the ratio of the S. aureus-induced increase is eventually larger in CGD than normal neutrophils;
although GSH significantly decreased both spontaneous and S. aureus-induced apoptosis in normal neutrophils, GSH did not significantly decrease such apoptosis in CGD neutrophils;
with PI staining S. aureus-induced apoptosis in CGD neutrophils showed a significantly lower rate than that in normal neutrophils.
These observations lead us to conclude that S. aureus-induced apoptosis is not primarily due to H2O2 but to other oxygen independent signals, although we cannot completely exclude the possibility of oxygen-related signals participating. Further studies will be needed to elucidate the precise process of PMN apoptosis.
Acknowledgments
This study was supported by Grants-in-Aid for General Scientific Research (B)07457187 (C)06807066 and (C)10672192 of the Ministry of Education, Science, and Sports and Culture of Japan, the Morinaga Houshikai and the Mami Mizutani Foundation.
REFERENCES
- 1.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation programmed cell death in the neutrophil leads to recognition by macrophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.William R, Watson G, Redmond PH, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–92. [PubMed] [Google Scholar]
- 3.Rotstein D, Parodo J, Taneja R, Marshall JC. Phagocytosis of Candida albicans induces apoptosis of human neutrophils. SHOCK. 2000;14:278–83. doi: 10.1097/00024382-200014030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kasahara Y, Iwai K, Yachie A, Ohta K, Konno A, Seki H, Miyawaki T, Taniguchi N. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–53. [PubMed] [Google Scholar]
- 5.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–8. [PubMed] [Google Scholar]
- 6.Hasui M, Hirabayashi Y, Kobayashi Y. Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils of whole blood. J Immunol Meth. 1989;117:53–8. doi: 10.1016/0022-1759(89)90118-x. [DOI] [PubMed] [Google Scholar]
- 7.Imajoh-Ohmi S, Tokita K, Ochiai H, Nakamura M, Kanegasaki S. Topology of cytochrome b558 in neutrophil membrane analyzed by anti-peptide antibodies and proteolysis. J Biol Chem. 1992;267:180–4. [PubMed] [Google Scholar]
- 8.Tsuchida H, Takeda Y, Takei H, Shinzawa H, Takahashi T, Sendo F. In vivo regulation of rat neutrophil apoptosis occurring spontaneously or induced with TNF-α or cycloheximide. J Immunol. 1995;154:2403–12. [PubMed] [Google Scholar]
- 9.Homburg CHE, de Haas M, dem Borne AEG, Kr Verhoeven AJ, Reutelingsperger CPM, Roos D. Human neutrophils lose their surface FcgRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–5. [PubMed] [Google Scholar]
- 10.Van Heerde WL, Degroot PG, Reutelingsperger CPM. The complexity of the phospholipid binding protein annexin V. Thromb Haemost. 1995;73:172–9. [PubMed] [Google Scholar]
- 11.Nicoletti I. A rapid and simple method for meauring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 12.Martin SJ, Finucane DM, Amarante-Mendes GP, O'Brien GA, Green DR. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem. 1996;271:28753–6. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- 13.Harter L, Keel M, Hentze H, Steckholzer U, Ungethum U, Trentz O, Erter W. Spontaneous in contrast to CD95-induced neutrophil apoptosis is independent of caspase activity. J Trauma. 2001;50:982–8. doi: 10.1097/00005373-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–35. [PubMed] [Google Scholar]
- 15.Wang Jh. Protective effects of oxygen free radical scavengers during superior mesenteric artery occlusion shock in rats. Clin J Pathophysiol. 1992;8:195–9. [Google Scholar]
- 16.Abello PA, Fidler SA, Bulkley GB, Buchman TG. Antioxidants modulate induction of programmed endothelial cell death (apoptosis) by endotoxin. Arch Surg. 1994;129:134–41. doi: 10.1001/archsurg.1994.01420260030003. [DOI] [PubMed] [Google Scholar]
- 17.Rollet-Labelle E, Grange MJ, Elbim C, Marquetty C, Gougerot- Pocidalo MA, Pasquier C. Hydroxyl radical as a potential intracellular mediator of polymorphonuclear neutrophil apoptosis. Free Radic Biol Med. 1998;24:563–72. doi: 10.1016/s0891-5849(97)00292-x. [DOI] [PubMed] [Google Scholar]
- 18.Ottonello L, Gonella R, Dapino P, Sacchetti C, Dallegri F. Prostaglandin E2 inhibits apoptosis in human neutrophilic polymorphonuclear leukocytes; role of intracellular cyclic AMP levels. Exp Hemtol. 1998;26:895–902. [PubMed] [Google Scholar]
- 19.Gamberale R, Giordano M, Trevani AS, Andonegui G, Geffner JR. Modulation of human neutrophil apoptosis by immune complexes. J Immunol. 1998;161:3666–74. [PubMed] [Google Scholar]
- 20.Hannah S, Mecklenburgh K, Rahman I, Bellingen GJ, Greening A, Haslett C, Chilvers ER. Hypoxia prolongs neutrophil survival in vitro. FEBS Lett. 1995;372:233–7. doi: 10.1016/0014-5793(95)00986-j. [DOI] [PubMed] [Google Scholar]
- 21.Aoshiba K, Nakajima Y, Yasui S, Tamaoki J, Nagai A. Red blood cells inhibit apoptosis of human neutrophils. Blood. 1999;93:4006–10. [PubMed] [Google Scholar]
- 22.Fadeel B, Ahlin A, Henter JI, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis; Regulation by reactive oxygen species. Blood. 1998;92:4808–18. [PubMed] [Google Scholar]
- 23.McLeish KR, Klein JB, Coxon PY, Head KZ, Ward RA. Bacterial phagocytosis activates extracellular signal regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. J Leukoc Biol. 1998;64:835–44. [PubMed] [Google Scholar]
- 24.Ottonello L, Frumento G, Arduino N, Dapino P, Tortolina G, Dallegri F. Immune complex stimulation of neutrophil apoptosis: investigating the involvement of oxidative and nonoxidative pathways. Free Radic Biol Med. 2001;30:161–9. doi: 10.1016/s0891-5849(00)00453-6. [DOI] [PubMed] [Google Scholar]