Abstract
The combination of allograft limbal transplantation (ALT) and amniotic membrane transplantation (AMT) has been applied in the treatment of severe ocular surface diseases. The beneficial effect of this combination has been thought to result from possible immunosuppressive ability of amniotic membrane (AM). However, the mechanisms of any such ability remain unknown. In this study, we investigated whether human AM has the ability to suppress allo-reactive T cell responses in vitro. For mixed lymphocyte reaction (MLR), lymphocytes isolated from lymph nodes of C57BL/6 mice (Mls1b, Vβ6+) were cultured with irradiated splenocytes from DBA/2 mice (Mls1a, Vβ6−) with or without human AM. For carboxyfluorescein diacetate succinimidyl ester (CFSE) experiments, responder lymph node cells were labelled with a stable intracellular fluorescent dye and cultured with irradiated stimulator cells. The ratio of responder Vβ6+ T cells was then determined by FACS analysis, and the division profiles of responder Vβ6+ T cells were analysed by CFSE content. Furthermore, Th1 and Th2 cytokine synthesis by allo-reactive T cells in MLR culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA). Addition of AM to the MLR culture resulted in the significant inhibition of thymidine incorporation compared with control culture lacking AM. The population of responder CD4+Vβ6+ T cells was significantly reduced in the AM-treated culture in comparison to control. CFSE analysis revealed less division and lower proliferation of responder CD4+Vβ6+ T cells in cultures with AM than without. In addition, allo-rective T cell synthesis of both Th1 (IL-2 and IFNγ) and Th2 (IL-6 and IL-10) type cytokine was significantly decreased in the presence of AM. These results indicate that human AM has the ability to suppress allo-reactive T cells in vitro. This inhibitory effect likely contributes to the success of the ALT-AMT combination.
Keywords: amniotic membrane (AM), carboxyfluorescein diacetate succinimidyl ester (CFSE), mixed lymphocyte reaction (MLR), cytokine, flow cytometry
INTRODUCTION
When pathological insults destroy limbal epithelial stem cells, the corneal surface invariably heals with conjunctival epithelial in growth (conjunctivalization), neovascularization, chronic inflammation, and recurrent or persistent corneal epithelial de-fects [1–5]. These pathological conditions constitute the newly established disease called limbal (stem cell) deficiency. This can result from several causes, including total destruction of the limbal stem cell population by chemical or thermal injury, Stevens–Johnson syndrome, multiple surgical or cryotherapy procedures at the limbal region, contact lens wear and severe microbial infection [6,7]. Most cases of limbal deficiency require the use of limbal auto-grafts or allografts for corneal surface reconstruction [8–11]. Therefore, allograft rejection of transplanted limbal tissues is conventionally combated by admini-stering oral steroid and cyclosporin [9,10]. One newly emerging approach in the treatment of limbal deficiency is the use of amniotic membrane transplantation (AMT), first introduced by Kim and Tseng [12]. Amniotic membrane (AM), a thin semitransparent tissue forming the innermost layer of the fetal membrane [13], has a thick continuous basement membrane with a full complement of collagen types IV and V and laminin, the main basement membrane components [14]. AM has been used clinically to promote epithelialization in burns and skin ulcers, or as dressing in wounds or skin grafts [15–17]. It also has been proven suitable for epithelial cell culturing [18,19].
Previously, treatments such as simple penetrating keratoplasty or allograft limbal transplantation could not produce satisfactory results in Stevens–Johnson syndrome, pseudopemphigoid and chemical burns. However several groups have recently re-ported that the combination of allograft limbal transplantation (ALT) and AMT was useful for reconstructing the ocular surface in these diseases [20–22]. Accompanying conditions, including severe dry eye, lack of corneal stem cells, trichiasis, and persistent ocular surface inflammation, exacerbate these diseases’ refractoriness to the treatment. However, it has been hypothesized that AMT effectively facilitates epithelialization and reduces inflammation and scarring, desirable effects for promoting the success of ALT. Transplanted AM also seems to promote normal conjunctival epithelialization, in addition to preventing excessive subconjunctival fibrosis formation. Type IV collagen has been recognized histochemically in conjunctival, but not in corneal epithelial, basement membrane [12]. The collagen in AM therefore probably serves as a suitable substrate for conjunctival epithelialization. In fact, AMT has been regarded as substrate transplantation.
The beneficial effect of the ALT-AMT combination is also thought to result from possible immunosuppressive effect of AM, since placental tissues, including AM, have been shown to suppress the semiallo immune response against the fetus [23,24]. However, the immunological effects of AMT are not yet fully understood. In this study, we investigated whether human AM has the ability to suppress T cell proliferation in vitro. Our findings demonstrated that human AM inhibited allo-reactive T cell re-sponses, including proliferation, cell division and Th1 and Th2 cytokine synthesis. Further, novel results obtained by the study suggest the interesting possibility that soluble inhibitory factor secreted by human AM has the ability to suppress allo-reactive T cells in vitro. It is likely that the immunosuppressive function of human AM contributes to the success of the ALT-AMT combination.
METHODS
Mice
C57BL/6 (H-2b, Mls1b, Vβ6TCR+) and DBA/2 (H-2d, Mls1a, Vβ6TCR−) mice (Shimizu Laboratory Supplies, Kyoto, Japan) 6–12 weeks of age were used for the experiments. These strains differ at the major histocompatibility complex and at numerous minor histocompatibility loci. All animals were housed in the experimental animal facility at Kyoto Prefectural University of Medicine, and received sterilized food and autoclaved tap water. All experimental procedures conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Human amniotic membrane preparation
Human AM was obtained as described previously [25–27]. Briefly, human AM was obtained at the time of cesarean section, with proper informed consent. Under sterile conditions, the AM was washed 3 times in 200 ml phosphate-buffered saline (PBS) containing antibiotics (5 ml of 0·3% ofloxacin), washed once in 50% glycerol/DMEM and stored at −80°C in 50% glycerol/DMEM. AM for explant was then thawed, excised at 10 mm × 10 mm and examined in vitro for immunosuppressive properties. For some experiments, human AM was pretreated with 100% ethanol. This prefixed human AM was extensively washed with PBS prior to use for in vitro experiment.
Cell preparations
Mice were sacrified by cervical dislocation. Lymph node and spleen were then aseptically removed. Lymph node cells and splenic cells, obtained by dissociation using Nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ, USA), were suspended in RPMI 1640 medium (Invitrogen, Grand Island, NY, USA) [28]. Culture medium comprised 10 mm HEPES, 0·1 mm nonessential amino acids, 1 mm sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 × 10−5M 2-mercaptoethanol, 0·1% bovine serum albumin (Sigma, St. Louis, MO, USA), and ITS+ culture supplement (Becton Dickinson Labware).
Culture condition
Human AM on transwell (Corning Incorporatedm New York, NY, USA) was added to the MLR culture of C57BL/6 (Mls1b, Vβ6TCR+) responder and DBA/2 (Mls1a, Vβ6TCR−) stimulator. Lymph node cells (2 × 106) from C57BL/6 mice (as responders) were mixed with gamma-irradiated (20Gly) spleen cells (2 × 106) from histoincompatible DBA/2 mice (as stimulators); the mixture was then added directly to 24 culture wells containing transwell with AM. The positive control was MLR of C57BL/6 responder and DBA/2 stimulator without AM. The negative control culture consisted of C57BL/6 responder and C57BL/6 stimulator cells only.
Proliferation assay
Cultures were incubated for 96 h, including a final 8 h pulsed with tritiated thymidine (3·3 μCi/well). Cells were then divided into 96-well microplates, 200 μl/well, resulting in 1 μCi tritiated thymidine per well, and harvested by the Micro-Mate 196 Cell Harvester (Perkin Elmer Life Science Inc., Boston, MA, USA). Thymidine incorporation was measured by beta-counter (Matrix 9600, Perkin Elmer Life Science Inc.).
CFSE labelling and flow cytometry
The intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes Europe BV, Leiden, the Netherlands) was used to determine cell division in responder cells, as described previously [29,30]. The intracellular fluorescent dye CFSE was used to label cells before in vitro culture. The responder lymph node cells were resuspended in PBS with 0·1% BSA, CFSE was then added to make a final concentration of 5 μm. The suspension was vortexed immediately following CFSE addition, then incubated for 10 min in a 37°C water bath. The labelled cells were washed twice with PBS with 0·1% BSA and resuspended in culture medium. Human AM on transwell was then added to MLR of CFSE-labelled C57BL/6 responder and DBA/2 stimulator. After 4-day culture, cells were double-stained with PE-conjugated anti-Vβ6 mAb and APC-conjugated anti-CD4 mAb (BD PharMingen, San Diego, CA, USA). In addition, the division profiles of responder Vβ6+ T cells were analysed based on the CFSE content. Stained cells were analysed on a FACS Calibur (Becton Dickinson, San Jose, California), data were analysed using Cellquest software (Becton Dickinson).
Determination of Th1 and Th2 cytokine levels in MLR culture supernatants
Th1 and Th2 murine cytokine levels in MLR culture supernatants were determined by cytokine-specific ELISA, as described previously [31,32]. Briefly, Nunc MaxiSorp immuneoplates (Nagel Nunc International, Rochester, NY, USA) were coated with monoclonal anti-IFNγ (BD PharMingen) and left at 4°C overnight. After blocking, samples and serial 2-fold dilutions of standards were added to duplicate wells and incubated overnight at 4°C. The wells were washed and incubated with biotinylated monoclonal anti-IFNγ. After incubation, peroxidase-labelled antibiotin Ab (Vector Laboratories, Burlingame, CA) was added and developed with TMB (Moss, Pasadena, MD). Other cytokine levels IL-2, IL-4, IL-6 and IL-10 (BD PharMingen)] were determined by the same methods as IFNγ Standard curves were generated using mouse recombinant IFNγ (rIFNγ), rIL-2, rIL-4, rIL-6 and rIL-10 (Endogen, Woburn, MA). IFNγ was measured at a sensitivity of 150 pg/ml, IL-2 at a sensitivity of 300 pg/ml, IL-4 at a sensitivity of 150 pg/ml, IL-6 at a sensitivity of 400 pg/ml and IL-10 at a sensitivity of 800 pg/ml.
To assess human-specific cytokines, ELISA kits specific for IL-4, TGF-β (Amersham Biosciences KK, Tokyo, Japan) and IL-10 (BioSource International, California) were used. Human IL-4 was measured at a sensitivity of 10 pg/ml, human IL-10 at a sensitivity of 30 pg/ml and human TGF-β at a sensitivity of 125 pg/ml.
Data analysis
Data were expressed as mean ± SE, and were evaluated by student's t-test using the Excel program.
RESULTS
Human AM inhibits allo-reactive T cell responses in murine MLR
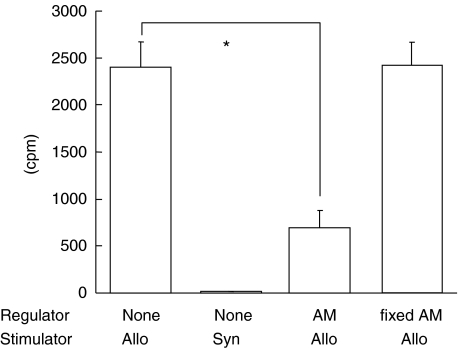
We initially investigated whether human AM suppressed MLR. Human AM on transwell was added to MLR cultures of C57BL/6 responder and histoincompatible DBA/2 stimulator. The positive control was the MLR culture of C57BL/6 responder and DBA/2 stimulator, without AM. The negative control culture consisted of responder and stimulator cells from C57BL6 mice. As expected, the positive control resulted in a high level of thymidine incorporation (2401 ± 272 cpm, Fig. 1). However, it was interesting to note that human AM in transwell significantly inhibited MLR (691 ± 189 cpm). This finding suggested the interesting possibility that human AM in transwell may produce an inhibitory factor that suppresses allo-reactive T cells in MLR. To examine this possibility we used AM fixed with 100% ethanol. Fixed human AM on transwell was added to MLR of C57BL/6 responder and DBA/2 stimulator, and compared with MLR containing untreated AM. Interestingly, MLR cultured with fixed AM showed thymidine incorporation levels (2416 ± 245 cpm) comparable to MLR without AM. MLR cultured with fixed AM was not significantly different from that without AM. Murine MLR was not suppressed by fixed human AM. These results suggest that cultured human AM might produce immunosuppressive factors, and that murine MLR might be suppressed by soluble factors produced by human AM.
Fig. 1.
Inhibition by human AM of proliferative responses of murine allo-reactive mixed lymphocyte reaction (MLR). Human AM was added to the transwell of MLR culture containing C57BL/6 responder and DBA/2 stimulator. Lymph node cells (2 × 106 cells/ml) from C57BL/6 mice (as responders) were mixed with gamma-irradiated (20Gly) spleen cells (2 × 106 cells/ml) from histoincompatible DBA/2 mice (as stimulators). These MLR cultures were then cocultured with human AM in the transwell of 24 wells. These culture plates were incubated for 4 days, during the last 8 h of incubation, 3H-thymidine was added. Positive allogenic control was MLR of C57BL/6 responder and DBA/2 stimulator without human AM. Negative syngenic control consisted of responder and stimulator cells from C57BL/6 without human AM. In some experiments, human AM was prefixed with 100% ethanol, dried, and washed with PBS 4 times. This fixed human AM was added to the transwell of allogenic MLR culture containing C57BL/6 responder and DBA/2 stimulator cells. Radioisotope incorporation detected in 4 separate experiments with triplicate wells is presented as mean cpm ± SEM. (*P < 0·001)
Inhibition of responder CD4+Vβ6+ T cells by human AM
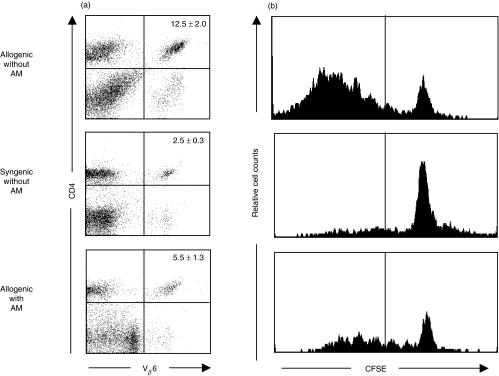
To directly determine whether human AM has the ability to suppress responder T cells in vitro, we next investigated AM effect on responder T cell proliferation by flow cytometry, using a combination of CFSE and mAbs specific for CD4 and Vβ6. Since stimulator DBA/2 mice have minor lymphocyte-stimulating (Mls) 1a superantigen [33], CD4+Vβ6+ T cells of responder C57BL6 mice were isolated as Mls1a superantigen reactive T cells. Results were expressed as percentages of CD4+Vβ6+ cells among lymphocytes isolated from allogenic MLR with AM, allogenic MLR without AM (positive control), and syngenic MLR without AM (negative control). The mean percentage of CD4+Vβ6+ T cells was 5·5% in allogenic MLR with AM, while the positive and negative control groups contained 12·5% and 2·5% of CD4+Vβ6+ T cells, respectively (Fig. 2a). FACS analysis revealed that the mean percentage of CD4+Vβ6+ T cells in MLR with AM was significantly less than in MLR without AM. The proliferation of responder CD4+Vβ6+ T cells was suppressed by the cultured human AM, possibly via their derived soluble factors.
Fig. 2.
Suppression of responder CD4+, Vβ6+ T cell division by human AM. FACS profile of CD4+Vβ6+ T cells (a) and analysis of responder CD4+Vβ6+ T cell division (b) in murine MLR in the presence or absence of human AM. Lymph node cells (2 × 106) from C57BL/6 mice (as responders) were mixed with gamma-irradiated (20Gly) spleen cells (2 × 106) from histoincompatible DBA/2 mice (as stimulators) in the presence or absence of human AM in the MLR culture transwell. Intracellular fluorescent dye, CFSE, was used to label responder cells before in vitro culture. After 4-day culture, cells were double stained with PE-conjugated anti-Vβ6 mAb and APC-conjugated anti-CD4 mAb. Results of mean percentages from 3 independent experiments are presented as mean (%) ±SEM. Data of CFSE histograms represent of 2 separate experiments.
We next labelled live CD4+Vβ6+ T cells in vitro with a stable, fluorescent dye, CFSE that segregates equally between daughter cells upon cell division, enabling fine monitoring of the proliferative history of any T cell present or generated during a response [34]. This system permits simultaneous evaluation of T cell surface markers, and concomitant assessment of cellular activation. T cell division in CFSE-labelled responder cell populations was kinetically analysed. The CFSE histograms were gated for CD4+Vβ6+ T cells on day 4 of the MLR culture, and showed the CFSE fluorescence profile (Fig. 2b). In the positive allogenic MLR culture, the CFSE histograms include large numbers of cells that had undergone more than 3 divisions (left of line). In the syngenic MLR culture without AM, the CFSE histograms revealed the absence of those divided cells. In human AM-treated allogenic MLR cultures, the CFSE histograms demonstrated that smaller numbers of cells had undergone more than 3 divisions, as compared with the positive control (Fig. 2b). These results demonstrate that the cell division and proliferation of responder CD4+Vβ6+ T cells were suppressed by human AM soluble factors.
Suppression of MLR induced Th1 and Th2 cytokine syntheses by human AM
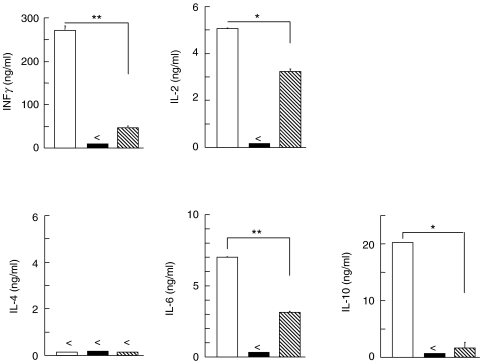
To examine the effects of AM on MLR-induced T cell cytokine production, the levels of mouse-derived Th1-(IFNγ and IL-2) and Th2-(IL-6 and IL-10) type cytokines in MLR culture supernatants were measured on the basis of their maximal production results of time-course study. To this end, IL-2 synthesis was measured in day 2 MLR supernatants, while the other cytokines were measured in day 4 MLR supernatants. Thus, the culture supernatants from the positive MLR control group contained high amounts of both Th1 and Th2 type cytokines. It should be noted that levels of IFNγ, IL-2, IL-6 and IL-10 synthesis decreased significantly when AM was added to the MLR culture transwell (Fig. 3). Moreover, undetectable levels of Th1 (IFNγ, IL-2) or Th2 (IL-6 and IL-10)-type cytokines were produced in the syngenic MLR without human AM group (negative control). Taken together, Th1 (IL-2, IFNγ) and Th2 (IL-6, IL-10)-type cytokines produced by allogenic MLR were significantly inhibited by the presence of human AM in the transwell. To determine whether known regulatory cytokines possessing inhibitory function were produced by human AM in the MLR culture transwell, we examined levels of human inhibitory cytokines (IL-4, IL-10 and TGF-β) in the human AM transwell. None of these human cytokines were detected in the culture supernatant (data not shown).
Fig. 3.
Inhibition by human AM of Th1 and Th2 cytokine synthesis in murine MLR. Levels of cytokine production in culture supernatants of allogenic MLR with AM ( ), allogenic MLR without AM (□) and syngenic MLR without AM (▪) were examined by murine Th1 and Th2 cytokine-specific ELISA. Following 2 day incubation, IL-2 synthesis level was measured. In case of IFNγ, IL-6 and IL-10, supernatants harvested from 4 day cultures were subjected to cytokine-specific ELISA. Data represent mean ± SEM from 1 experiment with duplicated wells. (<not detectable; *P < 0·01; **P < 0·001)
), allogenic MLR without AM (□) and syngenic MLR without AM (▪) were examined by murine Th1 and Th2 cytokine-specific ELISA. Following 2 day incubation, IL-2 synthesis level was measured. In case of IFNγ, IL-6 and IL-10, supernatants harvested from 4 day cultures were subjected to cytokine-specific ELISA. Data represent mean ± SEM from 1 experiment with duplicated wells. (<not detectable; *P < 0·01; **P < 0·001)
DISCUSSION
First, this study shows that human AM is capable of inhibiting allo-reactive T cell response in in vitro murine MLR. Murine MLR cultured with human AM in transwells showed significantly less thymidine incorporation than that cultured without AM. Next, this study demonstrates that human AM is capable of inhibiting responder CD4+Vβ6+ T cells in in vitro murine MLR. To this end, FACS analysis revealed that the mean percentage of CD4+Vβ6+ T cells in the culture of MLR with AM was significantly less than in the MLR without AM. CFSE analysis also revealed less division of responder CD4+Vβ6+ T cells in MLR cultures with AM than without. Furthermore, Th1 and Th2 cytokine production by allo-reactive T cells was also inhibited by human AM in the MLR culture transwell. Taken together, evidence that murine MLR was suppressed by the cultured human AM in transwell indicates that an immunosuppressive factor could be produced by human AM.
Our present results show that cultured human AM tissues inhibit MLR when separated from the mixed lymphocytes by a 0·4-μm pore membrane, indicating that MLR inhibition by human AM is mediated by a soluble factor. To support this view, pretreatment of human AM with 100% ethanol resulted in removal of the inhibitory effect. To our knowledge, this is the first report demonstrating immnosuppressive effects by human AM, possibly via secreted inhibitory factor. Since Streilein et al.[35] have proposed that soluble factors secreted by explanted ocular tissues in vitro represent the ability of these tissues to create and sustain an immunosuppressive microenvironment in vivo within the eye, it is highly possible that our present demonstration of the immunosuppressive effect of human AM in vitro allogenic T cell responses reflects in immunoinhibitory function of human AM when transplantated to the eye. It has been demonstrated that murine iris/ciliary body tissues and cells display the ability to suppress murine MLR to which they have been added as regulatory cells [28]. In addition, another previous report showed that rat ciliary body cells were also capable of inhibiting Ag-driven Th lymphocyte proliferation [36]. This inhibitory activity was not species specific, since similar inhibitory effects were observed with bovine and human ciliary epithelial cells [36]. Taking these previous and our present findings together, it is interesting to suggest that human AM may produce a known or unknown soluble factor capable of inhibiting allogenic T cell responses without species specificity.
ALT is rejected easily, sometimes even with immunosuppressive treatment (e.g. steroid). As regards allo-immunogenicity of corneal epithelium, a previous study showed that an intact epithelial structure containing classII MHC-bearing cells (corneal limbus), is capable of inducing proliferation among allo-reactive T lymphocytes [28]. It has been suggested that Langerhans cells migrate out of the limbal tissue to where they can encounter responding lymphocytes for subsequent initiation of MHC-restricted alloantigen reaction [28]. Another previous study showed that full-thickness allogenic corneas induce vigorous delayed hypersensitivity for eventual rejection [37]. Similar re-sults were obtained with allografts of corneal epithelium alone and stromal allografts deprived of endothelium [37]. Furthermore, a previous study has shown that allogenic corneas deprived of epithelium and placed beneath the kidney capsule did not undergo immune rejection during prolonged follow-up [38]. Taken together, these results lead to the reasonable hypothesis that the epithelium is the site primarily responsible for the allo-immunogenicity of heterotopic corneal grafts; and that corneal epithelium has strong alloimmunogenicity. As well, outside the eye, afferent lymphatic vessels carry antigens in highly immunogenic form to regional lymph nodes in which naive T cell activation first occurs, leading to induction of conventional immunity [39]. The combination of ALT and AMT is more successful for the ocular surgery than ALT only, since AMT is thought to have the beneficial effect of AM's immunosuppressive ability. To this end, our present study directly and experimentally demonstrated that human AM possesses suppressive function that inhibits allo-reactive T cell response using murine MLR system. Further, the result suggested an interesting possibility that human AM may secrete inhibitory factor which can suppress allo-reactive T cell responses.
Previous studies have demonstrated that several factors contribute to the presence of immunological privilege in the eye. Biological fluid obtained from the anterior chamber of the eye has been shown to be immunosuppressive, an effect at least partly explained by the presence of TGF-β[40–43]. It has been demonstrated that mouse and rat iris/ciliary body cells can produce immunosuppressive factors, including TGF-β for the inhibition of MLR [28,36]. Human AM has been shown to express mRNAs for, and produce, TGF-β1 and -β2 [26]. In addition, a previous report has shown that in vitro, cytotrophoblasts, a placental tissue, produce IL-10, a cytokine that potentially inhibits alloresponse in MLR [44]. Furthermore, a previous study showed that amnion epithelial cells also expressed inhibitory cytokine such as IL-4 in protein and mRNA [45]. An obvious assumption therefore is that human AM-associated suppressor function could be due to the production of those inhibitory cytokines. To assess this possibility, we investigated the production by human AM of selected human cytokines thought to possess immune-suppressive effects, such as IL-4, IL-10, TGF-β1. However, we could not detect IL-4, IL-10 or TGF-β1 in the culture supernatant of MLR with human AM in transwells. Although those findings suggest that human AM in MLR culture did not produce the known inhibitory cytokines, including IL-4, IL-10 and TGF-β, additional confirmation is required as the lack of such inhibitory cytokine synthesis at the mRNA level. Further, it is important to examine whether stimulated human AM is capable of producing these inhibitory cytokines.
In the present study, we could not determine the AM soluble factor that suppresses allo-reactive T cells. In addition to the possibility of inhibitory cytokines, PGE2, which can inhibit IL-2 synthesis, may have immunosuppressive ability [46–48]. A previous study has indicated that human amnion cells produce PGE2 which can be enhanced by the presence of granulocyte supernatants. It has been also demonstrated that HLA-G inhibits the allogenic proliferative response [49], and that soluble HLA-G is present in amniotic fluids [50]. Furthermore, human fetal membrane expresses FasL, by which the fetus is afforded protection against the cytolytic actions of lymphocytes from the mother [51–54]. Thus, it is possible that the inhibitory effect of human AM could be explained by the presence of PGE2, HLA-G and FasL, in addition to inhibitory cytokines. In addition, we have to consider that these inhibitory factors could be induced by exposure of human AM to soluble factors derived from lymphocytes in MLR culture. To formally assume that human AM produces a new inhibitory molecule for suppression of allo-graft rejection in ALT, we must carefully perform a series of additional experiments. These lines of study are under investigation in our laboratory.
In summary, our present data demonstrate that human AM is capable of inhibiting allo-reactive T cell response including cell division, proliferation and Th1/Th2 cytokine synthesis in vitro. Further, our findings suggest the interesting possibility that human AM may secrete an undefined inhibitory factor for the suppression of allogenic response. This inhibitory effect of human AM likely contributes to the success of the ALT-AMT combination in ocular surgery.
REFERENCES
- 1.Kinoshita S, Kiorpes T, Friend J, et al. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982;23:73–80. [PubMed] [Google Scholar]
- 2.Shapiro M, Friend J, Thoft R. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981;21:135–42. [PubMed] [Google Scholar]
- 3.Schermer A, Galvin S, Sun T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotsarelis G, Cheng S, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 5.Huang A, Tseng S. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- 6.Tseng S. Concept and application of limbal stem cells. Eye. 1989;3:141–57. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita S. Chemical Burns. In: Brightbill F, editor. Corneal Surgery. St. Louis: Mosby; 1986. pp. 309–16. [Google Scholar]
- 8.Kenyon K, Tseng S. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 9.Tsai R, Tseng S. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tsubota K, Toda I, Saito H, et al. Reconstruction of the corneal epi-thelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–96. doi: 10.1016/s0161-6420(95)30841-x. [DOI] [PubMed] [Google Scholar]
- 11.Holland E, Schwartz G. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–56. [PubMed] [Google Scholar]
- 12.Kim J, Tseng S. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–84. [PubMed] [Google Scholar]
- 13.van Herendael B, Oberti C, Brosens I. Microanatomy of the human amniotic membranes. A light microscopic, transmission, and scanning electron microscopic study. Am J Obstet Gynecol. 1978;131:872–80. doi: 10.1016/s0002-9378(16)33135-0. [DOI] [PubMed] [Google Scholar]
- 14.Modesti A, Scarpa S, D’Orazi G, et al. Localization of type IV and V collagens in the stroma of human amnion. Prog Clin Biol Res. 1989;296:459–63. [PubMed] [Google Scholar]
- 15.Trelford J, Trelford-Sauder M. The amnion in surgery, past and present. Am J Obstet Gynecol. 1979;134:833–45. doi: 10.1016/0002-9378(79)90957-8. [DOI] [PubMed] [Google Scholar]
- 16.Prasad J, Feller I, Thomson P. Use of amnion for the treatment of Stevens–Johnson syndrome. J Trauma. 1986;26:945–6. doi: 10.1097/00005373-198610000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Subrahmanyam M. Amniotic membrane as a cover for microskin grafts. Br J Plast Surg. 1995;48:477–8. doi: 10.1016/0007-1226(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 18.Lwebuga-Mukasa J, Thulin G, Madri J, et al. An acellular human amnionic membrane model for in vitro culture of type II pneumocytes: the role of the basement membrane in cell morphology and function. J Cell Physiol. 1984;121:215–25. doi: 10.1002/jcp.1041210127. [DOI] [PubMed] [Google Scholar]
- 19.van der Linden PJ, de Groetj AF, Dunselman GA. Endometrial cell adhesion in an in vitro model using intact amniotic membranes. Fertil Steril. 1996;65:76–80. [PubMed] [Google Scholar]
- 20.Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens–Johnson syndrome. Am J Ophthalmol. 1996;122:38–52. doi: 10.1016/s0002-9394(14)71962-2. [DOI] [PubMed] [Google Scholar]
- 21.Shimazaki J, Yang H, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–76. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 22.Tseng S, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–41. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 23.Sionov R, Yagel S, Har-Nir R, et al. Trophoblasts protect the inner cell mass from macrophage destruction. Biol Reprod. 1993;49:588–95. doi: 10.1095/biolreprod49.3.588. [DOI] [PubMed] [Google Scholar]
- 24.Beer A, Sio J. Placenta as an immunological barrier. Biol Reprod. 1982;26:15–27. doi: 10.1095/biolreprod26.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Koizumi N, Fullwood N, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–13. [PubMed] [Google Scholar]
- 26.Koizumi N, Inatomi T, Sotozono C, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 27.Koizumi N, Inatomi T, Quantock A, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000;19:65–71. doi: 10.1097/00003226-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Williamson J, Bradley D, Streilein J. Immunoregulatory properties of bone marrow-derived cells in the iris and ciliary body. Immunology. 1989;67:96–102. [PMC free article] [PubMed] [Google Scholar]
- 29.Fulcher D, Wong S. Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory. Immunol Cell Biol. 1999;77:559–64. doi: 10.1046/j.1440-1711.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 30.Groth BdS, Smith A, Koh W, et al. Carboxyfluorescein diacetate succinimidyl ester and the virgin lymphocyte: a marriage made in heaven. Immunol Cell Biol. 1999;77:530–8. doi: 10.1046/j.1440-1711.1999.00871.x. [DOI] [PubMed] [Google Scholar]
- 31.Okahashi N, Yamamoto M, Vancott J, et al. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–25. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanCott J, Staats H, Pascual D, et al. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–14. [PubMed] [Google Scholar]
- 33.Sutmuller M, Baelde H, Ouellette S, et al. T-cell receptor Vbeta gene expression in experimental lupus nephritis. Immunology. 1998;95:18–25. doi: 10.1046/j.1365-2567.1998.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells A, Gudmundsdottir H, Turka L. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streilein J, Bradley D, Sano Y, et al. Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas. Invest Ophthalmol Vis Sci. 1996;37:413–24. [PubMed] [Google Scholar]
- 36.Helbig H, Gurley R, Palestine A, et al. Dual effect of ciliary body cells on T lymphocyte proliferation. Eur J Immunol. 1990;20:2457–63. doi: 10.1002/eji.1830201115. [DOI] [PubMed] [Google Scholar]
- 37.Hori J, Joyce N, Streilein J. Immune privilege and immunogenicity reside among different layers of the mouse cornea. Invest Ophthalmol Vis Sci. 2000;41:3032–42. [PubMed] [Google Scholar]
- 38.Hori J, Joyce N, Streilein J. Epithelium-deficient corneal allografts display immune privilege beneath the kidney capsule. Invest Ophthalmol Vis Sci. 2000;41:443–52. [PubMed] [Google Scholar]
- 39.Zierhut M, Elson C, Forrester J, et al. Mucosal immunology and the eye. Immunol Today. 1998;19:148–50. doi: 10.1016/s0167-5699(97)01229-2. [DOI] [PubMed] [Google Scholar]
- 40.Cousins S, McCabe M, Danielpour D, et al. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–11. [PubMed] [Google Scholar]
- 41.Knisely T, Bleicher P, Vibbard C, et al. Production of latent trans-forming growth factor-beta and other inhibitory factors by cultured murine iris and ciliary body cells. Curr Eye Res. 1991;10:761–71. doi: 10.3109/02713689109013870. [DOI] [PubMed] [Google Scholar]
- 42.Wilbanks G, Streilein J. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-beta. Eur J Immunol. 1992;22:1031–6. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- 43.Granstein R, Staszewski R, Knisely T, et al. Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation. J Immunol. 1990;144:3021–7. [PubMed] [Google Scholar]
- 44.Roth I, Corry D, Locksley R, et al. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–48. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones C, Williams K, Finlay-Jones J, et al. Interleukin 4 production by human amnion epithelial cells and regulation of its activity by glycosaminoglycan binding. Biol Reprod. 1995;52:839–47. doi: 10.1095/biolreprod52.4.839. [DOI] [PubMed] [Google Scholar]
- 46.Felli M, Moschella C, Farina A, et al. Prostaglandin E2 inhibits the interleukin-2 promoter activity through down-regulation of the Oct – dependent transcription of the octamer motif. Cell Immunol. 1996;172:229–34. doi: 10.1006/cimm.1996.0237. [DOI] [PubMed] [Google Scholar]
- 47.Choudhry M, Ahmad S, Ahmed Z, et al. Prostaglandin E2 down- regulation of T cell IL-2 production is independent of IL-10 during gram-negative sepsis. Immunol Lett. 1999;67:125–30. doi: 10.1016/s0165-2478(99)00003-6. [DOI] [PubMed] [Google Scholar]
- 48.Gurlo T, Huang W, Grafenstein Hv. PGE2 inhibits IL-2 and IL-4-dependent proliferation of CTLL-2 and HT2 cells. Cytokine. 1998;10:265–74. doi: 10.1006/cyto.1997.0288. [DOI] [PubMed] [Google Scholar]
- 49.Riteau B, Menier C, Khalil-Daher I, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43:203–11. doi: 10.1016/s0165-0378(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 50.Rebmann V, Pfeiffer K, Passler M, et al. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens. 1999;53:14–22. doi: 10.1034/j.1399-0039.1999.530102.x. [DOI] [PubMed] [Google Scholar]
- 51.Hammer A, Blaschitz A, Daxbock C, et al. Fas and Fas-ligand are expressed in the uteroplacental unit of first-trimester pregnancy. Am J Reprod Immunol. 1999;41:41–51. doi: 10.1111/j.1600-0897.1999.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 52.Hammer A, Dohr G. Expression of Fas-ligand in first trimester and term human placental villi. J Reprod Immunol. 2000;46:83–90. doi: 10.1016/s0165-0378(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 53.Runic R, Lockwood C, Ma Y, et al. Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J Clin Endocrinol Metab. 1996;81:3119–22. doi: 10.1210/jcem.81.8.8768884. [DOI] [PubMed] [Google Scholar]
- 54.Runic R, Lockwood C, LaChapelle L, et al. Apoptosis and Fas expression in human fetal membranes. J Clin Endocrinol Metab. 1998;83:660–6. doi: 10.1210/jcem.83.2.4600. [DOI] [PubMed] [Google Scholar]